| Research Article |
Open Access |
|
| Bidhu Kalyan Mohanti* and Aman Sharma |
| Department of Radiation Oncology, BRA-IRCH, All India Institute of Medical Sciences (AIIMS), New Delhi, India |
| *Corresponding author: |
Dr. Bidhu K Mohanti, MD
Professor
Department of Radiation Oncology
Room 241, Dr.BRA Institute Rotary Cancer Hospital
All India Institute of Medical Sciences
Ansari Nagar, New Delhi, 110029, India
Tel: +91-11-26593399
Fax: +911126588663/26588641
E-mail: drbkmohanti@rediffmail.com |
|
| Â |
| Received September 11, 2012; Published October 25, 2012 |
| Â |
| Citation: Mohanti BK, Sharma A (2012) Human Papilloma Virus in Head and Neck Cancers - The Present and the Future. 1:452. doi:10.4172/scientificreports.452 |
| Â |
| Copyright: © 2012 Mohanti BK, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. |
| Â |
| Abstract |
| Â |
| Molecular analyses suggest that Human Papilloma Virus (HPV) associated head and neck cancers represent a biologically distinct group of Head and Neck Squamous Cell Carcinomas (HNSCC). HPV-positive HNSCC have special predilection for lingual & palatine tonsils, present at young age and are often associated with high risk sexual behavior. The typical genetic alterations that result from longstanding exposure to the carcinogenic effects of tobacco and alcohol may not be found in HPV-positive tumors. HPV-positive tumors have markedly superior outcome as compared to HPV-negative tumors. The present review summarizes HPV induced carcinogenesis, its distinct clinical & biology behavior, prognosis & response to therapy and future perspectives for HPV positive HNSCC. |
| Â |
| Keywords |
| Â |
| Human papilloma virus; Head and neck cancers; Prognosis |
| Â |
| Introduction |
| Â |
| Head and Neck Squamous Cell Carcinoma (HNSCC) is the sixth most common cancer worldwide. Each year nearly 650,000 patients are diagnosed with HNSCC around half of which present in locally advanced disease. About 350,000 disease related deaths are reported annually [1]. HNSCC originate from the pluristratified squamous epithelium which lines the upper aero digestive tract. Apart from the nasopharyngeal carcinoma which is regarded as a separate clinical entity (based on its etiology, histology, natural history and prognosis) rest of the HNSCC have traditionally been clustered together, with tobacco use and alcohol being the most common etiological agents accounting for nearly eighty percent of these cancers. |
| Â |
| In recent past, despite marked decrease in the smoking patterns there is an internationally reported upsurge in the incidence of oropharyngeal cancers [2-5]. HNSCC is presenting in patients with unusual risk factors for the disease. Young, non-smoking population is at risk for head and neck cancer. High risk Human Papilloma Virus (HPV) has been linked to HNSCC. Sufficient evidence has accumulated for its etiological role in the pathogenesis of squamous cell carcinomas [6-9]. Just like the cervical cancers HPV-positive HNSCC is associated with high risk sexual behaviuor particularly the oro-genital sex and multiple sexual partners. HPV-associated HNSCC now represent a distinct clinico-pathological entity as well as an emerging viral epidemic of cancer. |
| Â |
| The HPV Structure and Subtypes |
| Â |
| HPV is epitheliotrophic oncogenic DNA virus. HPV belong to the Papillomaviridae family and is 8-kilobase, small, circular double stranded DNA virus. The HPV genome comprises of several early (E) and late genes (L) which are responsible for viral infection, replication, transcription and carcinogenesis. The late (L) genes encode for L1 and L2 capsid proteins whereas the early (E) genes encode for E1, E2, E5, E6 and E7 proteins [10,11]. The E1 and E2 proteins regulate viral replication, the E5, E6 and E7 proteins have growth simulating & transforming properties. |
| Â |
| More than 100 different HPV subtypes have been reported. Based on their malignant potential these viruses have been classified into high-risk and low-risk subtypes. Low risk subtypes are associated with benign lesions such as warts whereas high risk HPV subtypes have malignant potential. More than 15 high risk-HPV subtypes have been described however more than 90% of HNSCC are associated with a single subtype, HPV 16 [12]. |
| Â |
| Prevalence of HPV in HNSCC |
| Â |
