| Research Article |
Open Access |
|
| Said A. H. Vuai* |
| School of Mathematical Sciences, College of Natural and Mathematical Sciences, P.O. Box 259, Dodoma, Tanzania |
| *Corresponding authors: |
Said A. H. Vuai
School of Mathematical Sciences
College of Natural and Mathematical Sciences
P.O.Box 259, Dodoma, Tanzania
E-mail: saidhamadv@yahoo.co.uk |
|
| Â |
| Received July 31, 2012; Published November 25, 2012 |
| Â |
| Citation: Vuai SAH (2012) Geochemical Characteristics of Spaleotherm Formation in Caves from Zanzibar Island, Tanzania. 1:505. doi:10.4172/scientificreports.505 |
| Â |
| Copyright: © 2012 Vuai SAH. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. |
| Â |
| Abstract |
| Â |
| This study was conducted to investigate geochemical characteristics of Spaleotherm precipitation from caves in Zanzibar Island, Tanzania as influenced by hydro geological zones. Nine caves, three from each region of the Zanzibar Island were selected for investigation. From each cave, water, stalagmite, stalactite, sediments, and hard rock were collected depending on their availability. Water samples were analyzed for pH, TDS, major cations and anions while rock samples were analyzed for mineral composition and total chemical composition including major and trace elements. The results of chemical composition of water showed higher contribution of seawater as indicated by higher concentration of Na+. Saline water has concentration trend (Na+>Mg2+>Ca2+>K+) similar to that of sea water while fresh water has the concentration trend of Na+>Ca2+>Mg2+>K+ which can be explained by mixing of seawater and carbonate water. The influence of hydogeological zone was evident for Mg concentration and mineral precipitation in cave features probable due to difference in chemical composition of water. The analysis of spaleotherms showed domination of calcite minerals in all cave features. However quartz and aragonite minerals exist in some cave sediments and stalactite. Observation of quartz minerals was related to the concentration above 1.6% of SiO2 detected in chemical composition. Inclusion of Mg during stalactite formation showed that it was hindered by phosphorous concentration. The magnesium partitioning coefficient range agrees well with those reported in other studies. Moreover the partitioning coefficient found to be low in the cave containing saline water emphasizing the influence of ionic strength on the Mg partitioning. Therefore the results suggest that chemical composition of spaleotherm can be used to trace the paleo-hydrological changes associated with salinization of ground water in cave. |
| Â |
| Keywords |
| Â |
| Geochemical; Spaleotherm; Zanzibar; Cave water |
| Â |
| Introduction |
| Â |
| Formation of cave is the common geological features in Zanzibar islands which is dominated by Quaternary (Q1, Q2, Q3), Pliocene and Miocene (M1, M2 M3). Both dry and wet cave do exist. Historically, Zanzibar people have been using caves for various purposes including cultural such as worship, recreation, such as site seen as well as source of water for drinking, washing, bathing and other domestic uses. Recently booming of tourism in the Island turns caves into one among good attractive sites due to their traceable history to colonization period especially Portuguese regime. |
| Â |
| Zanzibar Island can be divided into three major hydrogeological zones. These are eastern lime stone/sand stone corridor, the western sedimentary corridor and coastal limestone deposit called coral rag. Zanzibar Island has no reliable source of surface water; it depends entirely on ground water including springs, tube and bore holes as well as cave water (Figure 1). Cave water is a major source of water supply in urban and rural area. The quality of cave water is affected by anthropogenic and natural processes such as weathering rocks. For instance, McDonald [1] argued, cave drip waters respond to variations in surface recharge via changes in discharge and chemistry. Such changes may be encoded in the chemical and physical properties of the speleothems deposited from these waters, allowing the extraction of palaeohydrological and other climate-related information. According to Musgrove and Banner [2] variations in isotopes and trace elements (Mg/Ca and Sr/Ca ratios) of drip waters and soils from different caves, as well as phreatic ground waters, provide the potential to distinguish between local