Review Article Open Access
Why Not Introducing the Third Dimension in Photodynamic Therapy Research?
Alemany-Ribes M1, García-Díaz M1, Acedo P3, Agut M1, Nonell S1, Sagristá ML2, Mora M2, Cañete M3, Villanueva A3, Stockert JC3 and Semino CE1*
1IQS School of Engineering, Ramon Llull University, Barcelona, Spain
2Department of Biochemistry and Molecular Biology, Faculty of Chemistry, University of Barcelona, Barcelona, Spain
3Department of Biology, Faculty of Sciences, Autonomous University of Madrid, Madrid, Spain
- *Corresponding Author:
- Carlos E Semino
IQS School of Engineering
Ramon Llull University
Via Augusta 390, 08017 Barcelona, Spain
E-mail: carlos.semino@iqs.url.edu
Received date: May 03, 2013; Accepted date: June 17, 2013; Published date: June 19, 2013
Citation: Alemany-Ribes M, García-Díaz M, Acedo P, Agut M, Nonell S, et al. (2013) Why Not Introducing the Third Dimension in Photodynamic Therapy Research? J Anal Bioanal Tech S1:004. doi: 10.4172/2155-9872.S1-004
Copyright: © 2013 Alemany-Ribes M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which ermits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
Photodynamic therapy (PDT) is a clinically approved procedure for the treatment of diseases characterized by uncontrolled cell proliferation, particularly cancer. It involves the administration of a photosensitizer (PS) that is able to produce reactive oxygen species (ROS) upon irradiation with light, leading to the selective killing of neoplastic cells. A major challenge in PDT is the development of new PSs and drug-delivery systems that improve therapy efficacy and selectivity. To succeed in drug screening, it is crucial to use cellular systems that precisely reproduce the phenotype of the target tissue in order to obtain reliable biomedical data that correlate with in vivo tests. In this way, three-dimensional (3D) cultures are particularly attractive since they integrate chemical and mechanical signals that arise from extracellular matrix (ECM) and adjacent cells. Importantly, 3D models can mimic in vivo gene expression pattern and molecular gradients. These features significantly affect the outcome of PDT, enhancing the predictive power of 3D models. Therefore, PDT research should rely on the exploitation of this third dimension, guaranteeing a custom-tailor design depending on the tissue to be modeled, an easy applicability and reproducibility. The review summarizes progress in this emerging area.
Keywords
Cancer; Photodynamic therapy
Taking A Glance at Photodynamic Therapy
Photodynamic therapy (PDT) is a clinically approved procedure for the treatment of neoplastic and other non-malignant diseases generally characterized by an abnormal cell growth [1-3]. It involves the interaction of three independent and individually non-toxic factors: a photoactive dye called photosensitizer (PS), visible light and molecular oxygen.
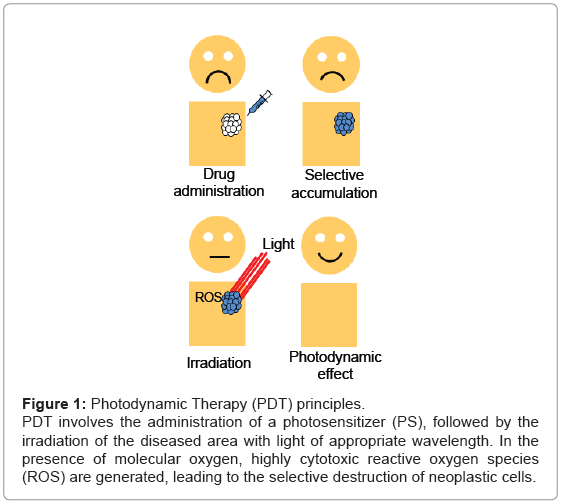
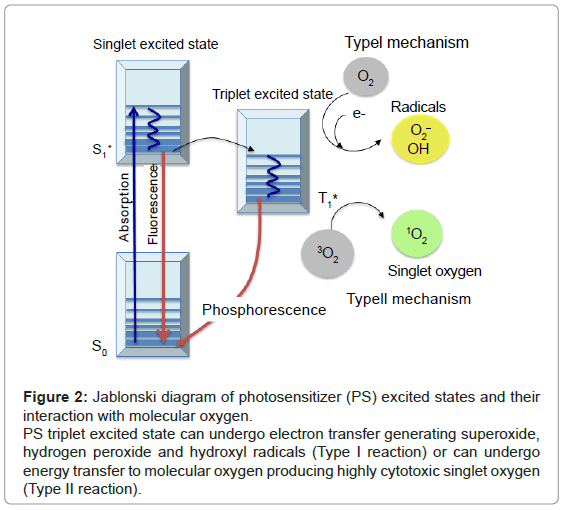
The principle of PDT relies on the administration of the PS, followed by the irradiation of the diseased area with light of appropriate wavelength. In the presence of molecular oxygen, highly cytotoxic reactive oxygen species (ROS) are generated, leading to the selective destruction of neoplastic cells (Figure 1). Specifically, once the PS has absorbed a photon, an electron is promoted from the ground state (S0) to an electronically excited state (S1). This short-lived specie can release its energy by emitting light (fluorescence), by internal conversion to heat or undergoing intersystem crossing to form a more stable triplet state (T1). The relatively long lifetime of the triplet state allows the interaction of the excited PS with the surrounding