| The first clue to viral etiology was suggested in 1983 by Syrjänen et al. [7], who noted that 40% of the cancers had histological & morphological similarities with HPV-associated lesions. HPV prevalence varies greatly depending on the target population, type of specimen and the detection method used. HPV DNA was identified in 3.9% of the oral cavity cancers and 18.3% of oropharyngeal cancers by the International Agency for Research on Cancer (IARC) study [9]. Nearly 20%-25% of the HNSCC are associated with HPV but the prevalence is much higher for oropharyngeal (18%-60%) sub-site [9-14]. |
| Â |
| Different Carcinogenic Pathways of HPV Positive and HPV Negative HNSCC Carcinogenesis |
| Â |
| Malignant transformation in HNSCC usually results from a series of genetic alterations produced by longstanding exposure to carcinogenic effects of tobacco and alcohol. Smoking and alcohol consumption have synergistic effect on development of HNSCC [15]. Carcinogens produced by tobacco induce nucleotide changes resulting in mutations of p53 tumor suppressor gene [16]. Alcohol induced carcinogenesis appears to be less clear but may act by acetaldehyde induced DNA damage, entrapment of glutathione and induction of cytochrome p450 enzyme CYP2E1 which is involved in inactivation of various procarcinogens [17]. Recent studies have pointed out the existence of at least two separate pathways for carcinogenesis of HNSCC one driven primarily by the mutagenic effects of tobacco and alcohol and the other driven by HPV-mediated transformation. |
| Â |
| HPV Related Carcinogenesis |
| Â |
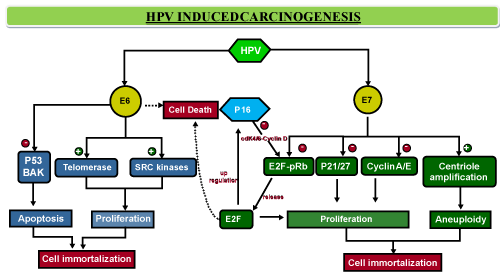
| Integration of high-risk HPV DNA, into the human cellular genome is an important step in malignant transformation [18]. The typical genetic alterations that result from longstanding exposure to the carcinogenic effects of tobacco and alcohol may not be found in HPV-positive tumors. Instead the oncoproteins expressed by HPV have the ability to deregulate the tumor suppressor function of p53 and pRb proteins resulting in defective apoptosis, DNA repair and cell cycle control resulting in cellular immortalization and malignant transformation [19-21]. The E6 oncoprotein disrupts the p53 pathway and causes subsequent degradation of the p53 protein, which results in loss of control at the G1/S and G2/M checkpoints making the cell susceptible to defective repair capacity as well as uncontrolled growth. |
| Â |
| The E7 oncoprotein is involved in the inactivation retinoblastoma tumor suppressor gene product pRb. Regulatory function of the Rb tumor suppressor protein appears to largely depend on the ability of Rb to bind to and inhibit the family of E2F transcription factors that encode proteins necessary for DNA synthesis, replication and maintenance of cell cycle regulation. The bound Rb-E2F protein serves as inhibitor of transcription for several genes regulation cell proliferation including p16 gene, phosphorylation of Rb leads to the release of the transcription factor E2F. The CyclinD-cdk4/6 complexes (CDKs), when not under the inhibition by p16 phosphorylate Rb leading to the release of the transcription factor E2F and initiating cell cycle progression. P16 regulates the activity of these CyclinD-cdk4/6 complexes. The E7 protein forms complexes with hypophosphorylated forms of the retinoblastoma tumor suppressor protein (pRb) resulting in release of E2F, thereby releasing p16 gene from its transcriptional inhibition and inducing transcription of various genes which lead to cell proliferation [22]. Consequently HPV-positive tumors are characterized by high expression levels of p16 (Figure 1). |
| Â |
|
|
Figure 1: The HPV oncoprotein E6 disrupts the p53 pathway and mediates its degradation thereby effectively silencing the action of TP53 tumor suppressor gene. This results in loss of control over the cell cycle at the G1/S and G2/M checkpoints making the cell susceptible to defective repair capacity as well as uncontrolled growth. The E7 oncoprotein is involved in the inactivation retinoblastoma tumor suppressor gene product pRb which results in cell immortalization & carcinogenesis. |
|
| Â |
| Molecular Analysis of HPV-Positive & HPV-Negative HNSCC |
| Â |
