| Research Article |
Open Access |
|
| Chunshui Pan1, Yingqing Huo1, Xiaojin An1,3, Gurbakhshish Singh3, Meng Chen1, Zhaoxiang Yang2, Junxue Pu2 and Jian Li1,3* |
| 1Laboratory of Vascular Biology, Institute of Molecular Medicine, Peking University, Beijing, China |
| 2Kunming Pharmaceutical Institute, Yunnan, China |
| 3Division of Cardiovascular Medicine, Cardiovascular Institute, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, USA |
| *Corresponding author: |
Jian Li
Division of Cardiovascular Medicine
CardioVascular Institute Beth Israel Deaconess Medical Center
Harvard Medical School
3 Blackfan Circle. Boston MA 02115, USA
E-mail: JLi@BIDMC.Harvard.edu |
|
| Â |
| Received March 16, 2011; Published October 30, 2012 |
| Â |
| Citation: Pan C, Huo Y, An X, Singh G, Chen M, et al. (2012) The Mechanism of Ginsenoside Rb1 and Rg1 in Vascular Reaction. 1:444 doi:10.4172/scientificreports.444 |
| Â |
| Copyright: © 2012 Pan C, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. |
| Â |
| Abstract |
| Â |
| Ginsenoside Rb1 and Rg1 are major components of Panax notoginseng (P.N.), an herb with a known clinical efficacy in hypertension and myocardial ischemia. This investigation is to elicit the mechanism of these components in vascularb reaction. Examination with Rb1 and Rg1 revealed significant vasodilatation in a dose dependent manner in a mouse microvessel reactivation assay. Rb1- and Rg1-induced vasodilatation was blocked by pre-incubation with eNOS and PI3K inhibitors. Coronaries of eNOS-/- mice showed attenuated vasodilatation with Rb1 and Rg1. Both Rb1 and Rg1 induce nitric oxide (NO) generation through increasing the phosphorylation of eNOS, activating Na+-independent L-arginine transport, and stimulating cationic amino acid transport (CAT)-1 mRNA expression in endothelial cells. In addition, P.N. extracts significantly lowered blood pressure in spontaneously hypertensive rats (SHR). Ginsenoside Rb1 and Rg1 increased endothelial-dependent vessel dilatation through the activation of NO by modulating the PI3K/Akt/eNOS pathway and L-arginine transport in endothelial cells. |
| Â |
| Keywords |
| Â |
| Panax notoginseng (P.N.); Rb1; Rg1; L-arginine/eNOS/ NO; PI3K/Akt; Vasodilatation |
| Â |
| Introduction |
| Â |
| Panax notoginseng (P.N.) is an herb that belongs to the acanthopanax gracilistylus family and is synonymous with stephania sinica and pseudoginsen radix. It is one of the famous traditional medicinal herbs that have been used for hundreds of years in many East Asian countries [1]. P.N. consists of two major ingredients: crude ginseng saponin and crude ginseng non-saponin. To date, thirty different types of saponins [2] have been isolated from ginseng and identified chemically. They can be classified into three major groups according to their chemical structure: 20 (S)-protopanaxadiol, 20 (S)-protopanaxatriol and oleanolic acid saponins. Ginsenoside Rb1 (Rb1) and ginsenoside Rg1 (Rg1) are contained in Panax notoginseng (P.N.), P. ginseng (P.G.), P. quinequefolius (P.Q.) and other Panax species. P.G. and P.Q. are generally categorized as adaptogenics, while P.N. is categorized as hemostatics [3-5]. Clinical reports suggest that P.N. plays an important role in the treatment of inflammation, coronary heart disease, stroke and immunological disease [5-7]. However, the mechanism of this medicine has not been fully studied. Recent reports suggest that Rg1 can serve as an agonist ligand for glucocorticoid receptors (GR), and the activated GR can induce rapid nitric oxide (NO) production in Human Umbilical Vein Endothelial Cells (HUVECs) from endothelial nitric oxide synthase (eNOS) via the non-transcriptional PI3K/Akt pathway [8,9]. Moreover, the PI3K/Akt and MEK/ERK pathways and androgen receptor are involved in the regulation of acute eNOS activation by Rb1 in human aortic endothelial cells [10]. The activated PI3K/Akt pathway leads to phosphorylation of eNOS and increased production of NO [11]. This pathway is a crucial regulator in cell proliferation, cell-cycle progression, and a mediator of cellular survival [12,13], which explains the NO-related endothelial proliferation and angiogenesis [14] that was shown to be activities of Rg1 and Rb1. This work reveals that P.N. could decrease blood pressure; Rb1 and Rg1 dilated the coronary arteries, and L-NAME, the selective inhibitor of eNOS, significantly attenuated their vasodilatory effect. Rb1 and Rg1 also increased CAT-1 mRNA involved in altering L-arginine transport. In summary, the major components of P.N. activate the relaxation of coronary arteries via the L-arginine/ eNOS/NO pathway. |
