| Research Article |
Open Access |
|
| Dan Chen1, Hai-e Chen1, Ying-chun Ma1, Shan Zhao1, Ya-kun Liu1, Li-na Lin2 and Wan-tie Wang1* |
| 1Department of Pathophysiology, Wenzhou Medical College, Wenzhou 325035, Zhenjiang, China |
| 2Department of Anesthesiology, First Affiliated Hospital of Wenzhou Medical College Wenzhou 325003, Zhejiang, China |
| *Corresponding author: |
Wan-tie Wang
Department of Pathophysiology
Wenzhou Medical College
Wenzhou 325025, Zhejiang, China
Tel: 0577-86689817
Fax: 0577-86689817
E-mail: wzwwt @tom.com |
|
| Â |
| Received October 29, 2012; Published November 05, 2012 |
| Â |
| Citation: Chen D, Chen H, Ma Yc, Zhao S, Liu Yk, et a. (2012) Effect of Propofol on Expression of PKC mRNA in Pulmonary Injury Induced by Ischemia-Reperfusion in Rabbits. 1:457. doi:10.4172/scientificreports.457 |
| Â |
| Copyright: © 2012 Chen D, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. |
| Â |
| Abstract |
| Â |
| Aim: This prospective study aimed at investigating the effect of propofol on the expression of protein kinase C (PKC) mRNA during pulmonary ischemia-reperfusion injury (PIRI) in the rabbits. |
| Â |
| Methods: Single lung ischemia-reperfusion animal model was administrated in vivo. The rabbits were randomly divided into three groups): sham-operated group (Sham); pulmonary I/R group (PIR) and PIR+propofol group (PIR+PPF). Changes of several parameters, including malondialdehyde (MDA) concentration, superoxide dismutase (SOD) activity, nitric oxide (NO) content, wet/dry ratio of lung tissue (W/D) and the index of quantitative assessment of histologic lung injury (IQA) were measured at 60 min after reperfusion. Meanwhile, the location and expression of PKC mRNA were observed, lung tissues were also harvested for histopathological evaluation. |
| Â |
| Results: As compared with PIR group, PKC mRNA is largely expressed in intima and extima of small pulmonary artery as well as thin-wall vessels (mostly small pulmonary veins). The average optical density values of PKC-α, δ and θ mRNA in small pulmonary veins in PIR+PPF group showed obviously higher than that in group PIR (all P<0.01); the activity of SOD increased, the concentration of MDA, W/D and IQA decreased at 60 min after reperfusion in lung tissue (P |
| Â |
| Conclusions: The results of this study suggested that propofol possesses and acts its notable protective effect on PIRI in rabbits by activating PKC-α, δ and θ mRNA expression in lung tissue, raising NO level, reducing OFR level and decreasing lipid peroxidation. |
| Â |
| Keywords |
| Â |
| Ischemia-reperfusion injury; Lung; Protein kinase C; Oxygen free radicals; Nitric oxide; Propofol |
| Â |
| Introduction |
| Â |
| With the rapid development of science and medicine recent years, pulmonary artery sleeve resection, lung and heart transplantation, pulmonary thrombolytic therapy and other new treatments are ongoing to establish and develop. However, the pulmonary ischemiareperfusion injury (PIRI) has always been a common complication for the thrombolytic therapy and prognosis after transplantation [1]. Herein, finding ways against PIRI becomes urgent and important [2,3]. The aim of this study is to explore the effect of propofol and its anti- PIRI mechanism on the rabbit PIRI models in vivo through observing protein kinase C (PKC) mRNA expression and location in lung tissue, determining SOD activity, MDA concentration, nitric oxide (NO) content, wet to dry ratio (W/D), evaluating the index of quantitative assessment of histological lung injury (IQA) and morphological changes in lung tissue, to provide a theoretical basis for strengthening the perioperative lung protection. |
| Â |
| Materials and Methods |
| Â |
| Animal models |
| Â |
