
Page 112
conferenceseries
.com
Volume 11
Journal of Proteomics & Bioinformatics
ISSN: 0974-276X
Structural Biology 2018
September 24-26, 2018
September 24-26, 2018 | Berlin, Germany
14
th
International Conference on
Structural Biology
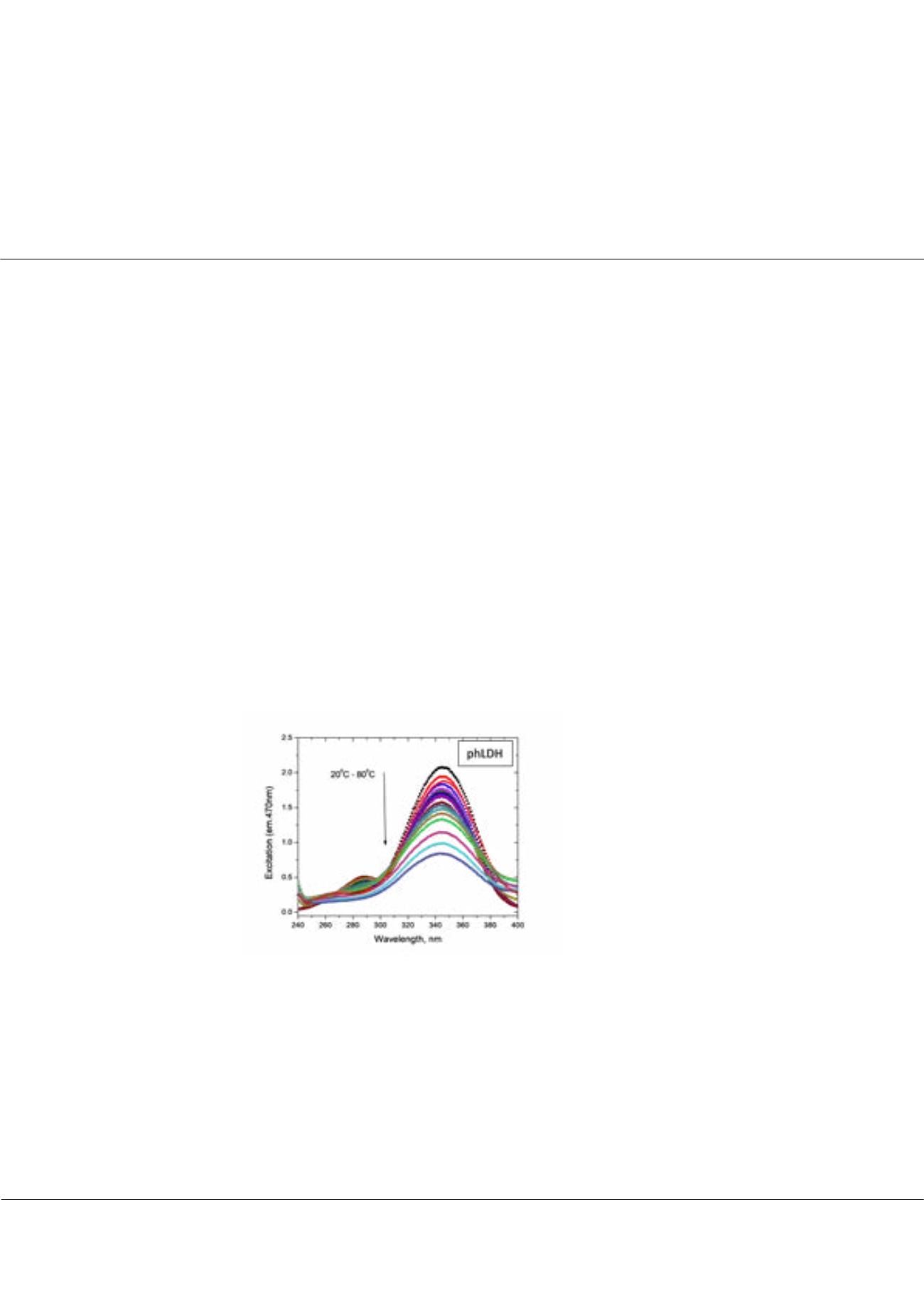
Thermo-regulated set of functional subpopulations of lactate dehydrogenases
Sergei Khrapunov, Eric Chang,
and
Robert H Callender
Albert Einstein College of Medicine, USA
T
he thermodynamics of the apoenzyme, holoenzyme (LDH-NADH) and ternary (LDH-NADH-oxamate) complex of
the glycolytic enzyme lactate dehydrogenase (LDH) from bsLDH (moderate thermophilic
Bacillus stearothermophilus
),
porcine heart, phLDH (mesophilic Sus scrofa), and from mackerel icefish, cgLDH (
psychrophilic Champsocephalus gunnari
)
have been investigated. A novel fluorescence assay was elaborated, which simultaneously monitors changes to the global
protein structure, structural changes near the active site, and aggregation of the enzyme in response to increasing temperature.