| Â |
| Materials and Methods |
| Â |
| Extraction and quality control of P.N. and its components |
| Â |
| Panax notoginseng (P.N., San-Chi in Chinese, Yunnan, China) was collected and subjected to ethanol extraction. The yield rate of Panax notoginsenosides (P.N.S.) from Panax notoginseng (P.N.) was around 10%. In P.N.S, the yield rate of Rb1 was around 30-35%, and Rg1 was around 20-29%. To ensure quality control, HPLC was carried out with a Waters HPLC system using an ODS-A column with a solvent system consisting of methanol-water. The UV detection was monitored at 203 nm and the following chromatogram was obtained (Supplementary figure 1). The molecular weight of Rb1 and Rg1 is 1109.29 and 801.01, respectively. |
| Â |
| Vasorelaxation of mouse coronary artery |
| Â |
| The left anterior descending coronary arteries were dissected from the heart of wild type (C57/BL6) or endothelial Nitric Oxide synthase (eNOS) knock-out mice (Jackson Laboratories) and placed in ice-cold physiological salt solution (PSS) composed of (in mmol/L): NaCl 119, KCl 4.7, CaCl2·2H2O 2.5, MgSO4·7H2O 1.2, NaHCO3 25, KH2PO4 1.2, and glucose 5.5. Microvessels were placed in a microvessel chamber and cannulated with dual glass micropipettes, as previously described [15]. For all measurements, the vessels were washed three times and allowed to equilibrate in the Krebs’ buffer solution 15-30 min between interventions. The vessel was visualized using an inverted microscope connected to a video camera (VM-902; Hitachi Denshi Ltd), and the internal lumen diameter was measured with an electronic imaging apparatus (Living Systems Inc., VT). Vessels were pre-contracted with the thromboxane A2 analog U46619 by 30% of the baseline diameter prior to application of a vasodilator agent [16]. Rb1 (10-9~10-5 g/mL) or Rg1 (10-9~10-5 g/mL) was used to examine the vasorelaxation of mouse coronary artery. To study the effects of Rg1and Rb1 on NO signaling, pharmacological inhibitors including L-NAME (10-5M, Alexis, MA), the inhibitor of eNOS, and LY294002 (2*10-5M, Cayman Chemical, MI), the inhibitor of PI3K/Akt, were pre-perfused for 30 min before adding Rg1 or Rb1 in PSS (10 mL/min) through the artery. To test whether eNOS or Ca2+ was involved in the vasodilatory effect, dose–response curves for sodium nitroprusside (SNP, NO donor, 10-9~10-4 g/mL, Sigma, MO), and calcium ionophore A23187 (10-9~10-4 g/mL, Sigma, MO) were established in eNOS-/-mice. |
| Â |
| Endothelial cell culture and treatments |
| Â |
| Porcine aortic endothelial cells (PAECs) were harvested from porcine aorta by the method of enzymatic dissociation [17]. PAECs were then cultured in M199 media (Gibco, CA) supplemented with 10% fetal bovine serum (FBS) (Hyclone, MA) and antibiotics (0.1 mg/mL streptomycin, 100 U/mL penicillin). PAECs were incubated in serum-depleted medium for 20 hours prior to incubation with Rb1 or Rg1. The experiments were performed on cells between passages 4 to 6. PAEC viability was determined by trypan blue exclusion test. |
| Â |
| L-arginine uptake assay |
| Â |
| L-arginine uptake was determined in cells that had previously been depleted of intracellular free amino acids by incubation for 2 h in Krebs solution. Cells were then incubated in the presence or absence of Rb1 (10-9~10-5 g/ml) or Rg1 (10-9~10-5 g/ml) for 3 h. L-arginine uptake was measured by incubating the cells for 5 min at 37°C with Krebs bicarbonate solution containing [3H] L-arginine (GE healthcare, 50 mM, 1 mCi/mL) and L-arginine (Sigma, 64 μmol/L). L-arginine transport was also measured in Krebs solution in which NaCl was replaced equimolarly by choline chloride [10]. Radioactivity of the ambient incubation buffer and solution was measured by beta-scintillation counting (Bechman FJ-2170, Mississauga, ON). To correct for nonspecific uptake and binding, cells were incubated in parallel wells with buffer containing [3H] L-arginine, the fraction of the radioactivity associated with the cells was determined, and this fraction was then subtracted. |
| Â |
| NO Production assay |
| Â |
