| Research Article |
Open Access |
|
| M Masarat Dar*, Wani Idrees and FA Masoodi |
| Department of Food Science and Technology, University of Kashmir, Srinagar, Kashmir, India |
| *Corresponding author: |
M Masarat Dar
Department of Food Science and Technology
University of Kashmir
Srinagar, Kashmir, India
E-mail: mmasarat786@gmail.com |
|
| Â |
| Received August 04, 2012; Published December 27, 2012 |
| Â |
| Citation: Dar MM, Idrees W, Masoodi FA (2013) Detection of Sudan Dyes in Red Chilli Powder by Thin Layer Chromatography. 2:586 doi:10.4172/scientificreports.586 |
| Â |
| Copyright: © 2013 Dar MM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. |
| Â |
| Abstract |
| Â |
| Sudan I, II, III & IV are suspected genotoxic carcinogens and reports have shown the adulteration of chilli and chilli containing products with these azo dyes in recent past. In this study, a Simple Thin Layer Chromatographic method is described for the qualitative detection of Sudan-III & IV in Red chilli powder. The study includes extraction of sample, preparation of Thin Layer Chromatography (TLC) plates and Separation cum detection of dyes on TLC plates. The dyes were extracted with solvent Acetonitrile. Sample extracts were filtered and analyzed by simple thin layer chromatography in benzene, hexane and acetic acid solvent system. The chromatograms of sample, standard and spiked samples were evaluated in term of Rf and relative Rf values. The relative Rf values of four samples analysed, relative to the two standards of Sudan (III- S2 and IV S1) were less than one (P1-0.13,0.25; P2-0.1 9,0.35; P3-0.30,0.35; P4-0.15,0.26 and P5-0.20,0.38), confirming the absence of Sudan III and IV in these samples. The efficiency of the method was checked by internal standard method, in which pure sample was spiked by known weight of standard dye. The relative Rf values of spiked sample was almost equal to one (b1-0.93, b2-0.80). Limit of detection (5 mg/kg) was calculated on the basis of visual evaluation of spots (i.e. spot visible or not at given concentration of standard dye) under normal light without any derivetization. The chilli samples analysed though varied in color intensity, but none of them was found to contain Sudan III or IV. |
| Â |
| Keywords |
| Â |
| Thin Layer Chromatography; Chilli powder; Sudan I, II, III and IV |
| Â |
| Introduction |
| Â |
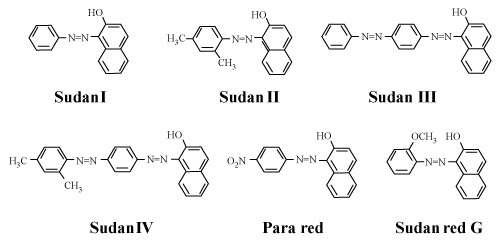
| The coloring matter in food may be natural or synthetic. They may also be classified as water soluble and oil soluble. Natural colors consist of chlorophyll, Carotenes, Curcumin, Caramel, Annatto, Capsainthin etc. Synthetic colors are of importance as they are widely used in different foods. Synthetic colors are further classified as acidic and basic dyes. Only eight coal-tar food colors are permitted in certain food products under the provision of Food Adulteration Act (1954). They include three Red shades namely, Carmoisine, Ponceau 4R, Erythrosine, two yellow shades Sunset yellow-FCF and Tartrazine, two blue shades Brilliant Blue FCF and Indigo Carmine and one green shade i.e. Fast green FCF. However certain unpermitted colors such as metanil yellow, Rhodamine B, Orange G, Blue VRS and certain oil soluble colors are often added in foods as adulterants. Sudan dyes (Sudan I, Sudan II, Sudan III and Sudan IV), Sudan red B, Sudan red 7B, Sudan red G, Sudan orange G, Sudan black, Di-methyl yellow, Para red are a familiar of compounds in the class of azo dyes that are used for different industrial and scientific applications. they are widely used in industrial applications and research. They have been used in paints and cosmetic products [1,2]. Scientific applications include staining of lipids with Sudan IV for visualization and analytical purpose. Sudan dyes are however illegally used as food adulterants, particularly in red chili powder and in chilli containing foods such as curry, frozen meats and spice mixes because of their intense red-orange color and low price. However Sudan I-IV dyes as food additives are not permitted worldwide due to their carcinogenicity. Even though Sudan dyes are banned the European Union Rapid Alert System for food and feed have reported at least 20 cases each year of Sudan I and IV (there were few cases of Sudan III in 2006) discovery in certain food products that are imported from outside EU. Reports were mainly from Germany, UK, Greece and Italy. Often Sudan I and IV were found simultaneously in different chilli and curry products (powders, sauces) as well as in seasonings and spice mixtures. These products originated mostly from turkey, India and Russian federations. A large part of Sudan findings are from palm oil. Since 2003, for reported Sudan findings by RASFF, around 20% cases are from palm oil. Palm oil is contaminated mostly with Sudan IV (less often with Sudan I) and originates from various African countries [2]. |