| The Japanese male big ear rabbits, weighing (2.0~2.5) kg, were anesthetized with urethane (1.0 g/kg, IV.), then intubated and ventilated with 100% O2. The ventilation frequency was 30~40 times/min, tidal volume of bilateral lung ventilation was (15~20ml)/kg, single-lung ventilation, (8~10ml)/kg, and respiratory ratio 1:1.25. We separated one side of the jugular vein and intubated, then infused normal saline (0.5~1.5ml/min, IV). Left thoracotomy was administrated between the 4th and 5th inter costal, then the hilum of left lung separated, and followed by ligation thread placed at the hilum. Heparin (1.0 mg/kg body weight) was infused with intravenous injection, all the surgical procedures were based on Sekido's methods [4] to copy the rabbit lung ischemia- reperfusion model, which completely ligated the left hilum to block the blood flow and bronchus, then restore its blood flow and ventilation. |
| Â |
| Experimental groups |
| Â |
| The rabbits were randomized to 3 groups (n=9): sham control group (Sham group): separated the left hilum of lung without ligation; pulmonary ischemia reperfusion group (PIR group): ligation for 30 min, then blocked the left lung hilum for 60 min, and following 60- min reperfusion; pulmonary ischemia reperfusion+propofol group (PIR+PPF group), injected propofol 20 mg/kg/h through the jugular vein with micro-syringe pump (Sweden AstraZeneca Co, 200 mg/20 ml/bottle) until the end of surgery, the other procedures were the same with PIR group. |
| Â |
| PKC-α, δ θ in situ hybridization analysis in lung tissues |
| Â |
| Sample preparation: The lung tissues were fixed and followed by dehydration in 75% and 85%, 95%, 100% alcohol for 30 min, respectively, then embedded in paraffin-wax and cut into 5 μm sections, finally fixed on the glass slides coated poly-L-lysine. |
| Â |
| PKC isoenzyme oligo-nucleotide probe sequences: |
| Â |
| Objects |
Probe Sequence |
| PKC-Α |
5´--CAGGA CGTGG CCAAC CGCTT CGCCC GCAAA--3´ |
| 5´--GAATG ACTTC ATGGG ATCCC TTTCC TTTGG--3´ |
| 5´--CCAGC CTCTG CGGAA TGGAT CACAC TGAGA--3´ |
| PKC-Δ |
5´--CCTTC AACTC CTATG AGCTG GGCTC CCTGC--3 |
| 5´--CGCGT GATCC AGATT GTGCT AATGC GGGCA--3´ |
| 5´--TGGCT TCTCC TTTGT CAACC CCAAA TTCGA--3 |
| PKC-Θ |
5´--GGAGA CATCC GCCAG CACCC TTTGT TTCGG--3´ |
| 5´--GTGAA ATCAC CATTT GACTG CAGCA ATTTC--3´ |
| 5´--CAGAG CACTG ATCAA CAGCA TGGAC CAGAA--3´ |
|
| Â |
| In Situ hybridization steps |
| Â |
| • Baking the slices overnight at 60ºC, then washed with xylene for 15 min×2 times, 100% alcohol×2 times, 95% and 80% alcohol for 5 min×1 time, respectively; then dewaxed;Taking 3% H2O2-methanol to inactivate the endogenous peroxidase for 10 min at room temperature; rinsing with DEPC-treated water for 5 min×2 times; |
| Â |
| • Dropping pepsin diluted with 3% fresh citric acid at 37ºC to digest for 10 min, to exposes mRNA fragments of nucleic acid, rinsing with 0.5 mmol/L PBS (pH=7.4) for 5 min×3 times, water 5 min×1 time; |
| Â |
| • Dropping hybrid solution 20 μl at 37ºC for 3 h, and absorbing needless liquid, without rinsing; |
| Â |
| • Dropping with 20 μl hybrid solution contained PKC isoenzyme oligo nucleotide probe, and covering with a dedicated cover slip for situ hybridization at 42ºC overnight (about 17 h), then opening the cover slip, rinsing in succession at 37ºC with 2×SSC for 5 min×2 times; 0.5×SSC 15 min×2 times; 0.2×SSC 15 min×1 time. Negative control group dropping the hybrid liquid without probe; |
| Â |
| • Dropping non-specific antibodies blocked with normal sheep serum and diluted with 5% BSA at 37ºC for 30 min, shaking off excess liquid, no rinsing; |
| Â |
| • Dropping biotinylated mouse anti-digoxin at 37ºC for 60 min, rinsing with 0.5 mmol / L PBS for 5 min×4 times; |
| Â |
| • Dropping SABC liquid 37ºC for 20 min, rinsing with 0.5 mmol/LPBS for 5 min×3 times; |
| Â |
