| Research Article |
Open Access |
|
| Hael Al Ebrahim1*, Mohammad Ali Salameh1 and Hassan Hamed2 |
| 1Mechanical Design Engineering Department, Mechanical and Electrical Engineering faculty, Damascus University, Syria |
| 2Chemical Engineering Department, Petroleum and Chemical Engineering faculty, AL-Baath University, Syria |
| *Corresponding author: |
Hael Al ebrahim
Mechanical Design Engineering Department
Mechanical and Electrical Engineering faculty
Damascus University, Syria
E-mail: hael1977@yahoo.com |
|
| Â |
| Received August 08, 2012; Published October 28, 2012 |
| Â |
| Citation: Ebrahim HA, Salameh MA, Hamed H (2012) The Effect of Motion on the Behavior of Corrosion Stainless Steels in Industrial Phosphoric Acid. 1:453. doi:10.4172/scientificreports.453 |
| Â |
| Copyright: © 2012 Ebrahim HA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. |
| Â |
| Abstract |
| Â |
| Phosphoric acid (H3PO4) is produced at General Fertilizer Company in Syria by a reaction between phosphate and sulfuric acid (H2SO4) forming a thick slurry, which exposed to filtration to produce industrial Phosphoric acid. The corrosion behavior of stainless alloys was investigated in phosphoric acid using electrochemical techniques like Tafel polarization. The corrosion rates of these alloys were calculated in industrial phosphoric acid. Measurements & tests have revealed that the effect of industrial phosphoric acid at these alloys is different. Laboratory Tests showed the change in both corrosion rate and corrosion current densities (Icorr) according to the existing of alloying elements and their percent in the stainless steel alloys. Effect of motion on the behavior of Corrosion stainless steels changes according to rotation speed of alloy sample, some experimental procedures at phosphate fertilizer factory tend to a new type of corrosion that can be called dealloing erosion-corrosion. This research showed the great importance for following studies of the effect of industrial phosphoric acid, produced at phosphate fertilizer factories, at the high corrosion resisting alloys, to arrive suitable alloys for equipments and machines used for its production, because of its high corrosion rates that create many corrosion problems in these equipments and machines. |
| Â |
| Keywords |
| Â |
| Corrosion; Phosphoric acid; Electrochemical behavior; Stainless steel |
| Â |
| Introduction |
| Â |
| Corrosion of structural elements is a major issue for any industry because of the chemical environment of chemical processing. Wet process phosphoric acid (H3PO4) is produced by reacting ground phosphate rock with sulfuric acid (H2SO4), forming a thick, acidic slurry. Many problems in these plants are caused by the corrosive, erosive, and scale-forming components present in the process system such as Hydrofluoric Acid (HF), Silicon Dioxide (SiO2), and Aluminum Oxide (Al2O3) [1,2]. Several works studied the corrosion of various materials in the phosphoric acid. Michael Schorr, Benjamin Valdez, Roumen Zlatev, and Margarita Stoytcheva, showed that erosion–corrosion is a frequent event in H3PO4 production plants, and the resistance of Nibase alloys was better than austenitic Stainless Steel (SS) [3]. Sulfide ions increase corrosion rate of austenitic SS in industrial phosphoric acid (40 wt% H3PO4) [4]. Some alloys of stainless steel with high chromium gave good resistance corrosion in industrial phosphoric acid medium 30% P2O5 [5]. It is observed that corrosion current density decreased of Ti-6Al-4V (Ti–Alloy) in the mixture of concentrated phosphoric and formic acid with increasing the concentration