| Salient features of HPV positive and HPV negative tumors have been mentioned in table 1. HPV-positive cancers are characterized by loss of expression of pRb, cyclin D1 and overexpression of p16 [23-25]. In contrast, HPV-negative tumors consistently show overexpression of pRb and cyclin D1 and loss of p16. Mutation in the p53 gene is common in HPV-negative cancers whereas it is the inactivation of p53 gene that is more commonly seen in HPV-positive cancers. HPV-positive HNSCCs also differ from HPV-negative HNSCCs in their patterns of allelic and chromosomal loss and in their global gene expression profiles [26,27]. |
| Â |
|
|
Table 1: Molecular Difference of HPV Positive and HPV Negative HNSCC. |
|
| Â |
| Clinical Features of HPV Positive HNSCC |
| Â |
| HPV associated HNSCC have special predilection for the lingual and palatine tonsils [7,9,13]. The association between HPV16 and cancer is strongest for tonsil (Odds Ratio: 15.1), intermediate for oropharynx (OR: 4.3) and weakest for oral (OR: 2.0) and larynx (OR: 2.0) [28]. HPV preferentially targets the highly specialized reticulated epithelium of tonsillar crypts; however, the intrinsic properties of this epithelium that make it susceptible to HPV infection are yet to be elucidated. HPV positive HNSCC have prominent basaloid morphology [29], are poorly differentiated, lack keratinization, are not associated with dysplastic epithelial changes and predominantly affect males. Clinically HPV-positive tumors present in loco-regionally advanced disease (stage III or IV) with early T stage and advanced nodal stage. The nodal metastases are usually cystic and multilevel regional involvement is common [30]. The apparent clinical difference between HPV positive and HPV negative tumors have been highlighted in table 2. |
| Â |
|
|
Table 2: Clinical Difference between HPV Positive and HPV Negative HNSCC. |
|
| Â |
| Response and Prognosis |
| Â |
| The HPV tumor status is a strong and consistent determinant of improved survival, regardless of treatment strategy [8,31-43]. Despite presenting in loco-regionally advanced disease, patients with HPV-positive tumors have superior outcome and better respond to cancer directed treatment. Prominent studies showing favorable treatment outcomes of HPV positive HNSCC have been highlighted in table 3. In majority of these retrospective series the 5-year survival rates among patients with HPV-positive tumors are approximately 75 to 80%, versus 45 to 50% for HPV-negative cancers [8,31-38]. This superior outcome in survival based by the HPV tumor status is not entirely understood at present, but is most likely caused by a combination of several factors. Apparently a part of the survival benefit in the HPV-positive HNSCC may be ascribed to patient-related factors that include younger age at presentation, limited exposure to tobacco and alcohol, less co-morbidity and a better general condition, however even after adjustment for various prognostic factors HPV positive tumors status remains an important independent prognostic factor. |
| Â |
|
|
Table 3: Studies Favoring Better Prognosis for HPV Positive HNSCC. |
|
| Â |
| HPV positive tumor status not only appears to be an independent prognostic factor for overall and progression free survival but also is a predictive factor for inherent sensitivity to induction as well as concurrent chemo-radiation in patients with oropharyngeal squamous cell carcinomas. The presence of intact p53 is implicated as one of the potential mechanisms whereby removal of HPV E6 and E7 expression leads to the restoration of apoptotic pathways rendering the tumor more sensitive to chemo-radiation treatment. Better prognosis with HPV+ HNSCC can also be explained partially by the absence of "field cancerization", but the exact underlying mechanisms are yet to be clearly elucidated. |
| Â |
| Recent prospective study on ECOG protocol 2399 [39] also confirmed improved survival (95% vs 62%) with lower risks of progression and death for HPV + tumors. HPV-positive tumor status was independently associated with 64% reduced risk of death. Similar findings within the Radiation Therapy Oncology Group (RTOG)-0129study [40] confirmed the superior treatment outcomes in HPV-positive tumors than for HPV-negative tumors (3-year OS 82.4% vs. 57.1%; P14,41,42]. |
| Â |
| The favorable biologic behavior of HPV-positive tumor may be altered by the extent of tobacco use. In HNSCC there is significant increased risk of death with each additional pack year of smoking, genetic alterations induced by tobacco-associated carcinogens may render HPV-positive tumors less responsive to therapy [40]. |
| Â |
| Impact on Future Perspectives |
| Â |