| Various concentrations of Rb1 and Rg1 were prepared in phenol red-free DMEM medium to reduce assay interference by phenol red. Cell culture supernatants were collected after treatment with different compounds (or vehicle alone in the controls) for 3 hours. NO production was detected spectrophotometrically by measuring its final stable equimolar degradation products, nitrite and nitrate, by using nitrate reductase (Assay Design Inc.) and the acid-catalyzed diazotation reaction by using sulfanylamide and naphtylethylenediamine (Griess reaction). Total nitrite was quantified after the reduction of all nitrates with nitrate reductase. Nitrite levels in culture supernatants were within the linearity range of calibration curves that were generated from a solution of sodium nitrite. Total nitrite concentration was calculated from a standard curve constructed over the linear range of the assay and expressed as 100 pmol per 105 cells. |
| Â |
| Real- time Q-PCR |
| Â |
| The regulation of CAT-1 mRNA expression by Rb1 or Rg1 was determined by real-time quantitative polymerase chain reaction (Q-PCR) analysis. Total RNA was isolated using an RNA miniprep kit according to the manufacturer’s instructions (Stratagene, CA, USA). The specific primer pair for CAT-1 resulting in a 328-bp PCR product was: forward primer: 5’-CTCTCCTACATCATCGGTAC-3’, reverse primer: 5‘-GGCATAGATAACTCGCG-3’. The primer sequences for GAPDH were: forward: 5’-CACGACCATGGAGAAGGCTG-3’and reverse: 5’-TCCACGATGCCGAAGTTGTC-3’. Amplification was followed by melting curve analysis to verify the accuracy of the amplicon. A negative control without cDNA was run with every PCR to assess the specificity of the reaction. Analysis of data was performed using Light Cycler software version 3.5. |
| Â |
| Western blot analysis |
| Â |
| The mouse hearts and PAECs were lysed in RIPA buffer (Boston Bioproducts, MA) with protease inhibitors, and proteins were resolved by SDS/PAGE gel and transferred to a PVDF membrane (Millipore, MA). After being blocked for 1 h in TBS-T with 5% nonfat milk, the PVDF membrane was then probed with primary antibodies polyclonal rabbit anti-Akt (1:1000) or anti-phosphorylated Ser473-Akt (1:1000, Cell Signaling), mouse anti-eNOS/NOSIII (1:2500) or anti-phosphorylated Ser1177-eNOS (1:1000, BD), and rabbit anti-tubulin β (1:3000, Sigma, served as the internal control) at 4°C overnight and then incubated (1 hour) in TBST/0.2% BSA containing horseradish peroxidase-conjugated anti-rabbit or anti-mouse antibodies (1:5000). Cox-2 inhibitor NS398 (10−6 M, Sigma), PI3K inhibitor LY294002 (10 μM, Sigma) and a PKC inhibitor, GFX (1 μM, Sigma) were used. The proteins were visualized with an ECL detection system (Amersham). Semi-quantifications were performed with densitometric analysis by Metamorph software. |
| Â |
| Animals and measurement of blood pressure |
| Â |
| Male 11-week-old SHR and Wistar Kyoto (WKY) rats weighing 250 to 320g were purchased from Beijing Vital Laboratory Technology (Beijing, China) and housed under standard conditions at 25°C with a 12/12-hour light-dark cycle. SHR and WKY rats were randomly divided into 2 groups, weighed, and the conscious animals’ blood pressures were measured on a rat tail-cuff (Model RBP-1) for 30min, followed by administration of P.N. extracts (100 mg/kg) or isometric saline through the caudal vein. Final BP measurements were the mean of 5~6 determinations for each individual animal. All animal care and experimental protocols conform to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health, and approval was granted by the IACUC of PKU (approval reference number IMM-LiJ-1). |
| Â |
| Statistic Analysis |
| Â |
| All values are expressed as mean ± SEM. Relaxation in response to herbs is presented as percent change in tension from pre-constriction levels. When multiple vessel rings were studied from one mouse, responses were averaged, and n represents the number of mice per group. Comparisons were made by using a one-way ANOVA with repeated measures, followed by the Student-Newman-Keuls test to detect individual differences. P |
| Â |
| Results |
| Â |
| P.N., Rb1 and Rg1 have a vasodilator action via the eNOS/ NO pathway |
| Â |
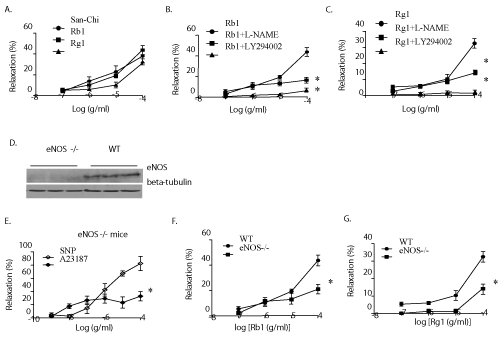
| The experiments used P.N. extract and its major components, Rg1 and Rb1, were analyzed by HPLC (Supplementary figure 1). Mouse coronary arteries with intact endothelium were pre-contracted by U-46619 (a thromboxane mimic), and treated with a fixed dosage of P.N. extract (10−7~10−4 g/mL), Rb1 (10−7~10−4 g/mL) or Rg1 (10−7~10−4 g/mL). Rb1 and Rg1 induced a significant vessel relaxation in a dose-dependent manner (Figure 1A). In order to further elicit the site of action of Rb1 and Rg1, either eNOS inhibitor (L-NAME) or PI3K inhibitor (LY294002) was injected for about 30 minutes before giving Rb1 and Rg1. It was observed that both L-NAME and LY294002 markedly diminished the vasodilator action of Rb1 (Figure 1B) as well as Rg1 (Figure 1C). |
