| Research Article |
Open Access |
|
| P Yazdanpanah1*, M R Nikbakht2, S Kheramin3 and R Hossini3 |
| 1Yasouj University of Medical Sciences, Yasouj, Iran |
| 2Kermanshah University of Medical Sciences, Kermanshah, Iran |
| 3Yasouj University of Medical Sciences, Yasouj, Iran |
| *Corresponding author: |
P Yazdanpanah
Associate Professor
Yasouj University of Medical Sciences
Yasouj, Iran
Tel: +987412231005
Fax: +987412231005
E-mail: parvan1339@yahoo.com |
|
| Â |
| Received February 13, 2012; Published August 25, 2012 |
| Â |
| Citation: Yazdanpanah P, Nikbakht MR, Kheramin S, Hossini R (2012) Effect of Oral and Intramuscular Injection of Methocarbamol and Placebo on Muscle Strain Pain. 1:573 doi:10.4172/scientificreports.573 |
| Â |
| Copyright: © 2012 Yazdanpanah P, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. |
| Â |
| Abstract |
| Â |
| Introduction: Muscle strain is caused by overstretching and/or partial or complete tear in musculo-tendinous junction. One series of treatment of muscle strain are non-steroidal anti-inflammatory drugs and muscle relaxants (such as methocarbamol). |
| Â |
| The aim of this study was survey the effect of oral and intramuscular injection of methocarbamol and placebo on muscle strain pain. |
| Â |
| Materials and methods: This clinical trial study was carried out on 120 patients with diagnosis of muscle strain. |
| Â |
| The objects were classified by randomized allocation in 3 groups with equal numbers and pain intensity was evaluated before starting and at the end of treatment. |
| Â |
| The first group received oral methocarbamol (2 tab(1000 mg), q4 h, for 3 days, then 3 tab, q8 h, for 4 days) ,the second group received 1 ampule (1000 mg) methocarbamol on buttocks and the third group received placebo (1 tab, q12h). |
| Â |
| The 3 groups received naproxen (1 tab (500 mg), q12 h for 7 days). |
| Â |
| Results: Results showed that the mean pain severities before treatment were a 4.64, 4.26, and 4.16 in 3 group respectively that was insignificant (p=0.087). And after treatment were 2.12, 2.16, and 1.57 in 3 groups respectively that were insignificant (p=0.133) too. Also, the mean pain severities before and after treatment were 4.49 and 1.95 respectively that was significant (p=0.001). |
| Â |
| Conclusions: The pain severity was decreased in 3 group’s. The pain reduction was more in patients who received placebo. These findings suggest that pain reduction was due to effect of naproxen and lapse of time. |
| Â |
| Keywords |
| Â |
| Muscle strain; Methocarbamol; Naproxen; Placebo |
| Â |
| Introduction |
| Â |
| A strain is an injury which damages the internal structure of the muscles. Muscle strain is caused by overstretching and/or partial or complete tear in musculotendinous junction [1]. Muscle strains and other musculoskeletal disorders are a leading cause of work absenteeism. Muscle pain, spasm, swelling, and inflammation are symptoms of strains [2]. Strain injuries are graded as follows: First degree (mild)- an overstretch with minimal disruption of musculotendinous unit integrity, second degree (moderate)-an actual (incomplete) muscle tear and third degree (severe) that is a complete rupture. Muscle function is essentially lost [3]. Back strain may account 60% to 70% of mechanical low back pain and almost 85% of neck pain results from acute or repetitive neck injuries or chronic stress and strain [4]. |
| Â |