of L-OH (an organic compound). Corrosion potential shifted toward more active potential and current densities increased significantly with increasing temperature from 20 to 50 ± 1°C [6]. The studies of the corrosion behavior of aged and annealed sample of 18 Ni 250 grade maraging steel have revealed that the corrosion rate of both aged and annealed samples increase with increase in temperature and increase in concentration of phosphoric acid in the medium. The corrosion rate of annealed sample is found to be less than that of aged sample [7]. The main conclusions of one study that graphite material was more resistant compared to stainless steels, with a low corrosion rate whatever the experimental condition (temperature and pollution), and the presence of impurities as chloride and sulphate ions accentuated the aggressiveness of phosphoric acid [8]. The pitting corrosion inhibition of 316L SS in NaCl achieved by addition 2-mercaptobenzimidazole (MBI), the protection efficiency increases with increasing MBI concentration but decreased with the increasing of the temperature [9]. Several inhibitors were investigated for the protection of stainless steel 316L at different inhibitor concentrations and temperature [10] the corrosion resistance of a highly alloyed austenitic stainless steel UNS N08031 effected by the solution temperature that the corrosion potential values shift to more anodic potentials as temperature increases. The same tendency was observed in the Heat Affected Zone (HAZ) of the base metal and the welded metal of this steel [11]. The chromium oxide rich layer of austenitic stainless steels type 304L, 316L and 310Nb which remain them in their passive state effected by some particular nitric media, and they can suffer intergranular corrosion [12]. The effect of nitrogen on the corrosion resistance of austenitic stainless alloys is to improve resistance of intergranular corrosion and increase resistance of localized corrosion for welded SS [13]. Galvanic corrosion of the pair AISI316L/welded AISI 316L affected by temperature and impurities in H3PO4solutions [14-16] the results was different, that these works didn’t similar the industrial process and parameters as addition to the different of phosphoric acid composition from place to another in the World, according to phosphoric rock. The objective of this work is to study behavior of corrosion stainless steels in industrial phosphoric acid 37.7% H3PO4 and the effect of motion on it. |
| Â |
| Experimental Part |
| Â |
| Material |
| Â |
| In this work, we tested little type of austenitic stainless steel and nickel alloy, the chemical composition of tested material is in table 1. |
| Â |
|
|
Table 1: Chemical composition of tested material. |
|
| Â |
| Medium |
| Â |
| Industrial phosphoric acid 37.7% H3PO4 produced by The General fertilizer Company in Syria, which contain 1.9% H2SO4, and 1.5% solid particles as addition to some impurities as it appeared in table 2. |
| Â |
|
|
Table 2: Chemical composition of industrial phosphoric acid 37.7% H3PO4. |
|
| Â |
| Electrochemical measurements |
| Â |
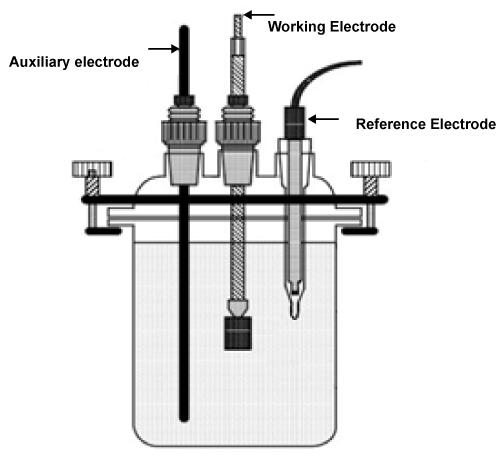
| Electrochemical measurements were carried out by using an electrochemical work station, that we can get Tafel polarization. Tafel plot measurements were carried out using conventional three electrodes: |
| Â |
| Reference electrode, auxiliary electrode, and working electrode (Figure 1), the working electrode had moved for different rotation speeds for every tested material alone then we had get Tafel polarization of all cases. |
| Â |
|
|
Figure 1: Reference electrodes for test. |