| Â |
|
|
Figure 1: P.N., Rb1, and Rg1 relaxed pre-contracted mouse coronary artery. Plots of P.N., Rb1 and Rg1-induced vasodilatation of pre-contracted mouse coronary arteries in-vitro (A). Diminishing of vasodilatation effect of Rb1 (B) and Rg1 (C) with prior treatment of microvessels by either the NOS inhibitor NG-nitro-L-arginine (L-NAME) or PI3K inhibitor (LY294002). Responses are percent relaxation of pre-contraction. The eNOS expression significantly abolishes in eNOS-/- mice. Anti–tubulin β served as the internal control (D). Plot of in vitro response of pre-contracted mouse coronary arteries to SNP and A23187 in eNOS-/- mice; the vasodilatations were significantly induced by SNP but not by A23187 (E). Plots of in vitro response of pre-contracted mouse coronary arteries to Rb1 (F) and Rg1 (G) in wild-type and eNOS-/- mice. Responses are percent relaxation of pre-contraction. Data (n=5-6) are expressed as mean ± SEM. (ANOVA) *P>0.0 5. |
|
| |
| Â |
| Rb1 and Rg1-induced vasodilatation is endothelial dependent |
| Â |
| In order to prove the exact mechanism for relaxation of the coronary microvessels by Rb1 and Rg1, eNOS-/- mice whose endothelium cannot constitutively generate NO were used (Figure 1D). The phenomenon of vascular relaxation by Rb1 and Rg1 was also present in the coronary vessels of eNOS-/- mice (Figure 1F, 1G). Infusion of SNP, which operates through an endothelium-independent cGMP-mediated pathway, to the eNOS-/- coronaries, causes vasodilatation (Figure 1E), while A23187, a calcium ionophore, whose activity required the presence of an intact endothelium, failed to do so (Figure 1E). |
| Â |
| Rb1 and Rg1 activate phosphorylation of eNOS via the PI3K/Akt pathway |
| Â |
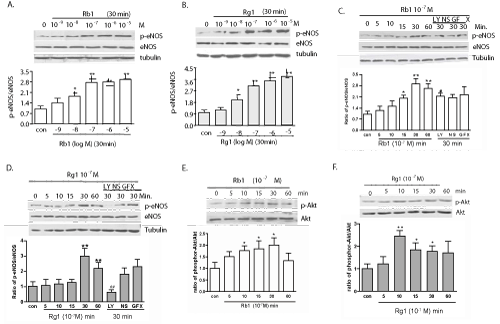
| Both Rb1 and Rg1 at concentration of 10-7 g/ml stimulated the eNOS phosphorylation in cultured PAECs in a concentration-dependent manner beginning after 10-15 minutes of incubation and peaking at 30 minutes (Figure 2A and 2B). When the endothelial cells were pretreated with an inhibitor of PI3K (LY294002), the Rg1 induced phosphorylated-eNOS (p-eNOS) was significantly diminished (Figure 2D). Interestingly, a similar effect on Rb1- mediated phosphorylation was not seen (Figure 2C). Cox-2 inhibitor (NS398) and a PKC inhibitor (GFX) did not show any interference with the amount of p-eNOS (Figure 2C and 2D). In addition, both Rb1 and Rg1 also rapidly increased the phosphorylation of Akt (Ser473) (Figure 2E and 2F), suggesting the involvement of the PI3K/Akt pathway in Rg1-mediated phosphorylation of eNOS. |
| Â |
|
|
Figure 2: Western blot analyses of signaling molecules in endothelial cells after treatment with Rb1 or Rg1. Rb1- (A) and Rg1- (B) induced phosphorylation of eNOS (Ser1177) were dose-dependent. Histograms and error bars represent means ± SEM of four independent experiments performed in duplicate. *P < 0.05 and **P < 0.01 vs control. Pretreatment with LY294002 significantly inhibited the phosphorylation of eNOS induced by Rg1 (D) but not Rb1 (C). The phosphorylated form of Akt (Ser473) was also found to be increased after treatment with 10-7 g/ml Rb1 (E) and Rg1 (F) with no change in the respective total forms. |
|
| Â |
| Rb1 and Rg1 stimulates production of NO |
| Â |
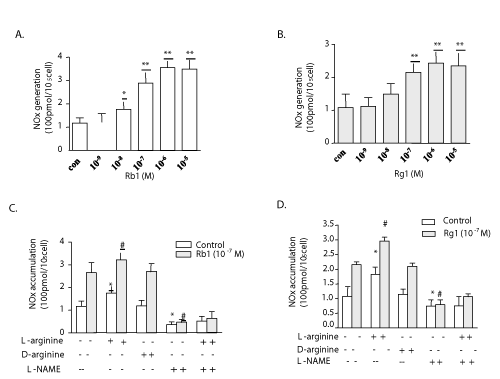
| Whether Rb1 or Rg1-induced eNOS activation resulted in enhancing the NO production in endothelial cells were examined. The increases in the NO level in cultured medium were measured after PAECs were incubated for 3 hours with or without Rb1 or Rg1, and the results were analyzed using the NO production assay. It was found that the NO production was unaffected with lower concentrations of both Rb1 (10−9 g/ml) and Rg1 (10−8 g/ml), whereas exposure to slightly higher concentrations of Rb1 (10−8~10−5 g/ml) and Rg1 (10−7~10−5 g/ml) significantly increased NO production (P<0.01) (Figure 3A and 3B). In order to explore the upstream cascades involved in NO generation and to exclude the role of other secondary pathways other than PI3K-phospho-Akt-eNOS, PAECs in the absence or presence of Rb1 or Rg1 were treated in culture with supplemental L-arginine, L-arginine and L-NAME, D-arginine, or no supplement. L-Arginine, but not D-arginine, is the natural substrate for NOS in nature. Culture medium containing supplemental L-arginine demonstrated a significant increase in NO levels compared with all other controls. This stimulatory effect of L-arginine was completely inhibited by L-NAME, and NO production was further enhanced in the presence of Rb1 or Rg1 (Figure 3C and 3D). |