| The demonstration of HPV as an etiological agent for a subset of head and neck squamous cell carcinoma and their precursor lesions has paved the way for the development of preventive and therapeutic HPV vaccines that may lead to the control of HPV-associated malignancies and their potentially lethal consequences. |
| Â |
| HPV-associated cancers constantly express E6 and E7 viral oncogenes even in the late stages of the disease, repression of viral oncogene expression by drugs that interfere with the expression or function of viral proteins can induce senescence and apoptosis of the malignant cells. |
| Â |
| In light of the HPV tumor status as an independent prognostic factor for survival there is a need for incorporation of HPV tumor status into the staging system for head and neck cancers for the oropharyngeal sub-site. |
| Â |
| A phase III study comparing treatment of loco-regionally advanced HNSCC with radiotherapy alone and radiotherapy plus cetuximab [43] reported significant improvement in survival in favor of cetuximab plus radiotherapy arm for patients with oropharyngeal cancers, early T stage, advanced N stage, higher performance status, male sex and age less than 65 years. All these factors are closely associated with HPV positive HNSCC. Uneven distribution of HPV-positive cancers can cause discrepancies in the observed results hence caution is warranted while interpreting results from previously well designed studies. |
| Â |
| HPV positive tumors present with early T stage and advanced N stage which at the extreme end would mimic secondary’s neck with an unknown primary tumor. Detection of p16 over expression or HPV DNA may help to localize the tumor to the primary oropharyngeal site & identify the subset of unknown primary tumors with a favorable outcome. |
| Â |
| HPV tumor status may provide insight into the tumor biology and help to individualize cancer directed therapies. The characteristic genotype and molecular biological profile of the HPV-associated HNSCC has been implicated to cause a differential response to hypoxic modification during radiotherapy suggesting that clinically relevant hypoxic radio-resistance may differ by tumor HPV status. Nimorazole (a hypoxic cell sensitizer) when given with radiation increased loco-regional control only in patients with p16-negative tumors [38]. Similar findings suggesting differential response to hypoxic modification with tirapazamine have demonstrated a trend for better loco-regional tumor control in p16-negative oropharyngeal carcinoma [44]. These studies may point out towards the underlying molecular mechanisms supporting the hypothesis that clinically relevant hypoxia may be more pronounced in p16-negative tumors as compared to p16-positive tumors. In such a situation treatment intensification might be more relevant in p16-negative tumors whereas de-intensification of treatment to reduce treatment morbidity seems a viable option for HPV + HNSCC. |
| Â |
| Important question that need to be addressed in further prospective studies have been mentioned in table 4. The impact of HPV status to cancer directed therapy of HNSCC appears to be so potent that tumor p16- or HPV status should always to be taken into account in a clinical practice. |
| Â |
|
|
Table 4: Questions that Need to be Addressed in Future. |
|
| Â |
| Summary and Conclusions |
| Â |
| • Epidemiologic evidence suggests that the incidence of HPV-associated oropharyngeal cancers is on the rise and now represents a distinct clinico-pathological entity as well as an emerging viral epidemic of cancer. |
| Â |
| • The HPV tumor status is a strong, consistent & independent factor for improved survival, regardless of treatment strategy. |
| Â |
| • Extent of Tobacco smoking can determent the favorable treatment outcome of HPV positive HNSCC. |
| Â |
| • Ongoing clinical studies will better help to define the role of de-escalating cancer directed therapy, role of HPV vaccination & targeted approaches in HPV-positive tumors. |
| Â |
| • HPV status may better help to individualize treatment therapy such as hypoxic modification during radiotherapy which may differ by the tumor HPV status. |
| Â |
| |
| References |
| Â |
- Parkin DM, Bray F, Ferlay J, Pisani P (2002) Global cancer statistics. CA Cancer J Clin 55: 74-108.
- Shiboski CH, Schmidt BL, Jordan RC (2005) Tongue and tonsil carcinoma: increasing trends in the U.S. population ages 20–44 years. Cancer 103: 1843-1849.