variability and regional processes controlling fluid geochemistry, and a frame work for understanding the links between climatic and hydrologic processes. In this regard, chemical composition of cave water can vary, depending on the nature of the caves, rocks or soil, as well as weathering process taking place at the particular site. Stalagmite trace element concentration profiles can reveal evidence for climatic events that disrupt the local hydrological cycle [3]. The aim of this study is to investigate the geochemical characteristics of spaleotherms from cave in Zanzibar. Specifically the study intend to investigate of source of chemical species of cave water, determination of mineralogical and chemical composition of spaleotherms (stalagmite, stalactites, sediments and hard rock) and investigation of precipitation and dissolution of characteristics of minerals during spaleotherms formation. |
| Â |
|
|
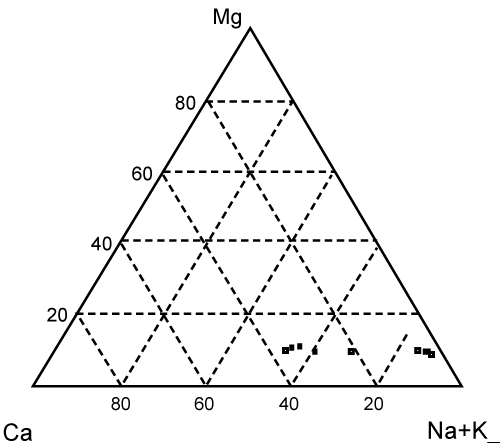
Figure 1: Triangular diagram showing chemical composition of cave water. |
|
| Â |
| Materials and Methods |
| Â |
| Study site |
| Â |
| Location and climate: This study focused on whole of Unguja Island in Zanzibar. Zanzibar is a union of two islands namely Unguja and Pemba, plus a number of small islets. Unguja Island is located between latitudes 6° south of the equator and 39.5’ east. It lies off the Tanzania Mainland 40 km (29miles) from Bagamoyo. Unguja Island is flat, and low lying covering an area of 1,554 sq km (598 sq miles) being about 85 km (53 miles) long and 20 km (12 miles) wide. Rainfall is strongly seasonal, related to change of monsoon and movement of the tropical convergence zone. The seasons of heavy rains (Masika) comes during March - May. Relative cool and dry season (Kusi) occurs during June- September while the light rains (Vuli) starts from October and December. The north east monsoon (Kaskazi) blows during January and February when the weather is dry and hot. The average annual rainfall varies between 1000-2,250mm and average annual temperature is 26°C. |
| Â |
| Geology: Zanzibar is a part of the ancient Miocene Rufiji / Ruvu river delta. Due to periods of isostatic movement and block faulting over the coastal Tanzania and off shore deltaic zone, only Zanzibar, Mafia and Latham Island areas remained above the sea level as land blocks of the original delta. It is a complex junction of four blocks evidenced by past artesian leakage with ferruginous and siliceous cements, and anhydrite deposits over the most of eastern Zanzibar. |
| Â |
| Geologically, Zanzibar is composed of lower Miocene M1, M2, and M3 rocks overlain by Quaternary Q1, Q2, and Q3. Q1 is complex of red, brown and black soils, thin tropical laterites, reworked Miocene sands, gravels, unsorted colluvial sands, clays and limestone fragments. The soil cover maintains a water table and acts as confining layer to the Q2 aquifer. It has a maximum of 25m thickness. Q2 is a white cream and coralline reef limestone with a thickness of 30 to 35m. It forms a thin veneer over the coastal platform where it is frequently cavernous and with many solution channels. In coastal areas, the Q2 is open to the sea. Q3 is marine fluviatile sand, cemented sandstone, and shell fragments, shark’s teeth with garnets, kyanites and tourmalines. It has a maximum thickness of 25m in the northeast Upenja-Kibokwa region. |
| Â |
| Miocene are rhythmic fluviatile sediments of the dissected Rufiji river delta. M1 is hard, dense, pearly white crystalline limestone in strata and lenses, detrital limestone of colluvial origin and recemented. It has a thickness of 2560m. M2 are grey, white course clean, angular siliceous sands often sugary in texture and lightly cemented. Closed and ponded drainage show the interesting mode of formation of local and isolated limestone lenses. M3 includes marls, sandy clays, clay sands and frequent gravel stringers (bluish grey to bluish green) which weather to a red, yellow or brown color. M3 constitute the main base rock of Unguja Island. |
| Â |
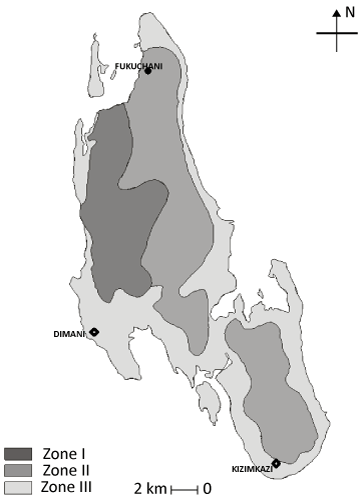