|
| Â |
| Results and Discussion |
| Â |
| The effect of industrial phosphoric acid 37.7% H3PO4 at the corrosion rate of austenitic stainless steel and nickel alloy was studied using Tafel polarization technique. |
| Â |
| The electrochemical behavior of 308L AISI in industrial phosphoric acid |
| Â |
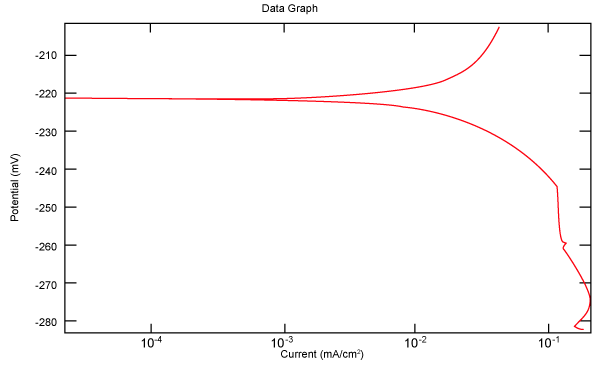
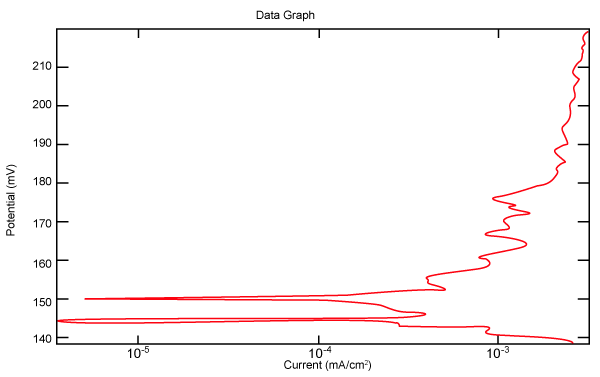
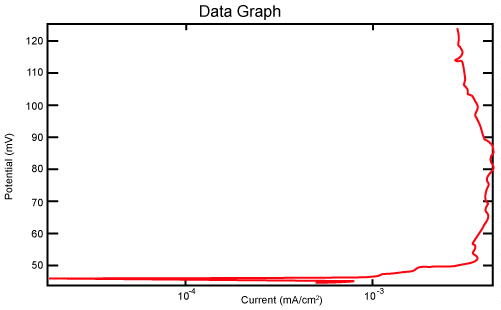
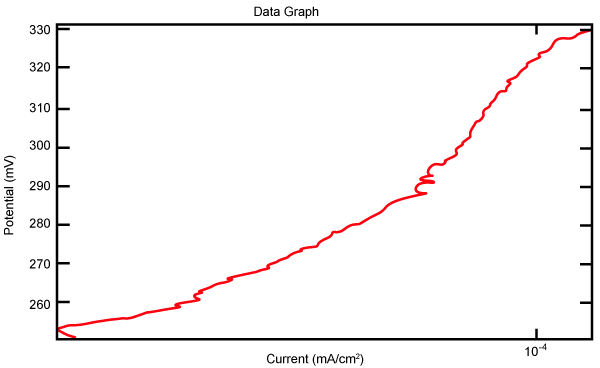
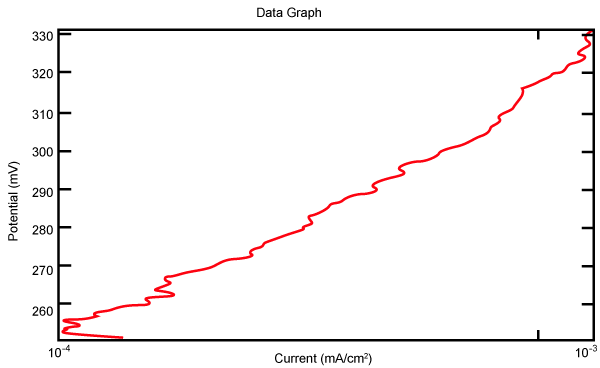
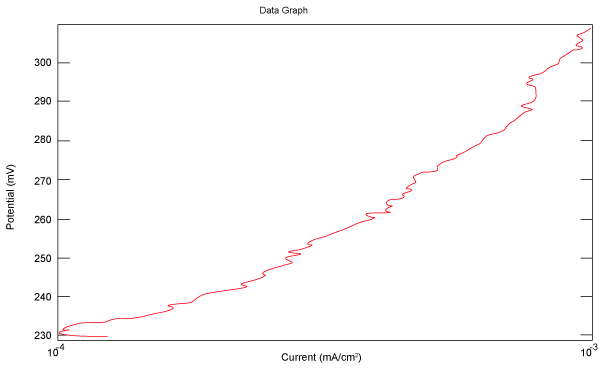
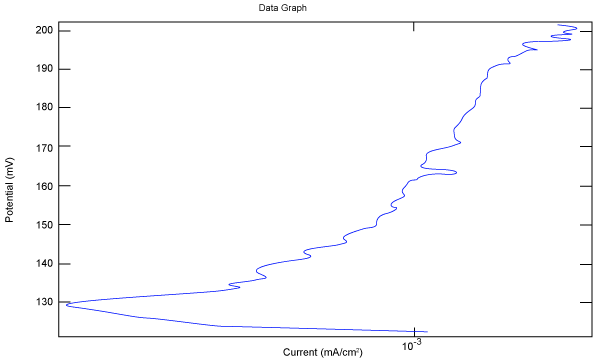
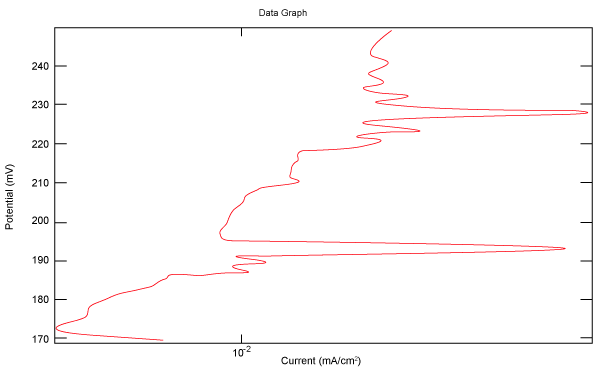
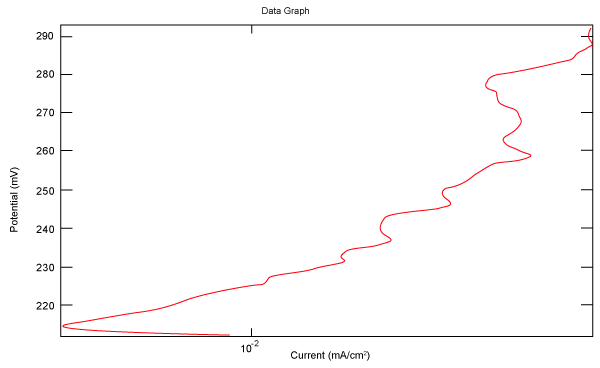
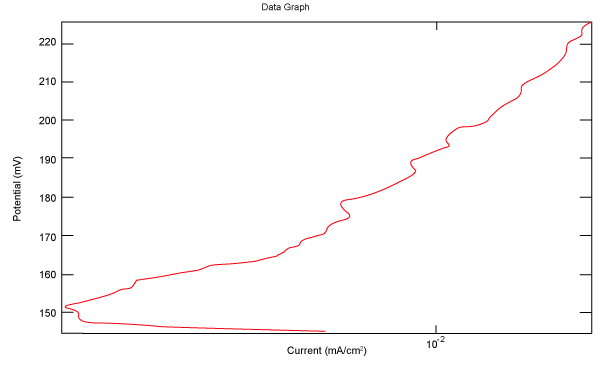
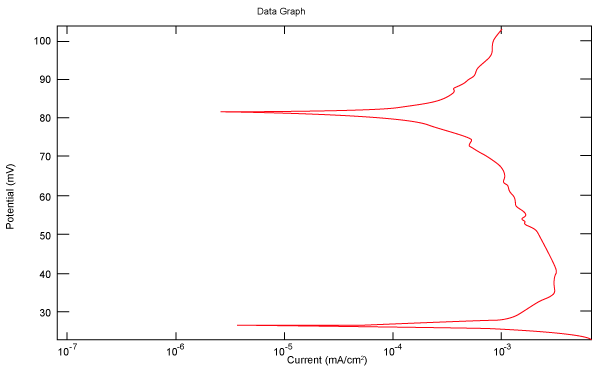
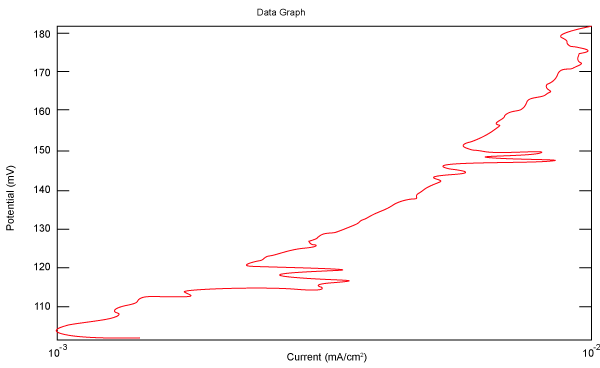
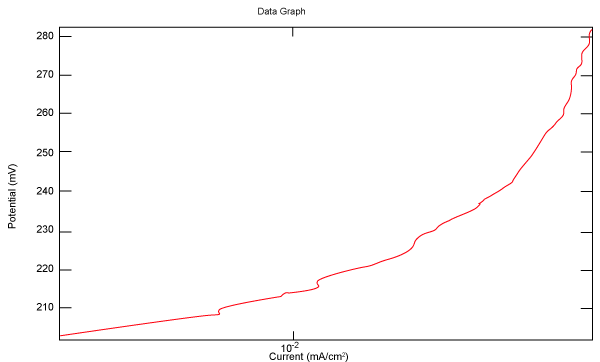
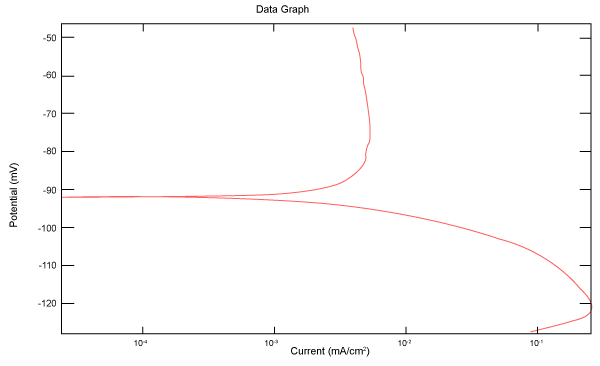
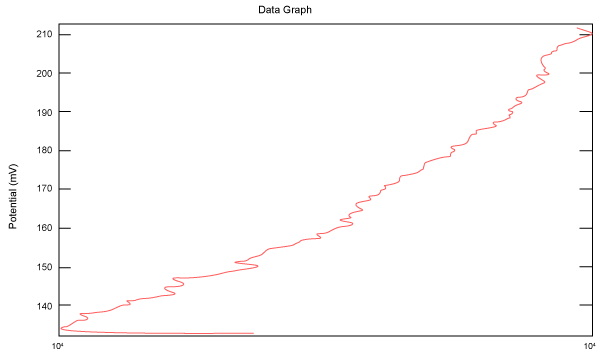
| Figure 2 shows Tafel polarization of 308L AISI when we had immersed a specimen in industrial phosphoric acid for a time (Table 3). Then we moved this specimen for different rotation speed, and got Tafel polarizations of 308L for several rotation speeds (500, 1000, 2000, 3000, 4000 rpm) as shown in figures 3-7. |
| |
| Â |
|
|
Figure 2: Tafel polarization of 308L in industrial phosphoric acid at static case. |
|
| Â |
|
|
Figure 3: Tafel polarization of 308L in industrial phosphoric acid at 500 rpm. |
|
| Â |
|
|
Figure 4: Tafel polarization of 308L in industrial phosphoric acid at 1000 rpm. |
|
| Â |
|
|
Figure 5: Tafel polarization of 308L in industrial phosphoric acid at 2000 rpm. |
|
| Â |
|
|
Figure 6: Tafel polarization of 308L in industrial phosphoric acid at 3000 rpm. |
|
| Â |
|
|
Figure 7: Travel polarization of 308L in industrial phosphoric acid at 4000 rpm. |
|
| Â |
|
|
Table 3: Corrosion and corrosion rate of 308L specimen at different rotation speeds. |
|
| Â |
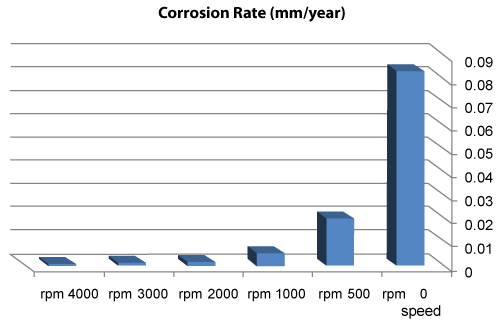
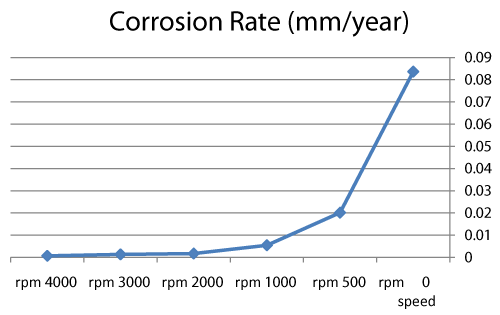
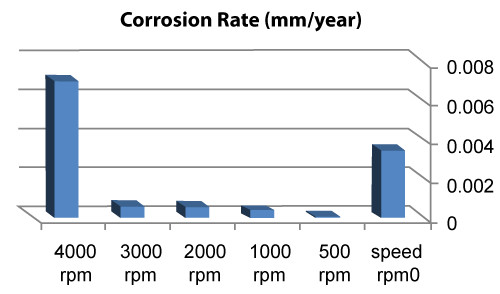
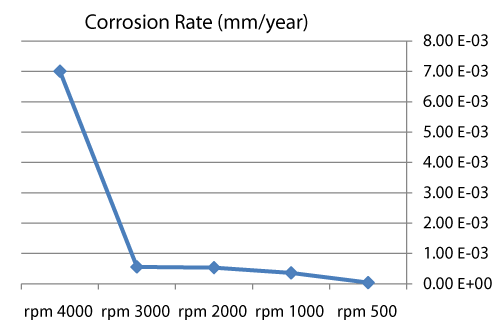
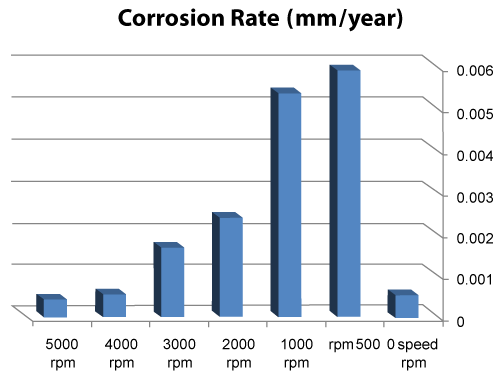
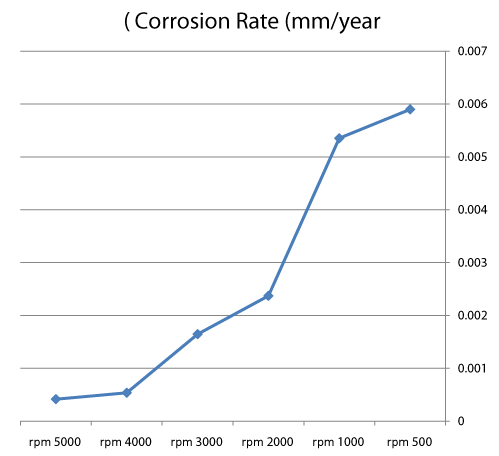
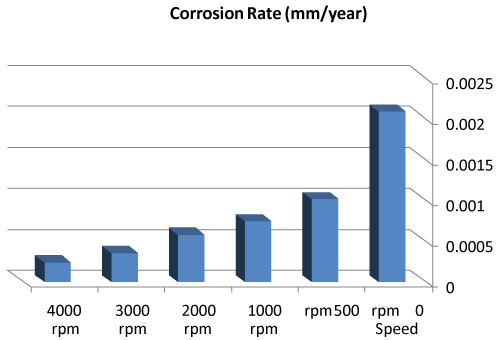
| We can observe from our tests that: |
| Â |
| • The highest value of corrosion rate for 308L is in static state. |
| Â |
| • There is a decrease in corrosion rate when the speed increases. |
| Â |
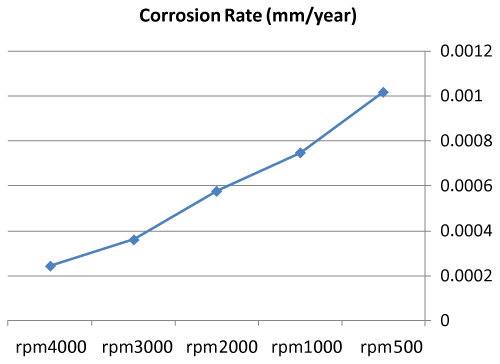
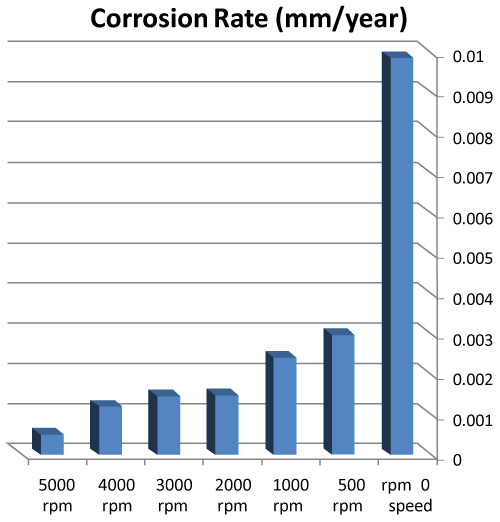
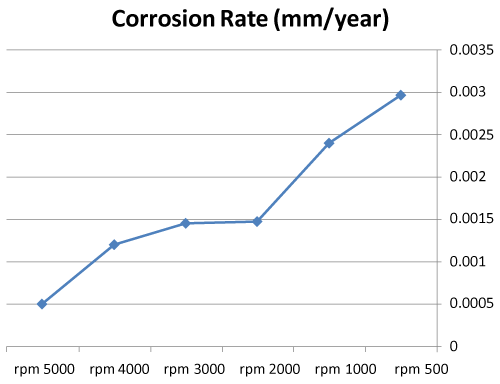
| • The corrosion rates of 308L are low in high rotation speeds (Figures 8 and 9). |
| Â |
|
|
Figure 8: The relation sheep between rotation speed and corrosion rate of 308L. |
|
| Â |
|
|
Figure 9: Graph between rotation speed and corrosion rate of 308L. |
|
| Â |
| The electrochemical behavior of 310S AISI in industrial phosphoric acid |
| Â |
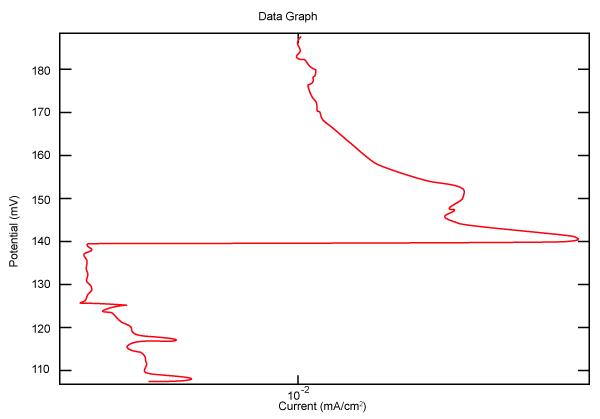
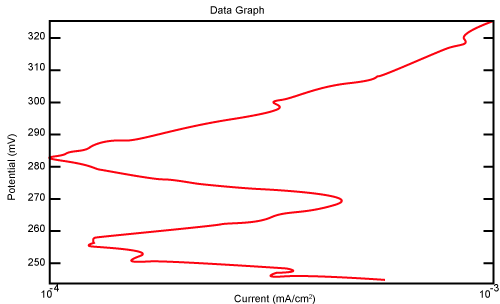
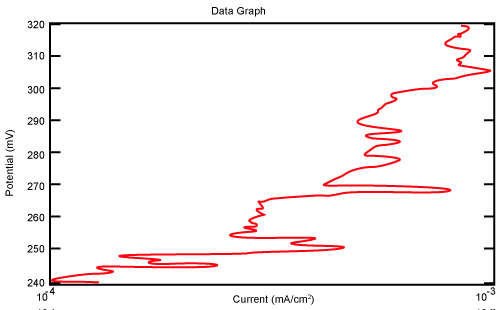
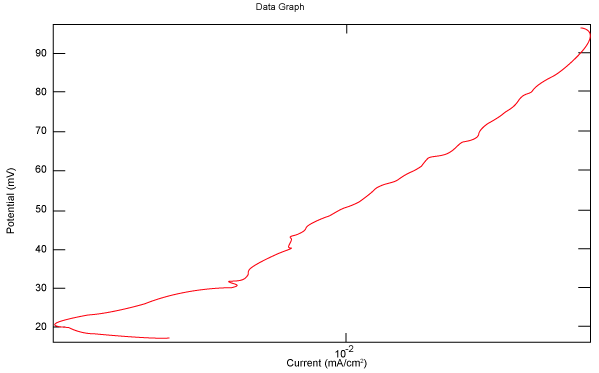
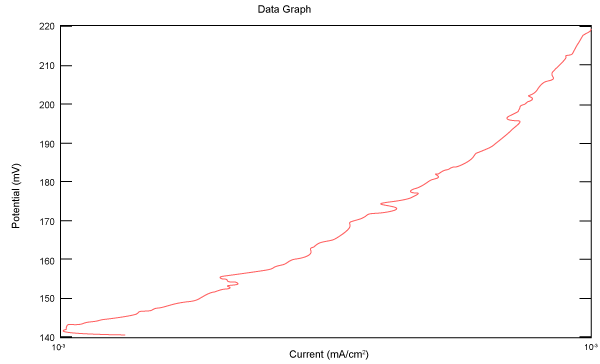
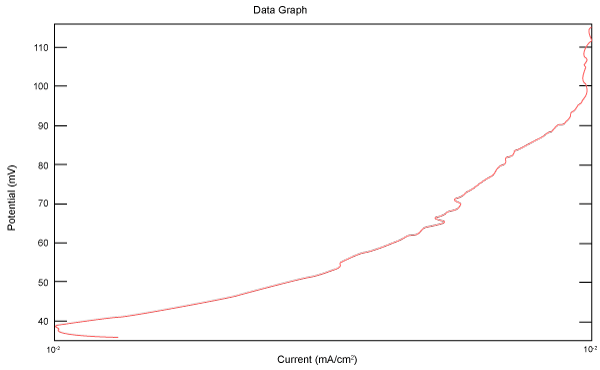
| Figure 10 shows Tafel polarization of 310S AISI when we had immersed a specimen in industrial phosphoric acid for a time, then we moved this specimen for different rotation speed, and got Tafel polarizations of 310S for several rotation speeds (500, 1000, 2000, 3000, 4000 rpm) as shown in figures 11-15. |
| Â |
|
|
Figure 10: Tafel polarization of 310S in industrial phosphoric acid at static case. |
|
| Â |
|
|
Figure 11: Tafel polarization of 310S in industrial phosphoric acid at 500 rpm. |