| • Dropping biotinylated peroxidase at 37ºC for 20 min, rinsing with 0.5 mmol/L PBS 5 min×3 times; |
| Â |
| • Dropping DAB reagent to colorate for 20 min, fully washed, hematoxylin staining, and then fully washed. 75%, 85%, 95% alcohol dehydration for 5 min×1 time, respectively. 100% alcohol dehydration 5 min × 2 times. Using xylene to make the slices transparent for 5min × 3 times, taking Clearmount for mounting. |
| Â |
| Criteria: The lung tissues showed brown color indicated the positive expression of PKC isoenzyme mRNA. |
| Â |
| Absorbance analysis: Apply the absorption analysis software developed by East China University of Science and Technology to read the absorbance values of the slices. Taking the non-specific staining regional of the peripheral vascular connective tissues as the negative background, we can get the absorbance values by subtracting the non-specific staining background from the measured diameters of the blood vessels. Selecting 5-10 thin-walled blood vessels from each slice, (inner diameter 15-150 μm, and mainly pulmonary vein), repeating 3 times, and applying the average absorbance value. |
| Â |
| Determinations of MDA content, SOD activity, NO levels and W/D ratio |
| Â |
| The animals were immediately killed after 60-min reperfusion, about 100 mg left lung tissues were harvested for 10% homogenate to measure SOD activity through xanthine oxidase method, to detect concentrations of MDA through thiobarbituric acid method, and to assay nitric oxide metabolites (NO2-/NO3-) to reflect the level of NO by nitrate reductase method (3 kits all from Jiancheng Bioengineering Research Institute, Nanjing, China) according to the manufacturer’s instructions. Additionally, about 2000 mg left lung tissues were taken for weighing wet weight, then baked at constant 70 ºC oven for 24 h for weighing dry weight to determine the ratio of W/D (wet/dry weight). |
| Â |
| Light microscope findings and IQA |
| Â |
| The left lower lobe of lung tissues, about 1mm3, were harvested and fixed with 4% paraformaldehyde, then the paraffin-wax embedding procedures were used. Hand E-stained slides were prepared by using standard methods. Light microscopic analyses of lung specimens were carried out in a blinded manner. Based on the methods introduced by Murata et al. [5], we observed a continuous 10 fields of vision (×200), calculated the ratio of the damaged alveolar cells to the total alveolar cells (× %), (the damaged alveolar cells contain more than 2 RBC and/ or 2 neutrophils (PMN) . The ratio was taken as the index of quantitative assessment (IQA). |
| Â |
| Electron microscopy findings |
| Â |
| The hilum of left lung tissues in 4cases each group, about 1 mm3, were harvested and fixed with 2.5% glutaraldehyde, followed by 1% osmium tetroxide. After fixation, the samples were administrated with ethanol-acetone series gradient dehydration, then embedded with Epon812, sliced with LKB-V-thin slicing machine, and following H-600-transmission electron microscopy observation. |
| Â |
| Statistical analysis |
| Â |
| All data are presented as mean ± standard deviation (x ± s) and performed with analysis of variance. Pearson analysis was used to analyze the relationship between MDA, SOD, W/D, IQA and PKC isoenzyme parameters. |
| Â |
| Results |
| Â |
| The absorbance changes of PKC isozyme in situ hybridization in pro-pulmonary vein during PIRI in rabbits each group |
| Â |
| The PKC-α, δ and θ mRNA expressions of pulmonary vein in PIR group were up-regulated, while there was no statistically differences comparing with Sham group (P>0.05); the pulmonary vein PKC-α, δ and θ mRNA expressions of PIR+PPF group were significantly higher than that in Sham group (PTable 1). |
| Â |
|
|
Table 1: Comparison of mean ISH OA values of PKC isoenzymes in pulmonary small veins among three groups in rabbits.
