
Notes:
Page 43
Chromatography 2016
September 21-23, 2016
Volume 7, Issue 5(Suppl)
J Chromatogr Sep Tech 2016
ISSN: 2157-7064 JCGST, an open access journal
conferenceseries
.com
September 21-23, 2016 Amsterdam, Netherlands
World Congress on
Chromatography
N-acylatedchitosanBis(arylcarbamate):Aclassof chiral separationmaterialswithpowerful enantioseparation
ability and high tolerability to organic solvents
Sheng Tang, Jiande Liu, Qin Bin, Keqin Fu, Xiaochen Wang, Yingbin Luo and Zhengwu Bai
Wuhan Institute of Technology, China
D
ue to the fact that cellulose and amylose derivatives tend to dissolve in some organic solvents, the related polysaccharide
derivatives-based CSPs prepared by coating manner can only work in a limited range of mobile phases, thus, restricting
their widespread application in enantioseparation. Chitin, as one of the most abundant optically active biopolymers, is similar
to cellulose in primary structure. Much lower solubility of chitosan derivatives was observed compared with the derivatives
of cellulose and amylose, enabling the corresponding chitosan derived CSPs to be possibly worked in a wider range of mobile
phases. Herein, in order to develop new types of chitosan derivatives-based CSPs, which not only are capable of excellent
enantioseparation performance, but also bear a desirable tolerability, we introduce a class of coated-type CSPs, which are based
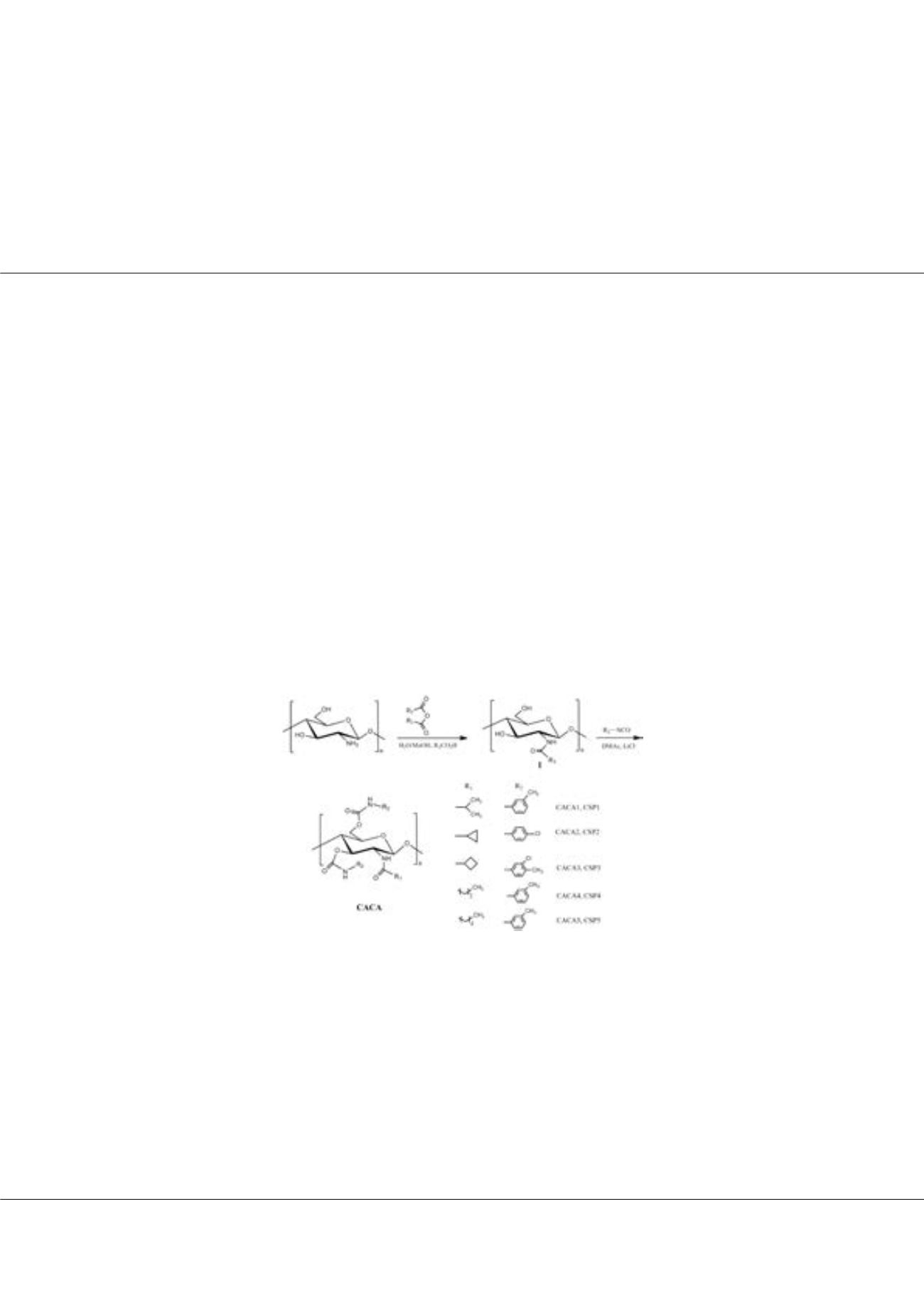
on chitosan bis(arylcarbamate)-(amide) (CACA). The N-acylated chitosan derivatives were synthesized by the reaction of
chitosan with carboxylic acid anhydrides in water/methanol in the presence of the corresponding carboxylic acids. CACAs were
prepared by further derivatizing N-acylated chitosan with aryl isocyanates. Fig. 1 shows the structures of the prepared CACAs,
which were then coated onto 3-aminopropyl silica gel, affording the corresponding CACAs-based CSPs. When the substituents
introduced on acyl group at 2-position and on aryl group of phenylcarbamates at 3- and 6-positions were perfectly coordinated,
the prepared N-acylated chitosan bis(arylcarbamate) would possess powerful chiral recognition and enatioseparation abilities,
and meanwhile exhibited a desirable tolerability towards a wider range of mobile phases, consequently resulting in a new class
of promising chiral separation materials.
The structures of the prepared CACAs.
Biography
Sheng Tang has completed his PhD in Analytical Chemistry from Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences. He serves as a teaching
and research fellow at Wuhan Institute of Technology. He has published more than 10 papers in reputed journals.
stang@wit.edu.cnSheng Tang et al., J Chromatogr Sep Tech 2016, 7:5(Suppl)
http://dx.doi.org/10.4172/2157-7064.C1.016