|
| Â |
|
|
Figure 12: Tafel polarization of 310S in industrial phosphoric acid at 1000 rpm. |
|
| Â |
|
|
Figure 13: Tafel polarization of 310S in industrial phosphoric acid at 2000 rpm. |
|
| Â |
|
|
Figure 14: Tafel polarization of 310S in industrial phosphoric acid at 3000 rpm. |
|
| Â |
|
|
Figure 15: Tafel polarization of 310S in industrial phosphoric acid at 4000 rpm. |
|
| Â |
| Table 4 and figures 16,17 show that: |
| Â |
|
|
Table 4: Corrosion and corrosion rate of 310S specimen at different rotation speeds. |
|
| Â |
|
|
Figure 16: The relation sheep between rotation speed and Corrosion Rate of 310S. |
|
| Â |
|
|
Figure 17: Graph between rotation speed and corrosion rate of 310S. |
|
| Â |
| • The highest value of corrosion rate for 310S is in the case of highest rotation speed of specimen (4000 rpm), then the second high value is in static state. |
| Â |
| • There is an increase in corrosion rate when the speed increases. |
| Â |
| The electrochemical behavior of 316L in industrial phosphoric acid |
| Â |
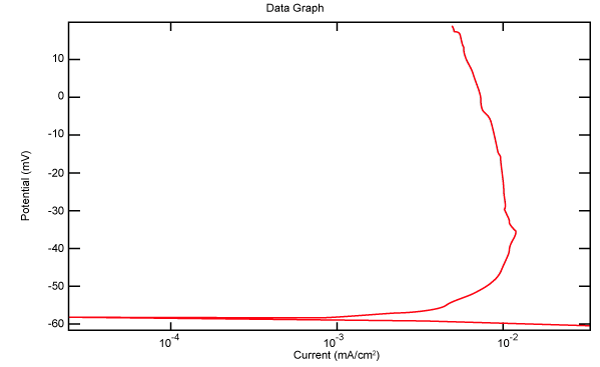
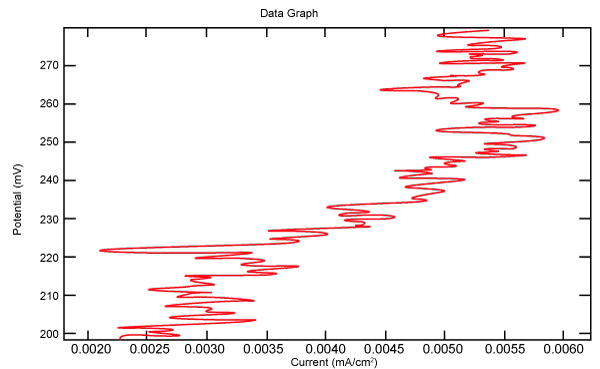
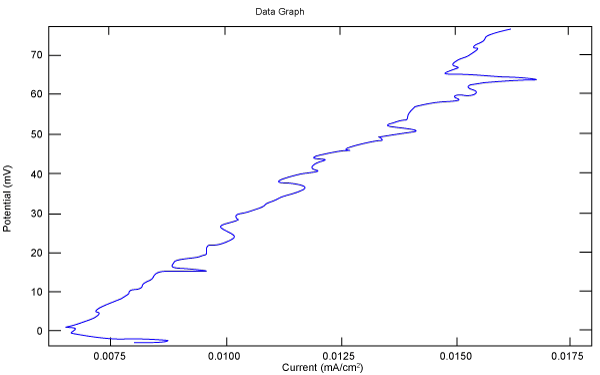
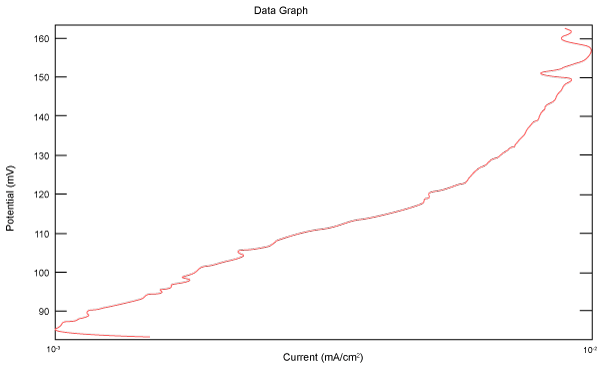
| Figure 18 shows Tafel polarization of 316L AISI in industrial phosphoric acid at static case. Figures 19-24 show Tafel polarizations of 316L for several rotation speeds (500, 1000, 2000, 3000, 4000, 5000 rpm). |
| Â |
|
|
Figure 18: Tafel polarization of 316L in industrial phosphoric acid at static case. |
|
| Â |
|
|
Figure 19: Tafel polarization of 316L in industrial phosphoric acid at 500rpm. |
|
| Â |
|
|
Figure 20: Tafel polarization of 316L in industrial phosphoric acid at 1000rpm. |
|
| Â |
|
|
Figure 21: Tafel polarization of 316L in industrial phosphoric acid at 2000rpm. |
|
| Â |
|
|
Figure 22: Tafel polarization of 316L in industrial phosphoric acid at 3000rpm. |
|
| Â |
|
|
Figure 23: Tafel polarization of 316L in industrial phosphoric acid at 4000rpm. |
|
| Â |
|
|
Figure 24: Tafel polarization of 316L in industrial phosphoric acid at 5000rpm. |
|
| Â |
| We can say as it appeared in figures 25, 26 and table 5 that corrosion rate of 316L in industrial phosphoric acid is decrease in case of increase in rotation speed, the lowest values at static case and 5000 rpm, the rise values at rotation speed (500,1000) rpm. |
| Â |
|
|
Figure 25: The relation sheep between rotation speed and Corrosion Rate of 316L. |
|
| Â |
|
|
Figure 26: Graph between rotation speed and corrosion rate of 316L. |
|
| Â |
|
|
Table 5: Corrosion and corrosion rate of 316L specimen at different rotation speeds. |
|
| Â |
| The electrochemical behavior of 904L AISI in industrial phosphoric acid |
| Â |
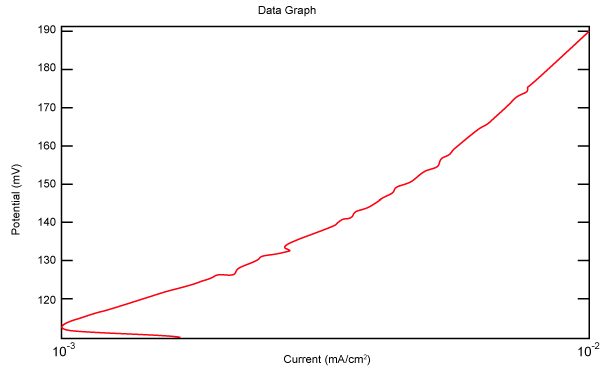
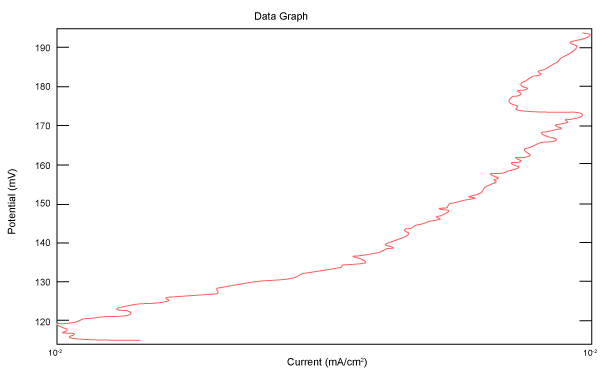
| Figure 27 shows Tafel polarization of 904L in industrial phosphoric acid at static case. Figures 28-32 show Tafel polarizations of 904L at several rotation speeds (500, 1000, 2000, 3000, 4000 rpm). |
| Â |
|
|
Figure 27: Tafel polarization of 904L in industrial phosphoric acid at static case. |
|
| Â |
|
|
Figure 28: Tafel polarization of 904L in industrial phosphoric acid at 500 rpm. |
|
| Â |
|
|
Figure 29: Tafel polarization of 904L in industrial phosphoric acid at 1000 rpm. |
|
| Â |
|
|
Figure 30: Tafel polarization of 904L in industrial phosphoric acid at 2000 rpm. |
|
| Â |
|
|
Figure 31: Tafel polarization of 904L in industrial phosphoric acid at 3000 rpm. |
|
| Â |
|
|
Figure 32: Tafel polarization of 904L in industrial phosphoric acid at 4000 rpm . |
|
| Â |
| We can observe from table 6 and figures 33, 34 that: |