| Â |
| Sudan dyes are fat soluble azo dyes but solubility data of Sudan dyes is not abundant in literature. Sudan dyes are insoluble in water and soluble in various organic solvents (1.49 mol/dm3 in trichloromethane, 0.57 mol/dm3 in dichloromethane, 0.30 mol/dm3 in toluene, 0.17 mol/dm3 in benzene, 0.04 mol/dm3 in acetonitrile, 0.02 mol/dm3 in ethanol and 0.017 mol/dm3 in methanol. There is no solubility information for Sudan II. Sudan III is 0.015 and Sudan IV is 0.09 mol/dm3 soluble in ethanol. |
| Â |
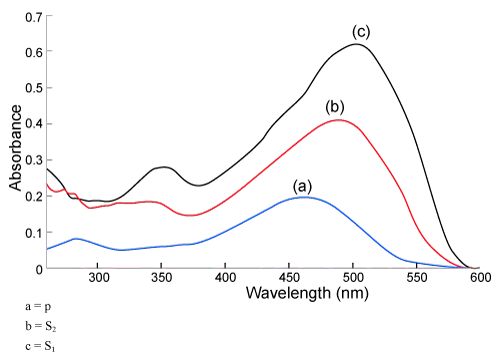
| The log-P value of Sudan I is 5.86 meaning that it is highly lipophilicity compound. No data is available for Sudan II-IV but given their molecular structures, their log-P values are expected to be similar or somewhat higher. UV-spectra of Sudan dyes I-IV differ from each other (Figure 3). The absorption maxima are at the following wavelengths; Sudan I 475 nm, Sudan II-489 nm, Sudan III-503 nm and Sudan IV-513 nm and 515 nm. |
| Â |
| Sudan dyes are weak acids, however the azo group in the I position that acts as a hydrogen bond acceptor leads to the formation of an intra-molecular hydrogen bond with the phenol-OH. This additional stabilization of the neutral Sudan molecule is responsible for rather high pKa value 11.65 of Sudan I and II [3]. The pka values of Sudan III and IV are expected to be similar. Sudan dyes thus can be considered to behave as neutral in most of the analytical methods. |
| Â |
| Sudan dyes are indirect carcinogens (classified as category 3 carcinogens by IARC) and are therefore banned from the use in foods in the EU [4,5]. They generate metabolites that are converted to several active mutagens and carcinogens in humans [6]. Sudan dyes are used in cosmetic products and animal testing has found that isomers of Sudan III cause allergic reaction [1]. |
| Â |
| Materials and Methods |
| Â |
| Reagents and materials |
| Â |
| Sudan III and Sudan IV were purchased from Merck. Acetonitrile, benzene, hexane, acetic acid and diethyl ether of HPLC grade was used. Silica gel TLC grade was used on plates of 10×10 cm. silica gel beaded HPLC grade was used for extraction of colors from the sample. |
| Â |
| Standard solutions |
| Â |
| Standard stock solutions were prepared by dissolving 5 mg/50ml of solvent (acetonitrile,) and stored at 10°C for one week. The working standard solutions were obtained from stock solutions by dilution (1:10) with the solvent. |
| Â |
| Preparation of chilli samples |
| Â |
| Four chilli samples were collected from the market including one pure control sample which was prepared by proper grinding of the whole pepper fruit to fine powder. 5 grams of sample was dissolved in 25 ml of the solvent acetonitrile. 10 grams of silica gel (beaded) and 2 grams of anhydrous sodium sulphate was added. The mixture was properly stirred for 5 min on a magnetic stirrer. The coloring components from the silica gel were recovered by 2-3 volumes of diethyl ether (15-20 ml). The ethereal extract was evaporated in a porcelain dish on hot water bath. In order to separate the colors from the interfering matter, the dried extract was re-dissolved in 1ml of diethyl ether and applied as band on a preparatory TLC plate (thickness 1.0mm) the plate was developed in a chamber containing hexane, allowed to air dry and colors were scrapped into a conical flask with addition of 10 ml of diethyl ether. The colored solution was filtered and stored in dark bottles of 5 ml capacity. |
| Â |
| Preparation of TLC plates |
| Â |
| TLC plates of 10×10 cm were prepared with the help of spreader, having provisions for varying the thickness of silica gel layer from 0.25 mm to 2 mm. Slurry of silica gel was prepared in distilled water and applied as a thin layer 0.25mm on TLC plates, allowed to air dry and then activated in oven for 1 hr at 110°C. |
| Â |
| Experiment |
| Â |
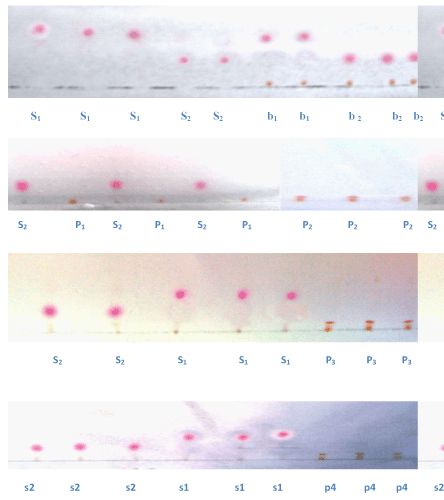
| Samples and standards were applied as spots in triplicate using either capillary or micro-pipette on a prepared TLC plates (10×10). Distance from the left and right edge of the plate was 10 mm and from the lower edge was 8 mm. The distance between the spots was kept at 8 mm. 9 spots were applied on a single plate. The scheme of the spot application is shown in table 1. Development of the plate was performed in saturated TLC jar with different solvent systems and the development time was 1½ hour. For visual evaluation the chromatograms were evaluated under normal light and the distances moved by solvent and the spots were measured by template scale (Figure 1). |