| Medications such as Non-Steroidal Anti-Inflammatory Drugs (NSAIDs), muscle relaxants and opioids are used for treatment of muscle strain [1]. Naproxen belongs to class propionic acids of NSAIDs which is perhaps the most popular NSAID class due to widespread prescription and use for treatment of muscle strain. This drug is beneficicial for treatment of inflammation and pain. The half-life of naproxen is 14 hours. The anti-inflammatory dose of naproxen is 375 milligrams bid [5]. The incidence of upper gastrointestinal bleeding in use of naproxen in over-the-counter is low but still double that of overthe- counter ibuprofen [5]. |
| Â |
| The use of muscle relaxants remains controversial. Despite its controversy, 35% of patients who visit a primary care physician for low back pain are prescribed muscle relaxants [6]. These medications fall into three classes of drug: the benzodiazepines, the nonbenzodiazepines that are antispasmodics, and antispasticity medication [4]. Methocarbamol is a non-benzodiazepine antispasmodic muscle relaxant. The mechanism of action of methocarbamol in humans has not been established, but may be due to central nervous system depression [7]. The methocarbamol are use as orally, intramuscular and intravenous forms the doses of methocarbamol in oral form 1500 mg qid for 48-72 h, then 1000-1500 mg qid. The doses of Intramuscular (IM) methocarbamol is 1000 to 1500 mg in two buttocks [8]. Methocarbamol was approved by FDA in July 16, 1957. |
| Â |
| There are multiple high-quality studies showing that muscle relaxants are effective for patients with acute low back pain for shortterm pain relief. The most common side effects are drowsiness and dizziness [5]. |
| Â |
| Currently there is no evidence that one type of muscle relaxant is more effective than another [1]. There exists not much literature on the use of muscle relaxants for chronic pain [7]. |
| Â |
| The placebo is administration of an inert material, with exactly the same physical response, odor, consistency, etc. as the active dosage form [5]. |
| Â |
| The aims of this study were: |
| Â |
| 1-Effect of oral and intramuscular injection of methocarbamol and placebo on muscle strain pain |
| Â |
| 2-Detection complications of oral and intramuscular (IM) methocarbamol |
| Â |
| Methods |
| Â |
| This was a clinical trial study which was conducted in S Mofateh clinic in Yasouj (Iran) from June 2008 to March 2010. 120 patients with diagnosis of muscle strain with duration of less than 2 weeks were classified by randomized allocation (3 blocks) in 3 groups with equal participants. The cases did not have any GI, renal and hepatic diseases. Differential diagnoses of muscle strain such as Radiculopathy, Spinal stenosis, Trauma, Discopathy, Myositis, Whiplash injury and others were unaccepted in study. Intramuscular methocarbamol was not administered to patients with known or suspected renal pathology. |
| Â |
| The cases were diagnosed by a physiatrist. This study was approved by ethics committee of Yasouj University of medical sciences. Iranian registration clinical trial ID was: IRCT138904014238N1. |
| Â |
| The demographic information, pain intensity (measured by visual analogue scale) were evaluated before start of treatment. The first group (33 cases) received oral methocarbamol (2 tab(1000 mg), q4 h, for 3 days, then 3 tab, q8 h, for 4 days) and naproxen (1 tab(500 mg), q12 h for 7 days). The second group (38 cases) received 1 ampule (1000 mg) methocarbamol on buttocks and naproxen (1 tab (500 mg), q12 h for 7 days). |