| Â |
|
|
Figure 33: The relation sheep between rotation speed and Corrosion Rate of 904L. |
|
| Â |
|
|
Figure 34: Graph between rotation speed and corrosion rate of 904L. |
|
| Â |
|
|
Table 6: Corrosion and corrosion rate of 904L specimen at different rotation speeds. |
|
| Â |
| • The highest value of corrosion rate for 904L is in static state. |
| Â |
| • The lowest value of corrosion rate for 904L is at highest rotation speed. |
| Â |
| • It can say that corrosion rate of 904L decrease when speed is increased. |
| Â |
| The electrochemical behavior of ALLOY 600 in industrial phosphoric acid |
| Â |
| Figure 35 shows Tafel polarization of ALLOY 600 in industrial phosphoric acid at static case. Figures 36-41 show Tafel polarizations of ALLOY 600 at several rotation speeds (500, 1000, 2000, 3000, 4000, 5000 rpm). |
| Â |
|
|
Figure 35: Tafel polarization of ALLOY 600 in industrial phosphoric acid at static state. |
|
| Â |
|
|
Figure 36: Tafel polarization of ALLOY 600 in industrial phosphoric acid at 500 rpm. |
|
| Â |
|
|
Figure 37: Tafel polarization of ALLOY 600 in industrial phosphoric acid at 1000 rpm. |
|
| Â |
|
|
Figure 38: Tafel polarization of ALLOY 600 in industrial phosphoric acid at 2000 rpm. |
|
| Â |
|
|
Figure 39: Tafel polarization of ALLOY 600 in industrial phosphoric acid at 3000 rpm. |
|
| Â |
|
|
Figure 40: Tafel polarization of ALLOY 600 in industrial phosphoric acid at 4000 rpm. |
|
| Â |
|
|
Figure 41: Tafel polarization of ALLOY 600 in industrial phosphoric acid at 5000 rpm. |
|
| Â |
| Table 7 and figures 42, 43 show that: |
| Â |
|
|
Figure 42: The relation sheep between rotation speed and Corrosion Rate of ALLOY600 (corrosion rate value for static case is shared on 10). |
|
| Â |
|
|
Figure 43: Graph between rotation speed and corrosion rate of ALLOY600. |
|
| Â |
|
|
Table 7: Corrosion and Corrosion Rate of ALLOY 600 specimen at different rotation speeds. |
|
| Â |
| • The highest value of corrosion rate for ALLOY600 is in static state. |
| Â |
| • The lowest value of corrosion rate for ALLOY600 is at highest rotation speed. |
| Â |
| • There is a decrease in corrosion rate when the speed increases. |
| Â |
| Comparison among corrosion rates of tested alloys: |
| Â |
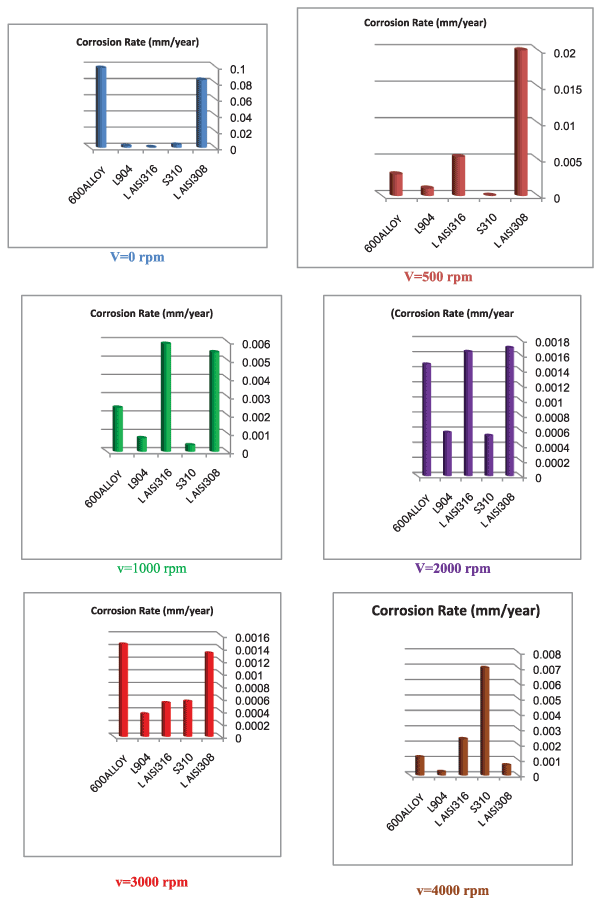
| Figure 44 shows that: |
| Â |
|
|
Figure 44: Corrosion rates of tested alloys in industrial phosphoric acid at several rotation speeds. |
|
| Â |
| • Types 904L and 316L show low corrosion rates in industrial phosphoric acid at static state, may be saying that their containment of chromium, nickel and molybdenum rise their corrosion resistance in industrial phosphoric acid. |
| Â |
| • Type 310S shows low corrosion rates in industrial phosphoric acid at static state. The recognized concentration of this alloy is high carbon concentration among tested alloys. |
| Â |
| • Type ALLOY 600 shows high corrosion rates in industrial phosphoric acid at static state, although it is great percent concentration of nickel. |
| Â |
| • Type 904L has low corrosion rates comparison with the tested types at all values rotation speed. |
| Â |
| • Type 310S has low corrosion rates comparison with the tested types at most of rotation speed values. |
| Â |
| Experimental Procedures At Phosphate Fertilizer Factory |
| Â |
| The dealloying erosion-corrosion |
| Â |
| The dealloying corrosion: Dealloying is a corrosion process in which the more active metal is selectively, removed from an alloy, leaving behind a porous weak deposit of the more noble metal. |
| Â |