| Hydrology: Hydrological Zanzibar Island can be grouped into three zones (Figure 2). Zone one is Miocene type water, typically of a low TDS/CaCO3 type. It is a mixture of two simple components of local recharge (90%) and cannate (10%) water. Flow component in most cases is zero, which suggests that the steep Miocene water table gradients are the results of step and stairs type aquifer. Sulphate is principal contaminant at low season water levels when connate water percentage rises. Zone two is chloride type water due to either past ages of higher sea level, island movement or more simply windblown sea spray from an active coast. Island tilting, as evidenced by raised abandoned sea cliffs is considered the main cause in Zanzibar. Zone three is areas where sea water invasion is a natural phenomenon at depth, and where good quality waters are due to the development of perched aquifers. In daytime the perched aquifer becomes depleted and serves it contacts with well and the deeper contaminated take over. Zone one sources mainly consists of Q1/M1, zone two consist of Q2/M1 while zone III mainly consists of Q2/M1/M3. |
| Â |
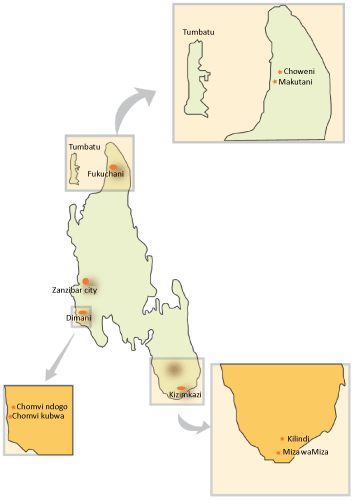
| Sample collection and analysis: Nine caves, three from each region of the Zanzibar Island of Tanzania were selected for investigation (Figure 2). The sampling covers two hydrological zones. Four samples (Diman 1-3 and Kizimkazi 3) were collected from hydrological zone II while the rest were collected from hydrological zone III. From each cave, water and rock samples were collected. Rock samples collected include stalagmite, stalactite, sediments, and hard rock depending on their availability. |
| Â |
|
|
Figure 2: Hydrological Zanzibar Island divided into three zones. |
|
| Â |
| Water samples were analyzed for temperature, pH, TDS, electrical conductivity (EC) and major cations and anions while rock samples were analyzed for mineral composition and total chemical composition including major and trace elements. Electrical conductivity and pH were measured in situ using HEC-100 EC meter and HM-21P pH meter, respectively. Samples were collected in polyethylene bottles and brought to the laboratory in a cool container. They were then filtered through a 0.45 μm Millipore cellulose filter. Water samples for cations analysis (Na+, K+, Mg2+ and Ca2+) were preserved in 1% v/v of 1.42 sp. density HNO3 and analyzed using atomic absoption spectrometry. Total chemical compostion for rock samples was measure using XRF using powdered sample while mineral compostion was measured using X-ray differection technique in random oriented powdered sample. The geochemical computer program PHREEQC [4] was used to calculate saturation indices and activities of dissolved species in cave water at formation temperature as measured during the time of sampling. |
| Â |
| Results and Discussion |
| Â |
| Water quality of cave |
| Â |
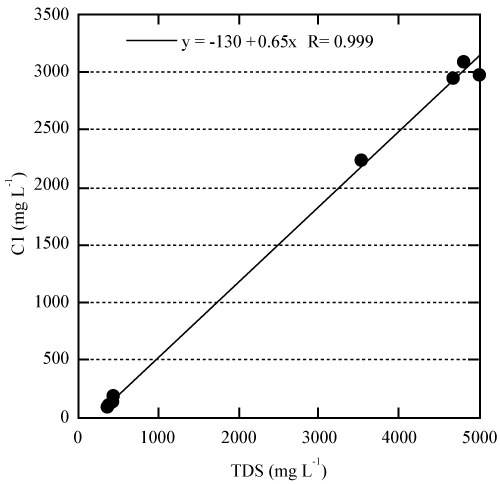
| Temperature variation of the water was very minimum; the value changes from 26.8 to 27.8oC. Similarly the pH also did vary slightly with values 7.29 to 7.74. The salinity of the water as indicated by Electrical Conductivity (EC) and total dissolved solids (TDS) range from 730 to 9500 μScm-1 and 359 to 4980 ppm, respectively. The chemical compositions differ according to hydrological zones with two different trends according to salinity. Zone III showed saline water strongly influenced by seawater as indicated by higher concentration of Na+. It has concentration trend (Na+>Mg2+>Ca2+>K+) similar to that of sea water. This was evident for samples collected from Fukuchani (1, 2 and 3) and Kizimkazi 1 (Table 1). Fresh water showed concentration trend of Na+>Ca2+>Mg2+>K+. The samples from Dimani (1, 2 and 3) and two from Kizimkazi (2 and 3) contained fresh water. Although Kizimakazi 1 is located in hydrological zone III, it showed relatively fresh water with chemical composition similar to those located in zone II. This suggest that the water from Kizimkazi 2 is from perched aquifer that is not affected by seawater intrusion and it chemical composition is mainly due to dissolution of Q2/M1/M3 rock by infiltration water enriched with chloride due to dissolution of sea salt aerosols. The amount of TDS varied proportional to the chloride concentration (R=0.999) suggesting salinization of cave water was due to seawater intrusion (Figure 3). The increase of chloride concentration in groundwater is most indicative of groundwater salinization caused by seawater intrusion due to its conservative nature whereas the concentration variations of other elements are frequently ignored because of their transformation and modification due to interactions with aquifer materials [5]. It is widely known that the hydrogeochemical