- Hammarstedt L, Dahlstrand H, Lindquist D, Onelöv L, Ryott M, et al. (2007) The incidence of tonsillar in Sweden is increasing. Acta Otolaryngol 127: 988-992.
- Braakhuis BJ, Visser O, Leemans CR (2009) Oral and oropharyngeal in The Netherlands between 1989 and 2006: increasing incidence, but not in young adults. Oral Oncol 45: 85-89.
- Conway DI, Stockton DL, Warnakulasuriya KA, Ogden G, Macpherson LM (2006) Incidence of oral and oropharyngeal in United Kingdom (1990–1999) -- recent trends and regional variation. Oral Oncol 42: 586-592.
- Syrjänen K, Syrjänen S, Lamberg M, Pyrhönen S, Nuutinen J (1983) Morphological and immunohistochemical evidence suggesting human papillomavirus (HPV) involvement in oral squamous cell carcinogenesis. Int J Oral Surg 12: 418-424.
- Snijders PJ, Cromme FV, van den Brule A, Schrijnemakers HF, Snow GB, et al. (1992) Prevalence and expression of human papillomavirus in tonsillar carcinomas, indicating a possible viral etiology. Int J Cancer 51: 845-850.
- Gillison ML, Wayne M. Koch, Randolph B, Spafford M, Westra WH, et al. (2000) Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst 92: 709-720.
- Herrero R, Castellsagué X, Pawlita M, Lissowska J, Kee F, et al. (2003) Human papillomavirus and oral cancer: the International Agency for Research on Cancer multicenter study. J Natl Cancer Inst 95: 1772-1783.
- Danos O, Katinka M, Yaniv M (1982) Human papillomavirus 1a complete DNA sequence: a novel type of genome organization among papovaviridae. Embo J 1: 231-236.
- Schwarz E, Durst M, Demankowski C, Lattermann O, Zech R, et al. (1983) DNA sequence and genome organization of genital human papillomavirus type 6b. Embo J 2: 2341-2348.
- Syrjänen S (2005) Human papillomavirus (HPV) in head and neck cancer. J Clin Virol 32: 59-66.
- Kreimer AR, Clifford GM, Boyle P (2005) Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomarkers Prev 14: 467-475.
- Dayyani F, Etzel CJ, Liu M, Ho CH, Lippman SM, et al. (2010) Meta-analysis of the impact of human papillomavirus (HPV) on cancer risk and overall survival in head and neck squamous cell carcinomas (HNSCC). Head Neck Oncol 2: 15.
- J. Decker, Goldstein JC (1982) Risk factors in head and neck cancer. N Engl J Med 306: 1151-1155.
- Brennan JA, Boyle JO, Koch WM, Goodman SN, Hruban RH, et al. (1995) Association between cigarette smoking and mutation of the p53 gene in squamous-cell carcinoma of the head and neck. N Engl J Med 332: 712-717.
- Poschl G, Seitz HK (2004) Alcohol and cancer. Alcohol Alcohol 39: 155-165.
- zur Hausen H (2002) Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer 2: 342-350.
- Mansur CP, Androphy EJ (1993) Cellular transformation by papillomavirus oncoproteins. Biochim Biophys Acta 1155: 323-345.
- Munger K, Phelps WC (1993) The human papillomavirus E7 protein as a transforming and transactivating factor. Biochim Biophys Acta 1155: 111-123.
- McCance DJ (2005) Transcriptional regulation by human papillomaviruses. Curr Opin Genet Dev 15: 515-519.
- Nevins JR (2001) The Rb/E2F pathway and cancer. Hum Mol Genet 10: 699-703.
- Klussmann JP, Gultekin E, Weissenborn SJ, Wieland U, Dries V, et al. (2003) Expression of p16 protein identifies a distinct entity of tonsillar carcinomas associated with human papillomavirus. Am J Pathol 162: 747-753.
- Slebos RJ, Yi Y, Ely K, Evjen A, Zhang X, et al. (2006) Gene expression differences associated with human papillomavirus status in head and neck squamous cell carcinoma. Clin Cancer Res 12: 701-709.
- Hafkamp HC, Speel EJ, Haesevoets A, Bot FJ, Dinjens WN, et al. (2003) A subset of head and neck squamous cell carcinomas exhibits integration of HPV 16/18 DNA and overexpression of p16INK4A and p53 in the absence of mutations in p53 exons 5-8. Int J Cancer 107: 394-400.