molecules, performing two classes of reactions, defined as Type I and Type II mechanisms. In the Type I pathway, the PS reacts directly with a substrate, transferring an hydrogen atom or an electron to form radical species. These free radicals further react with molecular oxygen, leading to ROS such as superoxide, peroxide or hydroxyl radicals. Alternatively, the Type II pathway is initiated by the energy transfer between the triplet excited state of the PS and nearby oxygen molecules, generating the singlet excited state of oxygen, referred as singlet oxygen, 1O2 (Figure 2). Both mechanisms can produce the photo-oxidation of certain amino acids residues in proteins, pyrimidine and purine bases of DNA/ RNA and unsaturated lipids. This process causes DNA damage and/or cytoplasmic membrane damage, allowing leakage of cellular contents or inactivation of membrane transport systems and, thus, triggering final cell death [4]. While it is generally accepted that 1O2 plays a key role in primary photodynamic effects, ROS generated by Type I mechanism become more important at low oxygen concentrations [5].
Figure 1: Photodynamic Therapy (PDT) principles.
PDT involves the administration of a photosensitizer (PS), followed by the
irradiation of the diseased area with light of appropriate wavelength. In the
presence of molecular oxygen, highly cytotoxic reactive oxygen species
(ROS) are generated, leading to the selective destruction of neoplastic cells.
Figure 2: Jablonski diagram of photosensitizer (PS) excited states and their interaction with molecular oxygen.
PS triplet excited state can undergo electron transfer generating superoxide, hydrogen peroxide and hydroxyl radicals (Type I reaction) or can undergo energy transfer to molecular oxygen producing highly cytotoxic singlet oxygen (Type II reaction).
PDT induces tumor regression by three mechanisms: direct killing of tumor cells, damage of tumor vasculature and triggering of an antitumor immune response [6,7]. The main advantage of PDT compared to other antitumoral therapies, such as chemotherapy or radiotherapy, is its dual selectivity. In particular, the PS is preferentially accumulated in the tumor tissue and its activation can be confined by restricting the illumination to a specific area, reducing undesired side effects. Paradoxically, this selectivity turns into an intrinsic limitation when having metastatic lesions, due to the impossibility of irradiating the whole body with appropriate doses [1].
Nowadays, there are few PS agents that have been approved for PDT treatments. Porfimer sodium, temoporfin, 5-aminolevulinic acid and verteporfin are the most commonly used [1]. However, none of them fulfills all the demands required for standardized applications in oncology. They present low selectivity between tumor and healthy tissue and a low therapy efficacy. Furthermore, porfimer sodium and temoporfin have been demonstrated to induce a pronounced and lengthy skin photosensitivity [1,8]. Based on these considerations, a concerted effort is being made to develop new PSs, with the overall aim of improving therapeutic outcomes for patients [9,10]. However, the drug development process is proved to be extremely inefficient. It is estimated that only 8% of all drug candidates that enter Phase I trials reach the bedside [11]. This high failure rate can be related with the continued use of cellular systems that altered or missed many tissue-related functions, impairing their predictive power. Therefore, a challenge in drug assays, including the ones for PDT, is set on developing the appropriate cellular models that precisely reproduce the phenotype of the target tissue in order to obtain reliable biomedical data that correlate with in vivo tests.