(x ± s, n=9) |
|
| |
| Â |
| The changes of SOD activity, MDA content and NO levels during PIRI in 3 groups |
| Â |
| In PIR group, the MDA concentration was significantly higher than that of Sham group, SOD activity was significantly lower than that of Sham group (P<0.01). In PIR+PPF group, MDA concentration was significantly lower than that of IR group, SOD activity and NO levels were significantly higher than that of PIR group (P<0.01); and there were no significant differences between SOD activity, MDA concentration and that in Sham group (P>0.05), while the levels of NO were significantly higher than that in Sham group (P<0.01) (Table 2). |
| Â |
|
|
Table 2: Comparison of SOD activity, MDA content and NO level in lung tissue among three groups in rabbits.
(x ± s, n=9) |
|
| Â |
| The changes of lung W/D and IQA |
| Â |
| In PIR group, the lung W/D ratio and IQA value were significantly higher than that in Sham group (P<0.01) at the point of 60min reperfusion. In PIR+PPF group, W/D ratio and the value of IQA were significantly lower than that of PIR group (P0.05) (Table 3). |
| Â |
|
|
Table 3: Comparison of W/D and IQA values in lung tissue among three groups in rabbits.
(x ± s, n=9) |
|
| Â |
| The relationship between the lung tissue MDA concentration, SOD activity, W/D ratio, IQA and PKC isoenzyme parameters |
| Â |
| Linear Correlation analysis indicated that there was negatively correlated between the MDA concentration and the absorbance values of PKC-α, δ and θ mRNA in situ hybridization, the correlation coefficient were -0.701, -0.651 and -0.626, P=<0.01, Pin situ hybridization, the correlation coefficient r were 0.574, 0.620 and 0.435, P=<0.01, Pin situ hybridization, the correlation coefficient “r†were -0.721, -0.698 and -0.478, P=<0.01, P in situ hybridization, the correlation coefficient r were -0.695, -0.743 and -0.494, P were <0.01, P |
| Â |
| Morphological changes in lung tissue |
| Â |
| Light microscope: In Sham group, the pulmonary interstitium and pulmonary alveoli are relatively complete and no infiltrated inflammatory cells. In PIR group, atelectasis coupled with emphysema, as well as pulmonary edema and widened, inflammatory cell infiltration were observed, and the pulmonary alveoli were filled with blood components effused, which indicated obvious injury. In PIR+PPF group, the lung injury and the inflammatory cells infiltrated were more slight and less than that of PIR group, and the structures of the alveoli were relatively intact. |
| Â |
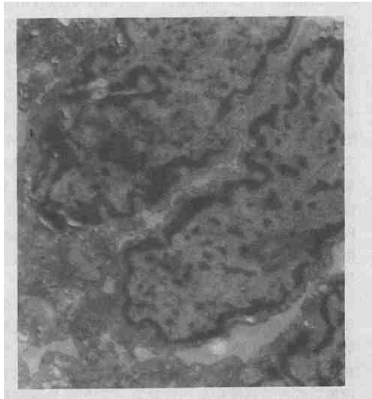
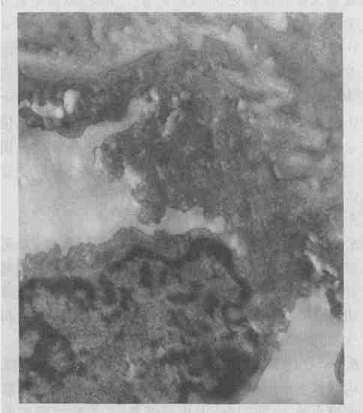
| Electron microscope: In PIR group, the endothelial cells with numerous pinocytosis vesicles were found in small pulmonary artery, and mitochondria became swelling and even vacuolar. Basilar membrane edema and vacuole degeneration, the dangling endothelial cells, and capillary lumen filled with PMN were seen. In type II epithelial cells, mitochondria became swelling and the number of lamellar body decreased (Figure 1). In PIR+PPF group, the structure of in small pulmonary artery looked normal, intact basilar membrane outside endothelial cells and dense connections of endothelial cells were observed. The structure of II-type cells showed no obvious abnormalities, no shed microvilli and no obvious PMN infiltration in alveolar septa were seen, but there were slight PMN adhesion and infiltration in capillary lumen (Figure 2). |
| Â |
|
|
Figure 1: Ultrastructure changes of left lung in PIR group. (TEM X15000) |
|
| Â |
|
|
Figure 2: Ultrastructure changes of left lung in PIR+PPF group. (TEM X15000) |
|
| Â |
| Discussion |
| Â |
