
Page 105
conferenceseries
.com
Volume 11
Journal of Proteomics & Bioinformatics
ISSN: 0974-276X
Structural Biology 2018
September 24-26, 2018
September 24-26, 2018 | Berlin, Germany
14
th
International Conference on
Structural Biology
3D modeling of BmpA, BmpB, BmpC and BmpD from
Borrelia burgdorferi
Mia Åstrand
1
, Julia Cuellar
2
, Jukka Hytönen
2
and
Tiina A Salminen
1
1
Åbo Akademi University, Finland
2
University of Turku, Finland
B
. burgdorferi
is one of the main Borrelia species causing Lyme disease in humans. The pathogens are transmitted by the
Ixodes ticks and there are 60,000-200,000 Lyme disease infections in Europe annually. The BmpA, BmpB, BmpC and
BmpD proteins are expressed by
B. burgdorferi
in infected patients, but the exact role of the proteins is still unknown. The Bmp
proteins are reported to be homologous to T. pallidum PnrA (Purine nucleoside receptor A), which has been characterized as a
substrate binding lipoprotein of the ATP binding cassette (ABC) transporter family, preferentially binding purine nucleosides.
Based on our 3D homology models, the Bmp proteins share the typical fold of the substrate-binding protein family. Moreover,
the residues involved in binding the ribose moiety of the nucleoside are highly conserved in the Bmp models, whereas the
residues in the purine binding site are less conserved. In particular, the BmpC model has differences in the residues binding
the base moiety of the nucleoside. In conclusion, the revealed differences indicate that the Bmp proteins could prefer different
nucleosides and thus, might have distinct biological functions.
Recent Publications:
1. Sykes R A (2014) An estimate of Lyme borreliosis incidence inWestern Europe. Journal of Public Health DOI: 10.1093/
pubmed/fdw017.
2. Bryksin A V, Godfrey H P, Carbonaro C A, Wormser G P, Aguero-Rosenfeld M E and Cabello F C (2005)
Borrelia
burgdorferi
BmpA, BmpB, and BmpD proteins are expressed in human infection and contribute to P39 immunoblot
reactivity in patients with Lyme disease. Clinical and Diagnostic Laboratory Immunology 12:935–40.
3. Ramamoorthy R, Povinelli L and Philipp M T (1996) Molecular characterization, genomic arrangement, and
expression of bmpD, a new member of the bmp class of genes encoding membrane proteins of
Borrelia burgdorferi
.
Infection and Immunity 64:1259–1264.
Mia Åstrand et al., J Proteomics Bioinform 2018, Volume 11
DOI: 10.4172/0974-276X-C2-116
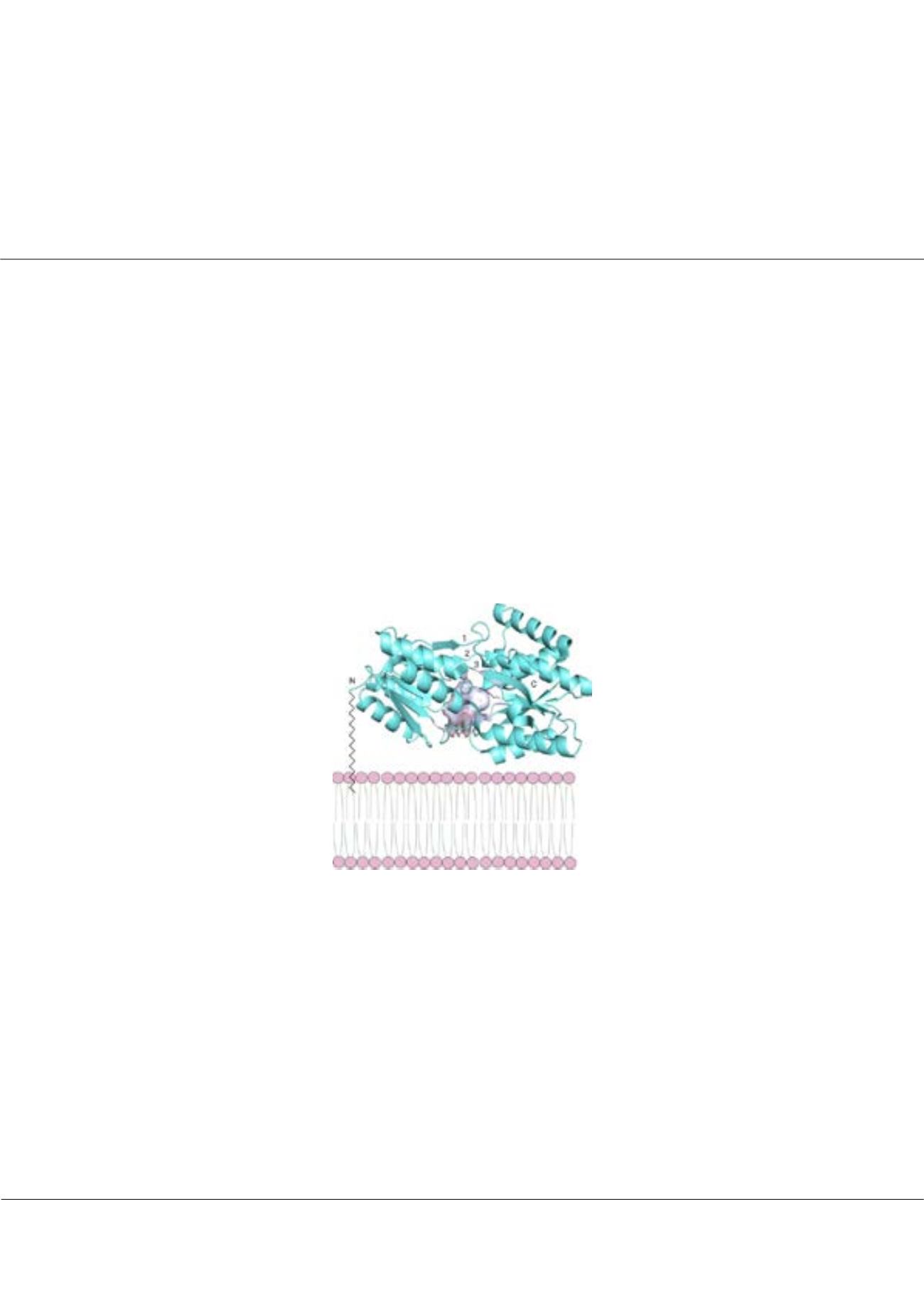
Figure 1:
A typical structure of an ABC-type substrate- binding lipoprotein. The
protein is attached to the membrane by a lipid anchor. The ligand-binding site is found
between the two domains (shown as gray surface).
