composition of coastal groundwater affected by seawater intrusion is mainly controlled by cation exchange reaction rather than simple mixing process [6]. The chemical composition of fresh groundwater unaffected by seawater intrusion is dominated by Ca2+ and HCO3- ions where as in sea water; Na+ and Cl- are the dominant ions. The cation exchange in the aquifer is therefore expected to increase Ca2+ in the water. If this takes place stochiometrically, the slope of the graph of Ca2+ verses Na+ in molar concentration is expected to be close to 2. The exchange process is always not linear and follows ligumier curve. The plot of Ca2+ against Na+ in this study showed value clearly deviating from 2. In addition to that, chemical composition of cave water (Figure 4) plots along the straight line of Na+ K and Ca line. The results suggest that mixing of seawater and carbonate water can explain the chemical composition of the cave water in Zanzibar. |
| Â |
|
|
Table 1: Chemical Characteristics of Cave Water. |
|
| Â |
|
|
Figure 3: Map of Zanzibar Island showing sampling points. |
|
| Â |
|
|
Figure 4: Evolution of Chemical Species in Cave water. |
|
| Â |
| Minerals and chemical characteristics of cave features |
| Â |
| In each cave the study intended to collect stalagmite, stalactite, hard rock and sediments from cave flow. However, in most caves these features could not be collected. Only four caves provided stalactite and stalagmite samples. The cave features showed uniform chemical composition (Table 2) reflecting carbonate minerals of the bed rock. They are dominated by loss of ignition and CaO. Ignition loss includes organic matters and loss of carbon dioxide during decomposition of CaCO3 into CaO. These two species account for more than 90% of the total weight with exception of one sediment sample from Dimani (Table 2). Other chemical species present in significant amount is MgO, silica and aluminum oxide. They account for less than 2% each with exception of one sediment sample. This sediment has chemical characteristics significantly different from the hard rock collected in the same cave. It is suggested that the sediment is foreign material brought in either by human activities or runoff during the rain event. Influence of hydrogeological zone on chemical composition of the cave features revealed that the contents of magnesium oxide were higher for hard rock and sediment of the caves found in the zone III compared to zone II. The trend was reversed for stalactite and stalagmite (Table 3). |
| |
| Â |
|
|
Table 2: Mineralogical and chemical composition of Cave features. |
|
| Â |
| Strontium and phosphorous were the only trace elements found in all samples. The values ranged from 0.03 to 0.19% and 0.1 to 0.5 as SrO and P2O5, respectively. One sediment sample showed enrichment of SrO value of 0.6%. There is no clear difference between chemical composition of trace elements in hard rock/sediments and spaleotherms (stalactite and stalagmite). The contents were not statistical different among the zone in which these caves were found. This might be due to their low content and presence of heterogeneous geological features among the zones. |
| Â |
| Generally, the cave features are dominated by calcite minerals reflecting the dominant carbonate terrine in the cave areas. Aragonite was found in sediment samples contained the highest content of SrO (0.6%) and phosphorous found in zone III. It is suggested that this chemical species is preferential incorporate in the aragonite lattice than calcite or has influence on the precipitation of aragonite. This results support findings of Huang & Fairchild [7] which suggested that Sr/Ca is affected by crystallography of the spaleotherm. Okumura, 1987 also reported that PO43- is preferentially incorporated in the aragonite than in calcite lattice. Quartz mineral was also found in those samples in which the SiO content is high. |
| Â |
| Spaleotherm formation |
| Â |
| Trace element variations in cave water reflect water-rock interaction time which is controlled by hydrological condition [7,8]. The link between trace element variation in spaleotherms and cave water is partition of the element during calcite precipitation. The ratio of Mg/Ca, P/Ca and Sr/Ca are shown in table 3. |
| Â |
|
|
Table 3: Calcium ratios and Magnesium portioning coefficients of Spaleotherm. |
|
| Â |