| PKC was a group of protein kinase with a single peptide chain structure of the serine/threonine, with 12 kinds of subtype, were widely distributed in tissues and cells, and involved in hormone release, muscle contraction, DNA and protein synthesis, cell proliferation and differentiation, and other physiological processes. A lot of material, such as adenosine, bradykinin, norepinephrine, and so on can activate PKC by different means, and the activated PKC played its protection by acting on the end-effectors, such as endogenous antioxidant enzymes, inducible Nitrogen oxide synthase (iNOS), heat shock proteins (HSP), ATP-sensitive potassium ion channels, and so on. The key researches [6] in the past have shown that PKC can induce fibroblast growth factor, reduce endothelial cells apoptosis resulted from the radiation injury. Tanigaki et al. [7] also found that the inhibitor of PKC, H-7 can induce acute lung injury. This study showed that PKC-α, δ, and θ were negatively correlated to the indicators, the W/D and IQA, which were more sensitive to reflect the extent of damage for lung tissues, indicated that PKC isozyme had lung cells protection during ischemia-reperfusion injury [8]. The results of this study showed that W/D and IQA in PIR group significantly increased, the abnormal changes of the morphological structure were found. By contrast, W/D and IQA in PPF group only slightly increased and were significantly lower than that of PIR group, and abnormal morphological changes in lung tissues also markedly decreased. So the study indicated that propofol had an obvious protective role on ischemia-reperfusion injuries in lung cells. As shown in the figures and tables, PKC-α, δ, and θ mRNA expression in PIR+PPF group were significantly higher than that of sham group and PIR group, MDA concentration was significantly lower than PIR group, SOD activity and NO levels were significantly higher than PIR group. Besides, the linear correlation between MDA, SOD, NO and PKC-α, δ, and θ mRNA expression were significant, which indicated that propofol can cause PKC translocation and activation, protein substrates phosphorylation and enhance the activities of the endogenous antioxidant enzymes, iNOS and so on through certain signal transduction pathways, which alleviated ischemia reperfusion injuries by playing its cell protection and inducing the increase of the endogenous vasodilation mediators, such as NO. Kahraman et al. [9] also held the view that propofol can effectively prevent PIRI by decreasing the level of oxygen free radicals, reducing lipid peroxidation. Meanwhile, propofol can directly react with free radicals to generate 2.6-isopropyl-p-groups and at the same time cause the inactivation of free radicals. Murphy et al. [10] believed that propofol mainly interfered with the process of hydrogen abstraction during lipid peroxidation to form phenol, the latter participated in the further lipid peroxidation to form a more stable and non-active product, which interrupted the lipid peroxidation chain reaction. In addition, a good fat-soluble propofol also makes it easier to accumulate on the double lipid membrane of cells, thereby enhancing the cells' ability to anti-oxidative damage [11]. Certainly, propofol can increase NO levels by indirectly inhibiting PMN adhesion and aggregation [12], or indirectly inhibiting xanthine oxidase, or alleviating the production of superoxide anions [13] to prevent the lung tissues from the damage induced by oxygen free radicals, which effectively attenuated PIRI. Besides, propofol can also effectively prevent and treat PIRI by reducing the calcium content to reduce calcium overload [14,15]. |
| Â |
| Conflict of Interests |
| Â |
| The authors declare no conflict of interests. The authors alone are responsible for the content of this paper. |
| Â |
| Funding |
| Â |
| This work was supported by Department of Education Fund for Scientific Research in Zhejiang Province (20000670). |
| Â |
| Acknowledgments |
| Â |
| The authors thank the Animal Care center of Wenzhou Medical Collage for their assistance with animals. |
| Â |
| |
| References |
| Â |
- Davis RD Jr, Pasque MK (1995) Pulmonary transplantation. Ann Surg 221: 14-28.