3D Models: Capturing Tissue Physiology In Vitro
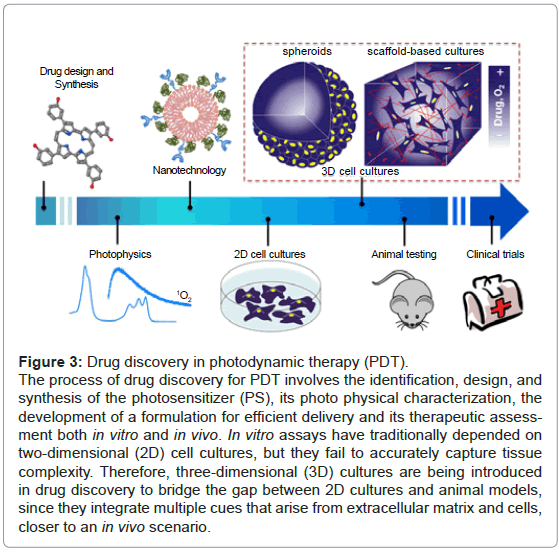
Pre-clinical assays have traditionally depended on two-dimensional (2D) cellular systems and animal models [12]. 2D cultures have provided valuable information on understanding cellular responses after early biological-activity assessment. However, cells in 2D grow in physiologically constrained conditions caused by being attached to rigid and flat substrates, which increases their surface exposed to cultured media and reduces cell-cell and cell-extracellular matrix (ECM) interactions [13]. At the opposite end of experimental platforms, animal models can display the integrated responses that result from complex interactions between tissues and organs. Nonetheless, they fail to capture important facets of human responses, are very costly, time-consuming and ethically controversial [12,14]. Therefore, there is a high demand for models that precisely recreate the complexity of human tissues while retaining the ability for high-throughput screening and cellular level imaging. As a result, three-dimensional (3D) cellular systems, which basically rely in the capacity of culturing cells in a 3D microenvironment, are currently under development [11,14] (Figure 3).
Figure 3: Drug discovery in photodynamic therapy (PDT).
The process of drug discovery for PDT involves the identification, design, and synthesis of the photosensitizer (PS), its photo physical characterization, the development of a formulation for efficient delivery and its therapeutic assessment both in vitro and in vivo. In vitro assays have traditionally depended on two-dimensional (2D) cell cultures, but they fail to accurately capture tissue complexity. Therefore, three-dimensional (3D) cultures are being introduced in drug discovery to bridge the gap between 2D cultures and animal models, since they integrate multiple cues that arise from extracellular matrix and cells, closer to an in vivo scenario.
Cells in the body grow within an organized 3D ECM, surrounded by other cells. Indeed, the interactions between cell-cell and cell- ECM can determine whether a given cell undergoes proliferation, differentiation, apoptosis or invasion [11,13-15]. 3D models provide this third dimension, essential to integrate mechanical and chemical signals, mimicking the in vivo microenvironment. In particular, 3D models present two key advantages in PDT research, with a focus on screening for novel antitumoral drugs [16] and delivery systems [17,18], since they have the capacity to (i) modulate the molecular gradients that exist in tissues for any soluble component, such as oxygen and drugs [14,19] and (ii) recreate the in vivo cellular morphology and physiology, including resistance to therapy [12,20]. Furthermore, ECM components offer additional targets to enhance drug internalization and tumor penetration [18] (Table 1).
| 2D cell cultures | 3D cell cultures | Animals | |
|---|---|---|---|
| Tissue Architecture | Cells are unnaturally polarized and mainly exposed to culture medium and rigid substrates | Cells interact with ECM and adjacent cells in 3D. They secrete and remodel ECM | Integrated response from interactions among all tissues and organs |
| Gene expression pattern | Physiological tumor properties are constricted in 2D and, thus, expression patterns | Key expression pattern in tumor biology is recreated (apoptosis resistance, etc.) | Human responses are not accurately captured (drug toxicity, metabolization, etc.) |
| Molecular gradients | Cells are submitted to uniformly rich oxygenation and drug accessibility | Oxygen and drug delivery is controlled by simple diffusion | Vascularization regulates oxygen and drug delivery, together with drug clearance |
| Drug discovery applicability | Inexpensive and simple to apply for high-throughput screening | More realistic drug response than 2D. Easier to apply than animal models | Expensive, time-consuming, poor cell-level imaging and ethically controversial |
Table 1: Comparison of cell morphology and physiology in two-dimensional (2D) cultures, three-dimensional (3D) cultures and animals.
Tissues experience mass transfer phenomena for any soluble agent. This situation is worsened in tumors, since they are characterized by an abundant and dense ECM microenvironment, together with a primitive vascular network. First of all, this complex tumor microenvironment is induced by an elevated deposition and remodeling of ECM components, such as fibrillar collagen and hyaluronic acid, by cancer cells and fibroblasts of the stroma [21,22]. Secondly, tumors present a poorly organized vascular architecture, due to the imbalance between pro- and anti- angiogenic factors and the stress generated by the uncontrolled proliferation rate that forces vessels apart [19,23,24]. The concomitance of these two phenomena causes an inefficient delivery of drugs and oxygen (hypoxia), significantly affecting therapy efficacy. Additionally, hypoxia is aggravated by any oxygen-consuming photoreaction which rapidly lowers even more the local partial oxygen pressure [25,26]. Experimentally, conventional 2D cultures are characterized by uniformly rich oxygenation and nutrition. Instead, 3D cultures can capture these mass transfer limitations and, therefore, better predict the outcome of oxygen- and drug-dependent therapies.