| Â |
|
|
Figure 3: The effect of Rb1 and Rg1 in NO production in culture medium. NO production was detected by measuring its final stable nitrite and nitrate. Both Rb1 (A) and Rg1 (B) induced the total NOX generation significantly. NO production induced by Rb1 (C) or Rg1 (D) plus L-arginine was inhibited completely by L-NAME. D-arginine did not alter NOx levels in the medium compared with the control. Values are mean ± SEM. *P < 0.05 and **P < 0.01 vs control; # P < 0.01 vs Rb1 or Rg1 alone. |
|
| Â |
| Rb1 and Rg1 stimulates L-arginine transport and uptake |
| Â |
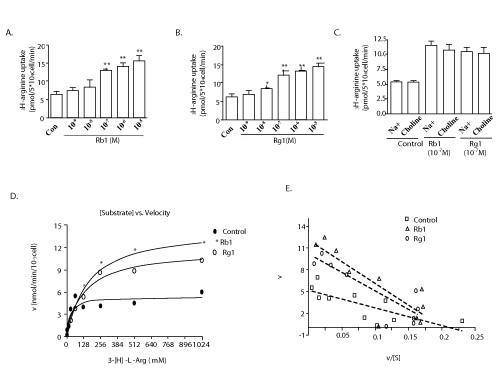
| In order to examine if Rb1 and Rg1 are also involved in modulating the transport of L-arginine into the endothelial cells, [3H] L-arginine (1 mCi/mL) and L-arginine were added to media containing PAECs in the presence or absence of Rb1 or Rg1. The results showed that both Rb1 and Rg1 significantly increased the L-arginine transport into PAECs in a dose-dependent manner (Figure 4A and 4B). To further elucidate whether the observed increase in [3H]-L-arginine uptake was occurring independently of extracellular sodium and only through the Na+-independent system y+ carrier, extracellular Na+ with equimolar choline was replaced and the experiment was repeated. Data in (Figure 4C) indicates that no change was observed in [3H]-L-arginine uptake with the latter. In addition, kinetic curve analysis of the data showed that both Rb1 and Rg1 do not affect L-arginine transport by increasing Vmax values but by decreasing Km values of the transporter (Figure 4D). Eadie-Hofstee analyses of transport were linear (Figure 4E), suggesting the presence of a single transport site for L-arginine in cells using either Rb1 or Rg1. |
| Â |
|
|
Figure 4: Effects of Rg1 and Rb1 on L-arginine transport. Rb1 (A) and Rg1 (B) significantly increased L-arginine transport in a dose-dependent manner. L-arginine uptake induced by Rb1 and Rg1 was not changed upon replacement of Na+ with equimolar choline (C). Values are mean ± SEM. *P < 0.05 and **P < 0.01 vs control. Saturable L-arginine transport showed an increase in the Vmax and a decrease in Km in PAECs treated with Rb1 or Rg1 compared with the control (D). Eadie-Hofstee analyses of transport were linear. Points are the means of three independent determinations within one representative experiment repeated with comparable results (E). |
|
| Â |
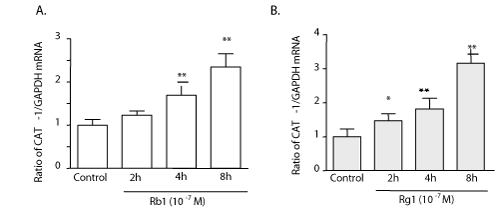
| Rb1 and Rg1 increased cationic amino acid transporter (CAT-1) mRNA expression |
| Â |
| Quantitative-PCR was performed on the PAECs isolated from cultures at incremental time intervals and analyzed for the expression of CAT-1. The evidence shows that both Rb1 and Rg1 (10-7 g/ml) increased CAT-1 mRNA levels (Figure 5A and 5B). |
| Â |
|
|
Figure 5: Rb1 and Rg1 increased cationic amino acid transporter (CAT-1) mRNA expression. Real-time PCR experiments show that both Rb1 (A) and Rg1 (B) increase CAT-1 mRNA expression. Values are mean ± SEM. *P < 0.05 and **P < 0.01 vs control. |
|
| Â |
| P.N. extract lowered the blood pressure in spontaneously hypertensive rats (SHRs) |
| Â |
| To further determine the activity of vasodilation and gene regulation driven by P.N. in hypertension, P.N. extract was injected into the tail of SHRs and WKY rats at the dosage of 100 mg/kg and a fall in the tail blood pressure in SHRs was noticed 25 minutes post-injection (215 ± 10.2 vs.172 ± 12.3 mmHg, p<0.01) (Table 1). Dosages of 10, 25, 50, 100 mg/kg were initially used and 100mg/kg had the best blood pressure-lowering effects. The same dosage of P.N. extracts did not significantly alter blood pressure in WKY rats (122 ± 10.0 vs.118 ± 9.3 mmHg, p>0.05). However, there was no significant change in the vehicle-treated SHRs (218 ± 7.3 vs.212 ± 8.8 mmHg, p>0.05). It would be significant here to note that the animals were restrained in a tail cuff BP measuring device for 30 minutes prior to the injection of the drug to minimize observations related to the acclimation of the apparatus as to the effects of the drug. The concomitant use of vehicle as control was also done to minimize any scope of error. |
| Â |
|
|
Table 1: P.N. lowered the blood pressure of spontaneously hypertensive rats (SHRs).