| Stalagmite showed wide variation of Mg/Ca ratios ranging from 16×10-4 to 78×10-4 while P/Ca ratios were almost constant. On the other hand stalactite showed wide variation in both Mg/Ca and P/Ca ratios. Stalagmite is highly influenced by cave water since it is precipitated on the surface of the cave and hence being in continuous interacting with water. In contrary, stalactite is precipitated on the roof of the cave as results of precipitation of pore water infiltrating through the soil and near surface rock. This different hydrological pathway affect water solute composition and hence the ratios. |
| Â |
| Magnesium partitioning coefficient for stalactite range between 0.0028 and 0.0259 while for the stalagmite ranged between 0.0069 and 0.035. The values agree fairly well with range of Kd reported by Huang & Fairchild at temperature of 25°C and that of Huang [7,8]. Magnesium partitioning coefficient during precipitation of spaleotherm is affected by temperature and ionic strength [9]. The temperature in our samples varies slightly (26.8-27.8). This one degree change could not be expected to course changes of one order of magnitude for stalactite and five fold for stalagmite. Thoroughly examination of the results showed that the lowest concentration of KD was found in the saline water emphasizing the effect of ionic strength. Our results concur with those of Huang and Fairchild which found that partitioning coefficient was low in high ionic strength solution [7]. |
| Â |
| Conclusion |
| Â |
| Hydrogeochemical characteristics of cave water and spaleotherms from Zanzibar Island, Tanzania were investigated. The results showed that chemistry of cave water varies from fresh water to saline water depending on the distance from the ocean as well as degree of mixing with sea water. Generally the chemistry of cave water can be explained by mixing of carbonate water and seawater. Chemical characteristics of cave features showed that they are dominated with calcium carbonate with relatively uniform chemical composition. This was also reflected by high degree of calcite minerals in all features. Aragonite and quartz mineral are found only in few samples in trace level. It was observed that the quartz mineral was related with SiO contents above 1%. Inclusion of Mg during stalactite formation showed that it was hindered by phosphorous concentration. The magnesium partitioning coefficient range agrees well with those reported in other studies. Moreover, the partitioning coefficient found to be low in the cave containing saline water emphasizing the influence of ionic strength on the Mg partitioning. |
| Â |
| Acknowledgement |
| Â |
| I would like to acknowledge Mr. Hassan R. Ali and Ali Omar Ali of the State University of Zanzibar for sample collection and processing and SEAMIC for sample analysis. |
| Â |
| |
| References |
| Â |
- McDonald J, Drysdale R, Hill D, Chisari R, Wong H (2007) The hydrochemical response of cave drip waters to sub-annual and inter-annual climate variability, Wombeyan Caves, SE Australia. Chem Geol 244: 605-623.
- Musgrove M, Banner JL (2004) Controls on the spatial and temporal variability of vadose dripwater geochemistry: Edwards Aquifer, central Texas. Geochimica et Cosmochimica Acta 68: 1007-1020.
- Baldini JUL, McDermott F, Fairchild IJ (2006) Spatial variability in cave drip water hydrochemistry: Implications for stalagmite paleoclimate records. Chem Geol 235: 390-404.
- Parkhurst DL, Appelo P (1999) UserÂ’s guide to PHREEQC (Version 2)-A Computer Program for Speciation, Batch-reaction, One-dimensional Transport and Geochemical Calculations. US Geo Surv.
- Stoessell RK (1997) Delineating the chemical composition of the salinity source for the saline ground waters: an example from East-Central Concordia Parish, Louisiana. Ground Water 35: 409-417.
- Appelo CAJ (1996) Multicomponent ion exchange and chromatography in natural systems. Reviews in Mineralogy and Geochemistr 34: 193-227.
- Huang J, Fairchild IJ (2001) Partitioning of Sr2+ and Mg2+ in to calcite under karst-analogue experimental condition. Geochimica et Cosmochimica Acta 65: 47-62.
- Huang Y, Fairchild IJ, Borsato A, Frisia S, Cassidy NJ, et al. (2001) Seasonal variation in Sr, Mg, and P in modern speleothems (Grotta di Ernesto, Italy). Chem Geol 175: 429-448.
- Burton EA, Walter LM (1990) The role of pH in phosphate inhibition of calcite and aragonite precipitation rates in seawater. Geochimica et Cosmochimica Acta 54: 797-808
|
| Â |
| Â |