
Page 107
conferenceseries
.com
Volume 11
Journal of Proteomics & Bioinformatics
ISSN: 0974-276X
Structural Biology 2018
September 24-26, 2018
September 24-26, 2018 | Berlin, Germany
14
th
International Conference on
Structural Biology
Heterologous production of α-rhamnosyl-β-glucosidases for crystallographic studies
Michael Kotik
1
, Gisela Weiz
2
, Kate Brodsky
1
, Laura S Mazzaferro
2
, Javier D Breccia
2
and
Vladimír Křen
1
1
Institute of Microbiology, Czech Academy of Sciences, Prague
2
Universidad Nacional de La Pampa, Argentina
α
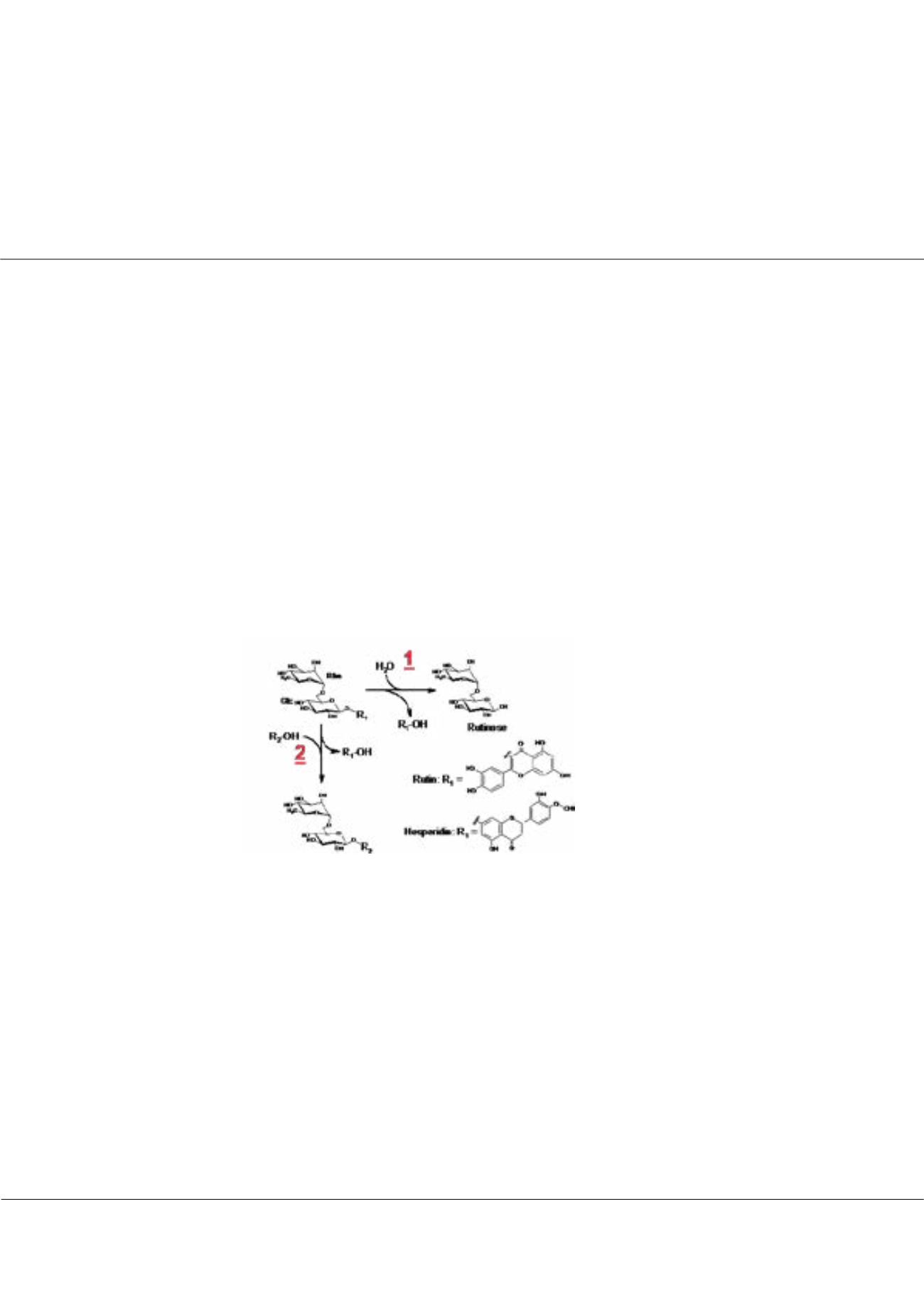
-Rhamnosyl-β-glucosidases are rather rare enzymes and occur in some bacteria, fungi and plants. They hydrolyze the
heterosidic linkage of diglycoconjugates such as rutin and hesperidin, generating rutinose (a disaccharide) and the
corresponding aglycon as products. Some enzymes exhibit transglycosylation activities, which provide access to various
novel diglycoconjugates. The objective of the first part of the project was to over express several fungal α-rhamnosyl-β-
glucosidases in a heterologous host and to characterize them. We report on the cloning of several α-rhamnosyl-β-glucosidase-
encoding genes from four fungal strains:
Aspergillus niger
, Mucor
circinelloides, Penicillium chrysogenum
and Acremonium
sp., we succeeded in heterologously expressing these enzymes in active form using
Pichia pastoris
as an expression host. The
enzymes were secreted to the cultivation medium, which greatly simplified the purification of the enzymes. It followed a basic
biochemical characterization of the purified enzymes, including determination of the pH optimum and optimal temperatures,
thermostabilities and substrate specificities and transglycosylation activities. Catalytically important active site residues
were assessed by site directed mutagenesis. The final goal of the project is an x-ray structure of at least one α-rhamnosyl-β-
glucosidase. Ideally the structure of the substrate bound to a catalytically inactive enzyme variant will be determined as well.
Recent Publications:
1. Šimčíková D, Kotik M, Weignerová L, Halada P, Pelantová H, Adamcová K and Křen V (2015) α-L-Rhamnosyl-β-D-
glucosidase (rutinosidase) from
Aspergillus niger
: characterization and synthetic potential of a novel diglycosidase.
Advanced Synthesis & Catalysis 357:107–117.
2. Neher B,MazzaferroLS, KotikM, Oyhenart J, Halada P, KřenVandBreccia JD(2016) Bacteria as source of diglycosidase
activity: Actinoplanes missouriensis produces 6-O-α-L-rhamnosyl-β-D-glucosidase active on flavonoids. Applied
Microbiology and Biotechnology 100:3061–3070.
3. Bassanini I, Krejzová J, Panzeri W, Monti D, Křen V and Riva S (2017) A sustainable one-pot two-enzymes synthesis
of naturally occurring arylalkyl glucosides. ChemSusChem 10:2040−2045.
Biography
Michael Kotik worked in academia and industry in the broad area of biotechnology during his entire career. His experience is in molecular biology, biochemical
characterization and directed evolution of enzymes. His research covered various enzymes, including epoxide hydrolases, haloalkane dehalogenases and
tyrosinases. His recent research activities involve among other things the heterologous expression and characterization of glycosidases and their use in
biotransformation reactions, including transglycosylations.
Michael Kotik et al., J Proteomics Bioinform 2018, Volume 11
DOI: 10.4172/0974-276X-C2-116
Figure 1:
Hydrolysis (1) and transglycosylation
reactions (2) catalyzed by retaining α-rhamnosyl-
β-glucosidases. R2: hydroxylic compound.
