The tail blood pressure of SHRs decreased approximately 20.0% (P<0.01) at 25 min after administration of 100 mg/kg of P.N., while the same dose of P.N. did not significantly alter blood pressure in Wistar Kyoto (WKY) control rats (P>0.05). n=9 for each group. Values are mean ± SD. *P |
|
| Â |
| Discussion |
| Â |
| Panax notoginseng has been explored for its clinically important role in multiple diseases. P.N. improved myocardial relaxation in rats by decreasing intracellular calcium overload and reducing left ventricular muscle mass [18]. However, the mechanisms of P.N.’s role in vasculature, especially in endothelium are not completely understood. In the present work, P.N. extract, specifically the major ingredients, Rb1 and Rg1, were used to elucidate their role in vasorelaxation in small animal models, demonstrated that these compounds relaxed murine coronary arteries primarily by increasing NO production through the PI3K/Akt/eNOS and L-arginine/eNOS/NO pathways, as well as increased cationic amino acid transporter expression. These observations provided additional evidences to previous reports that suggest an Rg1-induced increase of vascular endothelial growth factor in endothelial cells through the activation of a PI3K/Akt pathway [8,9], and revealed the mechanism of P.N.’s efficacy in the reduction of hypertension through endothelial-dependent vessel dilatation. It is well-known that the patho-physiology of the generation of high vascular pressure in SHRs and thrombooxaneA2-mediated vasoconstriction is similar to that of essential hypertension [19]. Integrity of the vascular endothelium is pivotal for health and its cardiovascular protective activity depends on its capacity to generate NO [12]. It is also known that inhibition of eNOS activity impacts the constitutive release of NO in the vessel walls. The results of the present study also show that both Rb1 and Rg1 mediate vasodilatation at clinically useful dosages using both intact endothelium and its activity of eNOS (Figure 1A-1G). WKY rats are essentially normotensive so, any significant hypotensive effect was not expected. However, even if there was any alteration in the BP in WKY rats, it might have been transient and could not be observed during BP monitoring with the cuff method that was used (Table 1). The dosage of the P.N. extract that is commonly used in clinics for adult patients without any significant side effects is 200-400 mg/day [20]. The accuracy of these observations is strengthened by the previous reports that suggest the role of Korean red ginseng (also panax gingseng, P.G.) in improving the function of endothelial cells by generation of NO [21] and the association of increases in the NO production upon ingestion of P.G. with the decreases in the systemic blood pressure [22]. eNOS is one of the isoforms of the NOS family responsible for NO production in endothelial cells [13]. The activity of eNOS is highly regulated by its interaction with various proteins such as calmodulin (CaM), heat shock proteins, the B2 receptor, caveolin, and dynamin-2 [23]. Phosphorylation of various protein kinases at serine residues and, to a lesser extent, threonine and tyrosine residues have been recently identified to be another important regulator of in eNOS activity [23]. The phosphorylation at Ser1177 is carried out by protein kinase A (PKA) or G- and AMP-dependent kinase. [23]. This study also suggests that Rg1 also mediates NO production via phosphorylating-eNOS at Ser1177 in a dose-dependent manner (Figure 2A and 2B). These findings are in line with previous studies which suggested that the PI3-kinase/Akt pathway is comprised of important signaling cascades that mediate eNOS activation in vascular endothelial cells. Leung K.W. et al showed that Rg1 is a potent stimulator of VEGF using the glucocorticoid receptors (GR), via the non-transcriptional PI3K/Akt and b-catenin T cell factor dependent pathways [8,9]. Moreover, the PI3K/Akt and MEK/ERK pathways as well as the androgen receptor are involved in the regulation of acute eNOS activation by Rb1 in human aortic endothelial cells [10]. Rg1 has also been shown to promote the regeneration of ischemic and damaged tissue via angiogenesis by increasing the endothelial proliferator cell (EPC) population in a time and dose-dependent manner in the in-vitro studies [24]. It is known that endothelial cells generate nitric oxide from L-arginine via the catalytic action of eNOS [25]. The intracellular L-arginine concentrations usually remain in the range of 0.8–2.0 mM, implying that the endothelium always maintains saturating levels of L-arginine as a substrate for supporting the constitutively active eNOS mediated NO synthesis. However, as the external supply of L-arginine decreases, L-arginine trans-cellular transport becomes the rate-limiting step in NO production. Rb1 and Rg1 do not affect the kinetic curve of