- Chengyun W, Zhengjie X, Wantie W (2003) L-arginine on rabbit lung ischemia - reperfusion injury in rats. Wenzhou Medical College33: 85-88.
- Guanchao J, Guoliang Z, Jun L (1999) Ischemic preconditioning on lung ischemia-reperfusion injury. Chinese Journal of Experimental Surgery 16: 354-356.
- Sekido N, Mukaida N, Harada A, Nakanishi I, Watanabe Y, et al. (1993) Prevention of lung reperfusion injury in rabbits by a monoclonal antibody against interleukin-8. Nature 365: 654-657.
- Murata T, Nakazawa H, Mori I, Ohta Y, Yamabayashi H (1992) Reperfusion after a two-hour period of pulmonary artery occlusion causes pulmonary necrosis. Am Rev Respir Dis 146: 1048-1053.
- Haimovitz-Friedman A, Balaban N, McLoughlin M, Ehleiter D, Michaeli J, et al. (1994) Protein kinase C mediates basic fibroblast growth factor protection of endothelial cells against radiation-induced apoptosis. Cancer Res 54: 2591-2597.
- Tanigaki T, Suzuki Y, Heimer D, Wang W, Sussman HH, et al. (1994) The protein kinase C inhibitor, H-7, induces acute lung injury in guinea pigs. Crit Care Med 22: 1167-1173.
- Junwei T, Zhengjie X, Wantie W (2003) Protein kinase C in the rabbit lung protection of ischemic preconditioning in the role. Wenzhou Medical College 33: 89-91.
- Kahraman S, Kilinç K, Dal D, Erdem K (1997) Propofol attenuates formation of lipid peroxides in tourniquet-induced ischaemia-reperfusion injury. Br J Anaesth 78: 279-281.
- Murphy PG, Myers DS, Davies MJ, Webster NR, Jones JG (1992) The antioxidant potential of propofol (2,6-diisopropylphenol). Br J Anaesth 68: 613-618.
- Yunfei C, Weifeng Y (1998) Propofol anti-oxidation. Anesthesiology and foreign medical recovery in volumes 19: 209-212.
- Kubes P, Suzuki M, Granger DN (1991) Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci U S A 88: 4651-4655.
- Fukahori M, Ichimori K, Ishida H, Nakagawa H, Okino H (1994) Nitric oxide reversibly suppresses xanthine oxidase activity. Free Radic Res 21: 203-212.
- Zhou W, Fontenot HJ, Liu S, Kennedy RH (1997) Modulation of cardiac calcium channels by propofol. Anesthesiology 86: 670-675.
- Amorim P, Chambers G, Cottrell J, Kass IS (1995) Propofol reduces neuronal transmission damage and attenuates the changes in calcium, potassium, and sodium during hyperthermic anoxia in the rat hippocampal slice. Anesthesiology 83: 1254-1265.
|
| Â |
| Â |