| Â |
| The third group (35 cases) received placebo (1 tab, q12 h) and naproxen (1 tab (500 mg), q12 h) for 7 days. |
| Â |
| The methocarbamol and naproxen drugs were the products of Darou Pakhsh Pharmaceutical Mfg. Co.-Tehran-Iran. The dosage and appearance of placebo was as same as naproxen. 14 patients missed the treatment courses due to different causes. The pain intensity and rate of recovery was evaluated at the end of treatment. |
| Â |
| The data were analyzed by SPSS, and χ square, T test and analysis of variance were used. |
| Â |
| Results |
| Â |
| The mean age of patients was 37.5 years age (80% of cases were younger than 45 years ago), 74.5% of patients were men and 25.5% were women. The jobs of patients were 34% white -collar workers, 31% unemployed, 19% workers and 16% unknown employment. The most common site of muscle strain was in the low back (81%). |
| Â |
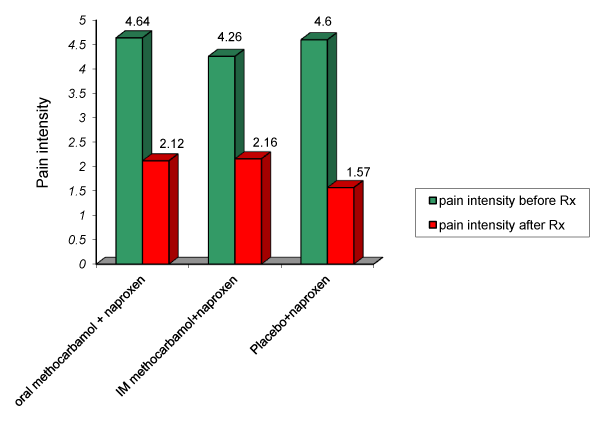
| The mean pain severity before treatment were 4.64, 4.26, 4.16 (with SD 0.699, 0.828, 0.812) in 3 groups respectively which was not significant (p=0.087). The mean pain severity after end of treatment were 2.12, 2.16, 1.57 (with SD of 1.635, 1.305, 1.119) in 3 groups respectively that was not significant (p=0.133) (Figure 1). The mean pain severity before treatment was 4.49 and after the treatment was 1.95 (with SD of 0.796, 1.376 respectively) that was significant (p=0.001). |
| Â |
|
|
Figure 1: Comparison of pain intensity before and after intervention in 3 groups. |
|
| Â |
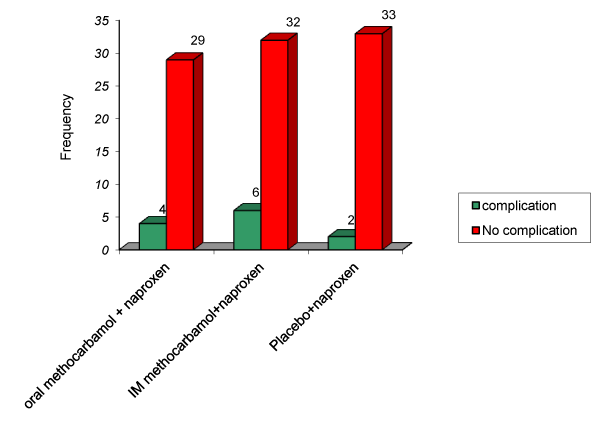
| There were complications in all 3 groups. The most complications were in the group which received IM methocarbamol and naproxen. The main complications were GI symptoms. There were 2 skin eruptions in the site of IM methocarbamol injection. There were no significant difference between complications in all 3 groups (P=0.392) (Figure 2) |
| Â |
|
|
Figure 2: Comparison of complications before and after intervention in 3 groups. |
|
| Â |
| Discussion |
| Â |
| The aim of the present study was to survey the effect of oral and intramuscular injection of methocarbamol and placebo on muscle strain pain. The pain severity was decreased in all 3 groups. The pain reduction was more seen in patients who received placebo. There was not significant improvement of pain in patients which received oral or IM methocarbamol. |
| Â |
| In Noonan study, initial treatment of muscle strain consisted of rest, ice, compression, and NSAIDs therapy. As pain and swelling subside, physical therapy should be initiated to restore flexibility and strength [9]. |
| Â |
| The number of scientific studies which have investigated the role of muscle relaxants in the care of patients with low back or neck pain, acute or chronic, are relatively small [10], and the number of studies which actually demonstrate efficacy is even smaller [11]. Only a limited number of high-quality, Randomized Controlled Trials (RCTs) provide evidence of the effectiveness of NSAIDs or skeletal muscle relaxants in the treatment of acute, uncomplicated musculoskeletal disorders [2]. There is strong evidence that any of muscle relaxants are more effective than placebo for patients with acute low back pain on short -term pain relief [6]. |
| Â |
