
Page 68
conferenceseries
.com
Volume 8, Issue 5 (Suppl)
J Chromatogr Sep Tech, an open access journal
ISSN: 2157-7064
Chromatography 2017
August 07-09, 2017
August 07-09, 2017 | Rome, Italy
4
th
World Congress on
Chromatography
UHPLC-MS/MS analysis of thyreostats in serum
Sebastian Witek, Barbara Wozniak, Iwona Matraszek-Zuchowska
and
Andrzej Posyniak
National Veterinary Research Institute, Poland
T
hyreostats are thioamide antithyroid drugs. Activity of these compounds consists in inhibiting the synthesis of thyroid
hormones triiodothyronine (T3) and thyroxine (T4), which favors the processes of animal fattening. Increase in weight
of animals is mainly due to the water retention in the tissues and the gastrointestinal tract. The consequence is not only the
production of lower meat quality, but also the risk of drug residues to human health. According to the International Agency
for Research on Cancer, some compounds of this group possess carcinogenic and teratogenic properties. In accordance with
the Council Directive 96/23/EC thyreostats belong to the group A2 - compounds with anabolic properties, which must be
controlled on slaughter animals. To extend the range of methods used in the national control plan, a fast and efficient UHPLC-
MS/MS method was developed and validated to detect five thyreostatic compounds: tapazole, thiouracil, methylthiouracil,
propylthiouracil and phenylthiouracil in bovine serum. Previously published method of thyreostats in urine was used as a
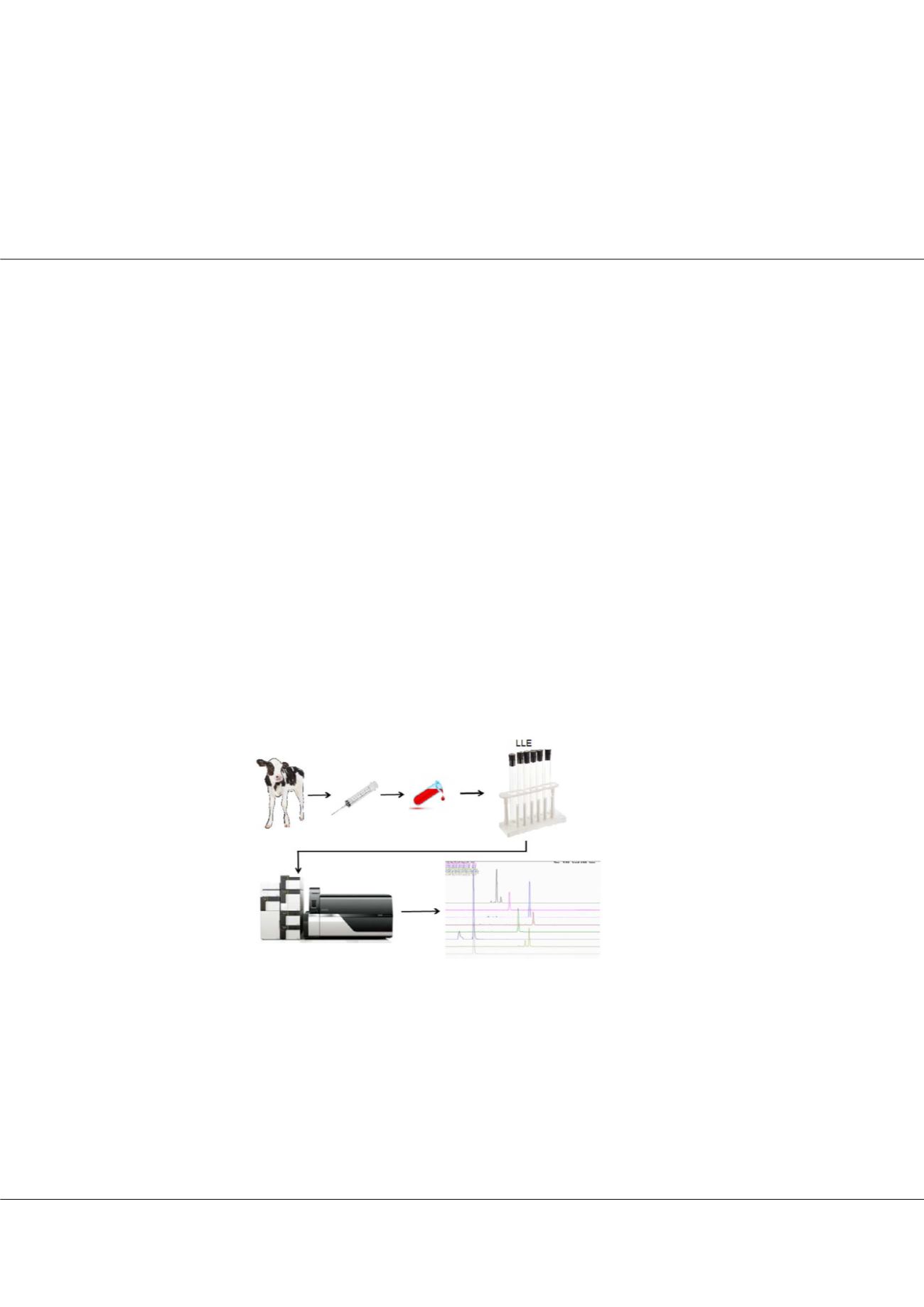
starting point for the development of our method. Thyreostats were extracted from serum samples with diethyl ether after
derivatization with 3-iodobenzylbromide in basic medium (pH 8.0) and analyzed by gradient elution within 7.5 min on a SB-
C18 column (50 x 2.1 mm; 1.8 μm, Agilent) using a mobile phase consisting of acetonitrile/0.1% acetic acid. The analysis was
performed on UHPLC Shimadzu NEXERA X2 with triple quadrupole MS 8050 instrument operating in positive electrospray
ionizationmode.The procedure was validated according to the CommissionDecision 2002/657/EC requirements.The recovery
and repeatability satisfy the performance criteria specified in this document for banned compounds. The recovery ranged from
97.5% to 113.5% for all examined compounds, and the repeatability did not exceed of 14.1%. The decision limits (CCα) did not
exceed 3.95 μg L
-1
, and also detection capabilities (CCβ) did not exceed 6.73 μg L
-1
. The developed procedure is sensitive and
robust, and therefore, useful for quantification and confirmation of thyreostats in residue control programme.
Fig.1:
Graphical scheme of determination thyreostats in serum.
Biography
Sebastian Witek works at National Veterinary Research Institute, Poland since 2009. He deals with residues of hormones and thyreostats in biological samples of
animal origin. He has a lot of experience in LC-MS/MS and GC-MS.
sebastian.witek@piwet.pulawy.plSebastian Witek et al., J Chromatogr Sep Tech 2017, 8:5(Suppl)
DOI: 10.4172/2157-7064-C1-032
