| In our research, we analyzed some pieces of impellers of centrifugal fans which had been use to pulled gases during the process of producing fertilizer. There were two areas for analyzing, the first one is the inertial part of metal, and the second area is the corroded aria at the surface of metal. Table 8 shows the results of analyzing, we can see the difference in the chemical composition between the two areas, that there is a change in percent concentrations of some elements (Si, Mo, Cu, Cr). |
| Â |
|
|
Table 8: The chemical composition of 904L after the erosion-corrosion of this alloy by gases results on the phosphate fertilizer industry. |
|
| Â |
| Si: The concentration of Si in the alloy 904L before the attack of pollution gases as in the inertial part of metal 0.33% Si, the concentration of Si decreases to became 0.23% Si, that mean the lose amount of Si about 30% from the first containment.We can say that there is a selective of Si from the alloy then there is a dealloyig corrosion. |
| Â |
| Mo: The concentration of molybdenum in the alloy 904L before the attack of corrosion agents (phosphoric acid, fluorides, chlorides, solids) which are companion to exhaust pollution gases and vapors as in the inertial part of metal 4.43% Mo, the concentration of Mo decreases to became 3.454% Mo, that mean the lose amount of Mo is about 22% from the first containment. We can say that there is a selective of Mo from the alloy then there is a dealloyig corrosion. |
| Â |
| Cu: We can see from the table 8 that there is a decrease in the percent of copper Cu about 10% that mean there is a selective of copper element from the alloy then there is a deploying corrosion. |
| Â |
| Cr: The decrease of chromium percent about 2.5%, so we can say that there is a slight dealloyig corrosion of chromium compared with the dealloying corrosion of molybdenum, silicon, and copper. |
| Â |
| We can explanation the increase in average nickel content because the decrease in content of the elements (Si, Mo, Cu, Cr). |
| Â |
| The special alloy 904L recognized by the elements (Cr, Mo, Ni) are present in it, and the addition of Cu. |
| Â |
| Chromium is an essential element to make the surface protection film, and the increase in concentration of chromium increase the balancing of surface film. |
| Â |
| Molybdenum improves the tensile strength. Molybdenum frequently used in conjunction with chromium to help in balancing of surface film. When used in alloy steels in combination with chromium and nickel, molybdenum may produce high yield point and tensile strength values. |
| Â |
| Silicon increases the mechanical strength and the elasticity. Copper raises the strength and the yield point. Steel with a minor content of Cu has high resistant of corrosion in sulfuric solutions. |
| Â |
| As we see in our tests there is a dealloying corrosion of Silicon, Molybdenum and copper. The high lose in concentration of Silicon (about 30%) decreases the mechanical strength and decreases the resistance of alloy against solids, which impacts surface of metal. The high removal average of Molybdenum affectst the conjunction of particles at the surface film and make the protection film easy to be destroyed. the influence of removal copper is so important because the addition of copper to alloy is little so the low removal in concentration of copper decrease the yield and tensile strength of the alloy 904L and makes this alloy more affected by acid gases. |
| Â |
| We can say as a result that the dealloying corrosion of the important elements (Si, Mo, and Cu) changes the resistance corrosion properties of this alloy especially the resistance of erosion- corrosion. |
| Â |