| In a double blind parallel study of 180 patients taking either methocarbamol or placebo for acute musculoskeletal disorders caused by trauma or inflammation, methocarbamol was significantly more effective (80%) than placebo (45%) after 2 days [12]. Studies about muscle relaxants were conducted in past such as Tisdale SA in 1975 [13], but new studies must be conducted. Few controlled comparison studies using methocarbamol have been conducted. A comparative placebo controlled study of 227 patients, treated with methocarbamol and cyclobenzaprine demonstrated a slight advantage to methocarbamol and significantly more effective than placebo [14]. Another study comparing methocarbamol, chlorphenesin, carisoprodol, and placebo did not demonstrate to be more effective than the other two active medications [15]. |
| Â |
| These findings suggested that pain relief is due to effect of naproxen and lapse of time. In fact, methocarbamol was not effective on pain relief of muscle strain. |
| Â |
| The major complications were due to naproxen (GI symptoms). There were no drowsiness and dizziness due to methocarbamol usage. Only two cases had skin eruption on buttocks due to IM methocarbamol. |
| Â |
| Therefore, methocarbamol is a safe drug without any significant complication. We suggest further studies will be done with more cases and with other muscle relaxants. |
| Â |
| Acknowledgements |
| Â |
| We thank medical students of Yasouj University of Medical Sciences and patients for collaboration of this study. |
| Â |
| |
| References |
| Â |
- Randall LB (2007) Physical Medicine & Rehabilitation (3rdedn), W.B.Saunders Company, Elsevier, Philadelphia: 906-907.
- Beebe FA, Barkin RL, Barkin S (2005) A clinical and pharmacologic review of skeletal muscle relaxants for musculoskeletal conditions. Am J Ther 12: 151-171.
- Jarvinen M (2000) Muscle injuries. In: Renstrom PAFH (Ed), Clinical Practice of Sport Injury Prevention and Care. Blackwell London 12: 115-124.
- Jackson R (1982) Cervical trauma: not just another pain in the neck. Geriatrics 37: 123-126.
- Bertram GK, Basic and Clinical Pharmacology (10thedn), Singapore: Mc Graw Hill, 2008; 68: 576-582.
- van Tulder MW, Touray T, Furlan AD, Solway S, Bouter LM, et al. (2003) Muscle relaxants for nonspecific low back pain: a systematic review within the framework of the cochrane collaboration. Spine(Phila Pa 1976) 28: 1978-1992.
- www.drugbank.ca/drugs/DB00423
- Joel AD, Bockenek WL, Frontera WR, Gerber LH, Geiringer SR, et al. (2005) Physical Medicine & Rehabilitation: Principles and Practice. Lippincott William & Wilkins 1218.
- Noonan TJ, Garrett WE Jr (1999) Muscle strain injury: diagnosis and treatment. J Am Acad Orthop Surg 4: 262-269.
- Valtonen EJ (1975) A double-blind trial of methocarbamol versus placebo in painful muscle spasm. Curr Med Res Opin 3: 382-385.
- Deyo RA (1983) Conservative therapy for low back pain: Distinguishing useful from useless therapy. JAMA 250: 1057-1062.
- David TC, John RS, Joseph WT (2002) Practical Pain Management. Lippincott William &Wilkins 284-286.
- Tisdale SA Jr, Ervin DK (1975) A controlled study of methocarbamol (Robaxin) in acute painful musculoskeletal conditions. Curr Ther Res Clin Exp 17: 525-530.
- Preston ES, Miller CB, Herbestone RK (1984) A double-blind, multicenter trial of methocarbamol and cyclobenzaprine in acute musculoskeletal conditions. Ther Trends 1: 1-11.
- Stern TH (1964) A controlled comparison of three muscle relaxant agents. Clin Med (Northfield II) 71: 367-372.
|
| Â |
| Â |