In our experiments the 2nd order of the monochromator grating was used to measure light scattering of the aggregated
protein solution (the setup of 240 nm/470 nm excitation/ emission monochromators). Thus, three properties, light scattering,
fluorescence resonance energy transfer (FRET), and NADH fluorescence could be measured simultaneously using respectively
excitation at 240 nm, 280 nm, 340 nm and a fixed emission at 470 nm. The reverse changes of stability and affinity for oxamate
were established for all orthologs. A reversible low-temperature (pre-denaturation) structural transition that precedes the
high-temperature (denaturation) transition was found for the Michaelis complexes. This transition was found to coincide
with a marked change in enzymatic activity for all LDHs. An observed lower substrate binding affinity for cgLDH compared
to phLDH was accompanied by a higher contribution of entropy to ∆G which reflects a higher functional plasticity of the
psychrophilic cgLDH compared to the mesophilic phLDH. The comparative study of the apoenzyme and holoenzyme has
shown that the basis for the pre-denaturation transition of the Michaelis complex is the flexibility of the global protein
structure. The hypothesis is expressed that the multiple active and inactive along with intermediate sub-state conformations
of the enzyme exist in equilibrium at the stage preceding irreversible thermal inactivation. This equilibrium is an essential
selective factor for the adaptation of an enzyme to the environmental temperature.
Recent Publications:
1. Khrapunov S, Chang E and Callender R H (2017) Thermodynamic and structural adaptation differences between the
mesophilic and psychrophilic lactate dehydrogenases. Biochemistry 56:3587-3595.
Biography
Sergei Khrapunov is a Research Professor of Biochemistry at Albert Einstein College of Medicine, USA. He completed his studies and graduated from National
Taras Shevchenko University, Kiev, Ukraine and received his PhD and Doctor of Science degree at O V Palladin Institute of Biochemistry, Kyiv, Ukraine. He
served as Professor and Chief in Department of General & Molecular Genetics at National Taras Shevchenko University, Kiev, Ukraine and then joined the
Biochemistry Department at Albert Einstein College of Medicine. His research focuses on structure and thermodynamics of protein-DNA complexes, structure and
thermodynamics of proteins, chromatin structure. His papers are published in the peer reviewed journals such as
Biochemistry, Biophysical Journal, Journal of
Molecular Biology
, PNAS,
Journal of Biological Chemistry
,
BBA
and others which can be viewed in PubMed archive of journal literature.
Sergei Khrapunov et al., J Proteomics Bioinform 2018, Volume 11
DOI: 10.4172/0974-276X-C2-116
Figure 1:
Temperature unfolding of Michaelis complex,
phLDH (40uM)-NADH (40uM)-Oxamate (0.5 mM).
Excitation, Emission 470nm, Temperature change from
20°C to 80°C.
