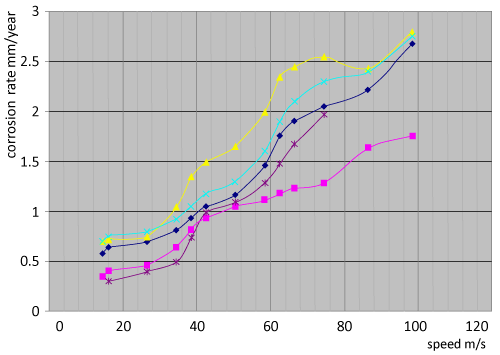
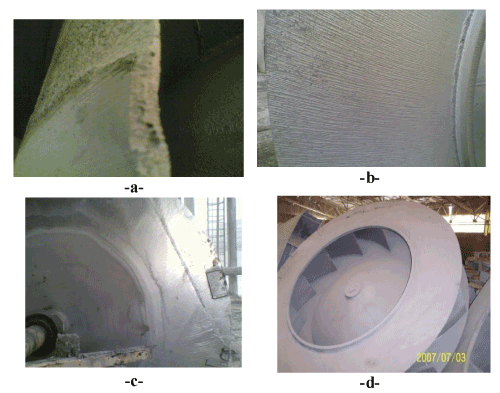
| The erosion corrosion: We calculated the corrosion rate of centrifugal fans made of 904L ALLOY by the Observation of change in corrosion rate during the service of fans, we take the thickness of metal in several places during stop of centrifugal fans for balancing, then we calculate the loss of metal against time as it shown in figure 45 which explain the change of corrosion rate against time for several centrifugal fans made of 904L serve in phosphate fertilizer factory. We Observe the high increase in corrosion during time, and there is a difference in erosion-corrosion rates of several places in the centrifugal fan according to line speed, that there is an increase in erosion-corrosion rates at high speed places (over than50 m/s), we find that there is a decrease in erosion-corrosion rates in low speed places (under than 50 m/s). the greater erosion-corrosion rates are in the places which have line speed about 95 m/s, the lowest greater erosion-corrosion rates are near the center of centrifugal fan where is the line speed is low. Figure 46c shows a semi circle line surrounds the area that have low erosioncorrosion rates where is the line speed under than 50 m/s, and we can see the strong attack of metal at the points that have greatest diameters, these points have high line speed. |
| Â |
|
|
Figure 45: Explain the change of corrosion rate against time for several centrifugal fans made of 904L ALLOY serve in phosphate fertilizer factory. |
|
| Â |
|
|
Figure 46: Shoes centrifugal fans which corroded by the flow of gases which contain (H3PO4, H2So4, F, solids). |
|
| Â |
| Gases include solids in phosphate fertilizer industry; these solids are fertilizer particles with different small diameters, the loss of metal surface as result of frequently shocks of these solids at the surface. The average of erosion-corrosion increase by the increase of speed, the highest average at the circulating points where is the speed about 95 m/s.Gases include some corrosive compounds such as phosphoric acid,sulfuric compounds, and fluoride and chloride compounds. These corrosive compounds affect the corrosion of 904L alloy that cause an increase in the affect of solids to rise erosion-corrosion average. we can say there is a united action of mechanical and chemical corrosive act.The most important effect on erosion-corrosion average is the the dealloying corrosion of (Si, Mo, Cu) which decrease the resistance corrosion of alloy and change the properties that the alloy had earn by the adnition of special elements, that make the way easy in front of particles and as a result there is high rate of abrasion and wear of alloy. The most important kinds of corrosion attack The special alloy 904L in phosphate fertilizer industry are dealloing corrosion and erosioncorrosion, that there is a united attack of dealloing and erosioncorrosion, the first kind changes the properties of alloy especially the yield strength and resistance corrosion, the second destroy and abrading the surface. We can say there is a dealloing erosion-corrosion attacks constructions in industrial Phosphoric acid (H3PO4) produced at General Fertilizer Company. |
| Â |
| Conclusion |
| Â |
| The objectives of this study were to investigate the corrosion behavior of stainless steels in industrial phosphoric acid with chloride and sulphate ions. In this paper, information was obtained with electrochemical Technique Tafel polarization. |
| Â |
| From this study, the following conclusions were: |
| Â |