- Braakhuis BJ, Snijders PJ, Keune WJ, Meijer CJ, Ruijter-Schippers HJ, et al. (2004) Genetic patterns in head and neck cancers that contain or lack transcriptionally active human papillomavirus. J Natl Cancer Inst 96: 998-1006.
- Martinez I, Wang J, Hobson KF, Ferris RL, Khan SA (2007) Identification of differentially expressed genes in HPV-positive and HPV-negative oropharyngeal squamous cell carcinomas. Eur J Cancer 43: 415-432.
- Hobbs CG, Sterne JA, Bailey M, Heyderman RS, Birchall MA, et al. (2006) Human papillomavirus and head and neck cancer: a systematic review and meta-analysis. Clin Otolaryngol 31: 259-266.
- Begum S, Westra WH (2008) Basaloid squamous cell carcinoma of the head and neck is a mixed variant that can be further resolved by HPV status. Am J Surg Pathol 32: 1044-1050.
- Goldenberg D, Begum S, Westra WH, Khan Z, Sciubba J, et al. (2008) Cystic lymph node metastasis in patients with head and neck cancer: an HPV-associated phenomenon . Head Neck 30: 898-903.
- Mellin H, Friesland S, Lewensohn R, Dalianis T, Munck-Wikland E (2000) Human papillomavirus (HPV) DNA in tonsillar cancer: clinical correlates, risk of relapse, and survival. Int J Cancer 89: 300-304.
- Schwartz SR, Yueh B, McDougall JK, Daling JR, Schwartz SM (2001) Human papillomavirus infection and survival in oral squamous cell cancer: a population-based study. Otolaryngol Head Neck Surg 125: 1-9.
- Lindel K, Beer KT, Laissue J, Greiner RH, Aebersold DM (2001) Human papillomavirus positive squamous cell carcinoma of the oropharynx: a radiosensitive subgroup of head and neck carcinoma. Cancer 92: 805-813.
- Ritchie JM, Smith EM, Summersgill KF, Hoffman HT, Wang D, et al. (2003) Human papillomavirus infection as a prognostic factor in carcinomas of the oral cavity and oropharynx. Int J Cancer 104: 336-344.
- Weinberger PM, Yu Z, Haffty BG, Kowalski D, Harigopal M, et al. (2006) Molecular classification identifies a subset of human papillomavirus—associated oropharyngeal cancers with favorable prognosis. J Clin Oncol 24: 736-747.
- Licitra L, Perrone F, Bossi P, Suardi S, Mariani L, et al. (2006) High-risk human papillomavirus affects prognosis in patients with surgically treated oropharyngeal squamous cell carcinoma. J Clin Oncol 24: 5630-5636.
- Sedaghat AR, Zhang Z, Begum S, Palermo R, Best S, et al. (2009) Prognostic significance of human papillomavirus in oropharyngeal squamous cell carcinomas. Laryngoscope 119: 1542-1549.
- Lassen P, Eriksen JG, Hamilton-Dutoit S, Tramm T, Alsner J, et al. (2009) Effect of HPV associated p16INK4A expression on response to radiotherapy and survival in squamous cell carcinoma of the head and neck. J Clin Oncol 27: 1992-1998.
- Fakhry C, Westra WH, Li S, Cmelak A, Ridge JA, et al. (2008) Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst 100: 261-269.
- Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, et al. (2010) Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 363: 24-35.
- Ragin CC, Taioli E (2007) Survival of squamous cell carcinoma of the head and neck in relation to human papillomavirus infection: review and meta-analysis. Int J Cancer 121: 1813-1820.
- OÂ’Rorke MA, Ellison MV, Murray LJ, Moran M, James J, et al. (2012) Human papillomavirus related head and neck cancer survival: A systemic review and meta-analysis. Oral Oncol.
- Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, et al. (2006) Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med 354: 567-578.
- Rischin D, Young RJ, Fisher R, Fox SB, Le QT, et al. (2010) Prognostic significance of p16INK4A and human papillomavirus in patients with oropharyngeal cancer treated on TROG 02.02 phase III trial. J Clin Oncol 28: 4142-4148.
|
| Â |
| Â |