| Â |
|
|
Table 1: Table showing Rf and relative Rf value. |
|
| Â |
|
|
Figure 1: Chromatograms of Samples and Standards. |
|
| Â |
| Results and Discussions |
| Â |
| Sudan IV and III were selected for the study because of their color, health risk and relevance in reported cases of chilli contamination. Four chilli powder samples (P1, P2, P3, and P4) were taken randomly that include a control sample P1, which was prepared by proper grinding of whole chilli fruit to fine powder. In order to detect the target compounds (Sudan IV and III) in chilli powders, it was important to either selectively extract them from the powder matrix or separate them from naturally occurring colorants, such as carotenoids which are extracted simultaneously. Hexane, ethanol, acetone, acetonitrile and diethyl ether were evaluated as extraction solvents and finally samples were extracted in acetonitrile. Nevertheless when separated with selected chromatographic system (benzene; hexane; acetic acid 40:60:2), most chilli powder extracts showed considerable amount of colored matrix that could potentially interfere with the target compounds. In order to avert the interferences of the colored matrix, the samples (P1, P2, P3 and P4) were prepared on preparative TLC plates to separate different compounds. The chromatograms of sample, standard and spiked sample are shown in figure 1. The results were expressed in terms of Rf (retardation Factor) and Relative Rf values (Table 1). Mean of the Rf values were calculated from three triplicate spots of the given sample/ standard. The standard S1 and S2 showed the grand mean Rf values 0.29 and 0.16 respectively. The relative Rf values of four samples analyzed were less than 1 (Table 1) confirming absence of the suspected dyes S1 and S2 in all the samples. |
| Â |
| The chromatogram of the sample (P3, P4) however showed two band separation. The top-most band (0f P3) was separated through preparative TLC method, eluted and further analyzed under UVvisible spectroscopy in wavelength scan mode. The wave length scan of the given spot however did not match with any of the standard (S1 and S2) (Figure 2). |
| Â |
|
|
Figure 2: UV-visible spectroscopy in wavelength scan mode. |
|
| Â |
|
|
Figure 3: Structure of Sudan dyes. |
|
| Â |
| The performance of sample extraction and TLC separation was cross checked by internal standard procedure, in which pure sample (P1) was spiked by 5 mg/kg of standard dye (S1 and S2). The relative Rf values of both spiked samples b1 and b2 were almost equal to 1 (table 1). The limit of detection was based on visual evaluation of coloring spot. When standard dyes were spiked in control (pure chilli powder), the spots were visible up to 5 mg/kg. |
| |
| Â |
| Conclusion |
| Â |
| • The Rf values of coloring spots from all the samples selected did not matched with neither of standards (S1, S2). |
| Â |
| • The relative Rf values of all the sample color spot were less than one. Only one sample showed the relative Rf value of 0.5. |
| Â |
| • The Rf values of both spiked samples (b1, b2) were almost similar to their respective standards. |
| Â |
| • The relative Rf values of spiked samples were almost close to 1. |
| Â |
| • The limit of detection based on the visual evaluation of coloring spot was found to be 5mg/kg. |
| Â |
| It is thus concluded that none of the four samples taken contained the suspected Sudan III or IV. The variation of color intensity may be due to some other reason. |
| Â |
| |
| References |
| Â |
- Cornet V, Govaert Y, Moens G, Van Loco J, Degroodt JM (2006) Development of a fast analytical method for the determination of sudan dyes in chili- and curry-containing foodstuffs by high-performance liquid chromatography-photodiode array detection. J Agric Food Chem 54: 639-644.
- Anderton MS, Incarvito DC, Sherma J (1997) Determination of Natural and Synthetic Colors in Alcoholic And Non Alcoholic Beverages by Quantitative HPTLC. J Liq Chromatogr R T 20: 101-110.
- González M, Gallego M, Valcárcel M (2003) Determination of natural and synthetic colorants in prescreened dairy samples using liquid chromatography-diode array detection. Anal Chem 75: 685-693.
- Anonymous (2009) Spice Board of India annual report.
- Anonymous (2005) Opinion of the scientific panel on food Additives, Flavourings, Processing Aids and Materials in contact with food (AFC) to review the toxicology of a number of dyes illegally present in food in the EU. EFSA journal 264: 1-71.
- Ertas E, Ozer H, Alasalvar C (2007) A rapid HPLC method for determination of Sudan dyes and Para Red in red chilli pepper. Food Chem 105: 756-760.
|
| Â |
| Â |