| Compared to stainless steels and nickel alloy, 316L was more resistant with a low corrosion rate in static state. |
| Â |
| Types 308L, 316L, 904L, and ALLOY 600 had a decrease in corrosion rates at rise of rotation speed. |
| Â |
| There is an increase in corrosion rate of 310S when the speed increases. |
| Â |
| It can say that there is a new kind of corrosion attacks constructions in industrial Phosphoric acid (H3PO4) called dealloing erosioncorrosion. |
| Â |
| These results can be used for the selection of suitable alloys for the exploitation in industrial phosphoric acid planet especially for moving equipment of plant as pumps, fans. |
| Â |
| Acknowledgements |
| Â |
| The authors acknowledge the Genera Fertilizer Company for the alloy samples. |
| Â |
| |
| References |
| Â |
- Lin IJ, Schorr M (1996) Problems and Solutions in WPA Production. A Review. Trends in Chemical Engineering 10.
- Stephen D, Cramer, Bernard S, Covino Jr (2006) ASM Handbook, Volume 13C: Corrosion: Environments and Industries. ASM International.
- Schorr M, Valdez B, Zlatev R, Stoytcheva M (2010) Erosion-corrosion in Phosphoric Acid Production. Materials Performance 49: 56-59.
- El Hajjaji S, Aries L, Pebere N, Dabosi F, Audouard JP, et al. (1996) Passive State Behavior of Special Austenitic and Ferritic Stainless Steels in Phosphoric Acid Polluted by Sulfide Ions. Corrosion 52: 865-871.
- Abdellah G, Mohamed-Adil H, El Miloudi J, Ali Ben B (2006) Study of corrosion erosion behaviour of stainless alloys in industrial phosphoric acid medium. Appl Surf Sci 253: 2362-2366.
- Hosseini SMA, Salari M, Quanbari M (2007) Electrochemical Behavior of Ti – Alloy in the Mixture of Formic and Phosphoric Acid in the Presence of Organic Compound. Int J Electrochem Sci 2: 935-946.
- Poornima T, Nayak J, Nityananda AS (2010) Corrosion of Aged and Annealed 18 Ni 250 Grade Maraging Steel in Phosphoric Acid Medium. Int J Electrochem Sci 5: 56-71.
- Iken H, Basseguy R, Guenbour A, Ben Bachir A (2006) Classic and local analysis of corrosion behaviour of graphite and stainless steels in polluted phosphoric acid. Electrochimica Acta 5: 2580-2587
- Refaey SAM, Taha F, Abd El-Malak AM (2006) Corrosion and Inhibition of 316L stainless steel in neutral medium by 2-Mercaptobenzimidazole. Int J Electrochem Sci 1: 80-91.
- Khaled MM (2010) The Effect of Molecular Weight on the Corrosion Protection Properties of Polyvinylpyrrolidone Polymers on Stainless Steel. The Arabian Journal for Science and Engineering 35: 29-39.
- Ibanez-Ferrandiza M, Blasco-Tamarita ME, Garcia-Garciaa DM, Garcia-Antona J, Guenbourb A, et al. (2009) Corrosion of Austenitic Stainless Steels in Phosphoric Acid Polluted by Chloride and Sulfate Ions. Meet Abstr.
- Fauvet P, Balbaud F, Robin R, Tran QT, Mugnier A, et al. (2008) Corrosion mechanisms of austenitic stainless steels in nitric media used in reprocessing plants. J Nucl Mater 375: 52-64.
- Kearns JR (1985) The effect of nitrogen on the corrosion resistance of austenitic stainless alloys containing molybdenum. J Mater Eng 7: 16-26.
- Sanchez-Tovara R, Garcia-Antona J, Montanesa MT, Guenbourb A, Bakour S (2009) Electrochemical Measurement of the Galvanic Corrosion Effects on the Pair AISI 316L/Welded AISI 316L in H3PO4 Solutions at Different Temperatures. Meet Abstr.
- Sanchez-Tovar R, Montanes MT, Garcia-Anton J, Guenbour A (2011) Galvanic Corrosion of the Base AISI 316l/Micro-Plasma Arc Welded AISI 316l in Polluted Phosphoric Acid Media at Different Temperatures. Int J Electrochem Sci 6: 5550-5564.
- Tuma JV, Celin R (2007) Erection of A Stainless-Steel Tank for Storing a Phosphoric Acid. Metabk 46: 173-178.
|
| Â |
| Â |