L-arginine transport by increasing Vmax values, but by decreasing its Km values (Figure 4D). Previous studies have shown the role of inflammatory media, cytokines, bioactive peptides, and hormones etc. on the function and/or expression of L-arginine transporters [26]. However, there is no known information about its interaction with Rb1 and Rg1. Therefore we are the first to show the direct effects of Rb1 and Rg1 on L-arginine transport. The Na+-independent system y+ carrier is thought to be one of the most important transporters for L-arginine inside endothelial cells [27]. There are other ways to link L-arginine uptake to the y+ system e.g. by using NEM, the inhibitors of y+ system CAT, which may also be used to replicate the results as shown in (Figure 4C), and confirmed by Eadie-Hofstee analyses (Figure 4E). The exact molecular mechanism of Rb1- or Rg1- activated L-arginine transport remains to be further studied. The nitric oxide pathway is thought to be involved in the regulation of vascular tone in hypertension and stoke [28]. NO is produced from the guanidino group of L-arginine in an NADPH-dependent reaction catalyzed by a family of NOS enzymes [29]. The activity of eNOS is not the only determinant in controlling NO production by endothelial cells. Recent reports indicate that NO production is dependent on the availability and delivery of extracellular L-arginine that can be mediated by several different classes of cationic amino acid transporters, which have been characterized in terms of their ion dependency, substrate specificity, and relative affinity [25]. In endothelial cells, the delivery of extracellular L-arginine is mediated by the Na+-independent system y+ carrier [30]. The activity of system y+ is encoded by four genes: CAT1, CAT2, CAT3, and CAT4. In endothelial cells, the vast majority of system y+-mediated Na+-independent L-arginine uptake is provided by the CAT1 transporter [31]. This work also confirms the role of L-arginine transport, its dependence on the y+ system, and the upstream regulation by CAT-1 mRNA in the generation of additional NO whenever needed by the cells (Figure 5A and 5B). |
| Â |
| Conclusion |
| Â |
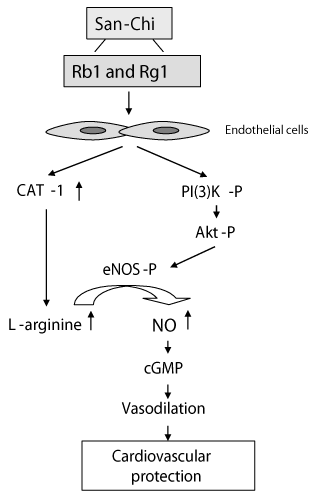
| The major findings include the following: (1) The vasodilator effect of Rb1 and Rg1 is apparent, as evidenced by significant lowering of the caudal blood pressure; (2) The vasodilator effect of P.N., Rb1 and Rg1 is endothelial-dependent, and uses a signal mechanism through the phosphorylation of eNOS via the PI3K/Akt pathway and the subsequent generation of NO; (3) Rb1 and Rg1 stimulates L-arginine transport and uptake to the endothelium cells, which is linked with increased cationic amino acid transporter (CAT-1) mRNA expression. All three points is graphically illustrated in (Figure 6). It is plausible that Rb1 and Rg1, the major components of P.N., could be responsible for the anti-hypertensive effects, as are other members of the ginseng family as previously reported [32,33]. |
| Â |
|
|
Figure 6: Percent cell reduction in MCF-7 cells by MTT assay post exposure to various concentrations of proteins isolated from roots of Boerhaavia diffusa and leaves of Clerodendrum aculeatum. |
|
| Â |
| |
| References |
| Â |
- Yoshikawa M, Murakami T, Ueno T, Yashiro K, Hirokawa N, et al. (1997) Bioactive saponins and glycosides. Viii. Notoginseng (1): New dammarane-type triterpene oligoglycosides, notoginsenosides-A, -B, -C, and -D, from the dried root of Panax notoginseng (Burk.) F.H. Chen 45: 1039-1045.
- Du Q, Jerz G, Waibel R, Winterhalter P (2003) Isolation of dammarane saponins from panax notoginseng by high-speed counter-current chromatography. J Chromatogr A 1008: 173-180.
- Li L, Sheng Y, Zhang J, Guo D (2005) Determination of four active saponins of panax notoginseng in rat feces by high-performance liquid chromatography. J Chromatogr Sci 43: 421-425.
- Li L, Zhang JL, Sheng YX, Guo DA, Wang Q, et al. (2005) Simultaneous quantification of six major active saponins of panax notoginseng by high-performance liquid chromatography-Uv method. J Pharm Biomed Anal 38: 45-51.
- Hofseth LJ, Wargovich MJ (2007) Inflammation, cancer, and targets of ginseng. J Nutr 137: 183S-185S.
- Xia ZY, Liu XY, Zhan LY, He YH, Luo T, et al. (2005) Ginsenosides compound (shen-fu) attenuates gastrointestinal injury and inhibits inflammatory response after cardiopulmonary bypass in patients with congenital heart disease. J Thorac Cardiovasc Surg 130: 258-264.
- Zhang YG, Zhang HG, Zhang GY, Fan JS, Li XH, et al. (2008) Panax notoginseng saponins attenuate atherosclerosis in rats by regulating the blood lipid profile and an anti-inflammatory action. Clin Exp Pharmacol Physiol 35: 1238-1244.
- Leung KW, Cheng YK, Mak NK, Chan KK, Fan TP, et al. (2006) Signaling pathway of ginsenoside-rg1 leading to nitric oxide production in endothelial cells. FEBS Lett 580: 3211-3216.
- Leung KW, Pon YL, Wong RN, Wong AS (2006) Ginsenoside-rg1 induces vascular endothelial growth factor expression through the glucocorticoid receptor-related phosphatidylinositol 3-kinase/akt and beta-catenin/t-cell factor-dependent pathway in human endothelial cells. J Biol Chem 281: 36280-36288.
- Yu J, Eto M, Akishita M, Kaneko A, Ouchi Y, et al. (2007) Signaling pathway of nitric oxide production induced by ginsenoside rb1 in human aortic endothelial cells: A possible involvement of androgen receptor. Biochem Biophys Res Commun 353: 764-769.
- Nathan C, Xie QW (1994) Nitric oxide synthases: Roles, tolls, and controls. Cell 78: 915-918.
- Blume-Jensen P, Hunter T (2001) Oncogenic kinase signalling. Nature 411: 355-365.
- Jia S, Liu Z, Zhang S, Liu P, Zhang L, et al. (2008) Essential roles of pi (3)k-p110beta in cell growth, metabolism and tumorigenesis. Nature 454: 776-779.
- Knowles RG, Moncada S (1994) Nitric oxide synthases in mammals. Biochem J 298: 249-258.
- Li J, Partovian C, Li J, Hampton TG, Metais C, et al. (2002) Modulation of microvascular signaling by heparan sulfate matrix: Studies in syndecan-4 transgenic mice. Microvasc Res 64: 38-46.
- Metais C, Li J, Simons M, Sellke FW (1999) Serotonin-induced coronary contraction increases after blood cardioplegia-reperfusion: Role of cox-2 expression. Circulation 100: II328-334.
- Bishop CT, Mirza Z, Crapo JD, Freeman BA (1985) Free radical damage to cultured porcine aortic endothelial cells and lung fibroblasts: Modulation by culture conditions. In Vitro Cell Dev Biol 21: 229-236.
- Feng PF, Qin NP, Qiao Q (1997) clinical and experimental study of improving left ventricular diastolic function by total saponins of panax notoginseng. Zhongguo Zhong Xi Yi Jie He Za Zhi 17: 714-717.
- Vanhoutte PM (2009) Cox-1 and vascular disease. Clin Pharmacol Ther 86: 212-215.
- Vogler BK, Pittler MH, Ernst E (1999) The efficacy of ginseng. A systematic review of randomised clinical trials. Eur J Clin Pharmacol 55: 567-575.
- Sung J, Han KH, Zo JH, Park HJ, Kim CH, et al. (2000) Effects of red ginseng upon vascular endothelial function in patients with essential hypertension. Am J Chin Med 28: 205-216.
- Han K, Shin IC, Choi KJ, Yun YP, Hong JT, et al. (2005) Korea red ginseng water extract increases nitric oxide concentrations in exhaled breath. Nitric Oxide 12: 159-162.
- Fulton D, Gratton JP, Sessa WC (2001) Post-translational control of endothelial nitric oxide synthase: Why isn't calcium/calmodulin enough? J Pharmacol Exp Ther 299: 818-824.
- Shi AW, Wang XB, Lu FX, Zhu MM, Kong XQ, et al. (2009) Ginsenoside rg1 promotes endothelial progenitor cell migration and proliferation. Acta Pharmacol Sin 30: 299-306.
- Closs EI, Simon A, Vekony N, Rotmann A (2004) Plasma membrane transporters for Arginine. J Nutr 134: 2752-2759
- Nelin LD, Nash HE, Chicoine LG (2001) Cytokine treatment increases arginine metabolism and uptake in bovine pulmonary arterial endothelial cells. Am J Physiol Lung Cell Mol Physiol 281: 1232-1239.
- Greene B, Pacitti AJ, Souba WW (1993) Characterization of l-arginine transport by pulmonary artery endothelial cells. Am J Physiol 264: 351-356.
- Kublickiene KR, Cockell AP, Nisell H, Poston L (2009) Role of nitric oxide in the regulation of vascular tone in pressurized and perfused resistance myometrial arteries from term pregnant women. Am J Obstet Gynecol 177: 1263-1269.
- Moncada S, Palmer RM, Higgs EA (1991) Nitric oxide: Physiology, pathophysiology, and pharmacology. Pharmacol Rev 43: 109-142.
- Zharikov SI, Block ER (1998) Characterization of l-arginine uptake by plasma membrane vesicles isolated from cultured pulmonary artery endothelial cells. Biochim Biophys Acta 1369: 173-183.
- Cui Z, Zharikov S, Xia SL, Anderson SI, Law AS, (2005) Molecular cloning, characterization, and chromosomal assignment of porcine cationic amino acid transporter-1. Genomics 85: 352-359.
- Han KH, Choe SC, Kim HS, Sohn DW, Nam KY (1998) Effect of red ginseng on blood pressure in patients with essential hypertension and white coat hypertension. Am J Chin Med 26: 199-209.
- Kim ND, Kang SY, Schini VB (1994) Ginsenosides evoke endothelium-dependent vascular relaxation in rat aorta. Gen Pharmacol 25: 1071-1077.
|
| Â |
| Â |