The morphology and physiology of cancer cells depend strongly on tumor microenvironment. In particular, their aggressiveness is enhanced by the mechanical and chemical signals that arise from both the ECM and neighboring cells residing within a 3D architecture pattern. For instance, the communication between quiescent and hypoxic cells located at the internal core of the tumor with highly proliferative cells at the surface promotes the transmission of growth factors, which are involved in key aspects of tumor biology such as cell survival, resistance to apoptosis and metabolic reprogramming. Some of these factors are vascular endothelial growth factor (VEGF), platelet derived growth factor (PDGF), placenta growth factor (PlGF) and transforming growth factor α (TGFα), among others [23,24,26-29]. Furthermore, hypoxic cells deal with a hostile metabolic environment that provides a selective pressure to favor the survival of the more malignant, aggressive and genetically unstable cancer cell types [23]. As a result, these growth conditions significantly affect RNA and protein expression profiles and, thus, cellular response to therapies, including the acquisition to drug resistance. Experimentally, 2D cultures depict a reductionist model based on a simple collection of relatively homogeneous cancer cells [28]. Instead, 3D cultures reestablish morphological and physiological properties of the tumor [11], mimicking multicellular resistance to apoptosis-inducing drugs [23,30,31] and, therefore, improving their predictive power for drug design and screening.
3D Models In PDT: Bringing PDT to the Next Dimension
The currently available 3D cell systems include tissue explants, cellular spheroids, scaffold based cultures, whole perfused organs and hollow-fiber bioreactors [11,32]. In drug discovery field, the most commonly used platforms are cellular spheroids and scaffold-based cultures. They provide tissue-specific information at different and complementary levels of complexity.
Up to date, the most popular 3D models for pre-clinical research consist in cellular spheroids. They are compact cell clusters based on the natural tendency of many cell types to aggregate. As a consequence, they contain both surface exposed and deeply buried cells, establishing zones of proliferating cells on the outside and quiescent cells on the inside due to nutrient and oxygen transport limitations. Therefore, these systems capture many aspects of the pathophysiology milieu in human tumor tissues [30,33,34]. Sutherland et al. [35,36] were pioneers in using spheroids in cancer research. Importantly, they characterized spheroids morphology and physiology through growth rate and oxygenation studies. In PDT field, the group of De Witte [37-40] used spheroids to mimic bladder carcinoma and evaluate the PDT outcome, comparing the results with classical monolayer cultures. Cells were grown until aggregates reach diameters between 450 and 500 μm. These dimensions could mimic mass transport limitations occurring in vivo, since simple diffusion typically allows for only 150-250 μm of oxygen and drug penetration [14,25]. In 2D cell systems PSs were readily taken; whereas 3D cell systems showed a heterogeneous distribution with high concentrations of the PSs at the periphery of the structure that decreased rapidly to a steady-state situation in the inner spheroidal regions. Furthermore, spheroids showed a dramatically low phototoxicity compared to 2D. This effect was due to poor drug penetration and oxygen depletion, which created a protective microenvironment for cells [37]. The authors went a step further using spheroids to study the cellular mechanisms underlying PDT. In particular, they studied the preferential localization of PSs in tumor cells, since it was only observed in vivo. In 2D monolayer conditions the cellular uptake by tumor and normal cells was similar. They demonstrated that the accumulation of PSs in cells inversely correlated with their intercellular adhesion proteins (E-cadherin) expression. Hence, while the first layers of cells in spheroids took up PSs directly, in deeper layers passive paracellular transport was activated and modulated by adhesion proteins, since these proteins configured the histoarchitecture pattern [38-40]. Therefore, spheroids have led to major conceptual advances in understanding discrepancies in PDT mechanisms and efficacy between conventional monolayer cultures and in vivo experiments. However, they are limited by slow spontaneous aggregation and uncontrolled final size and shape, which importantly difficult a precise control of oxygen and compounds gradients [34,41].
Scaffold-based cultures are considered a good alternative to spheroids models. Scaffolds are inspired on extracellular matrix in order to give cells the appropriate chemical, physical and mechanical cues and, therefore, mimic the in vivo microenvironment. They offer two important advantages compared to cellular spheroids. First of all, scaffolds can be designed depending on the tumor-tissue being modeled [28]. Secondly, scaffold architecture and dimensions can be precisely defined, controlling the spatial distribution of drug and oxygen [14]. On the basis of their origin, they can be classified as natural or synthetic materials. Natural scaffolds are extracted from animals, the most applied for cell culture are collagen and Matrigel™. Collagen is one of the main protein in ECM; whereas, Matrigel™ consists in Engelbreth- Holm-Swarm (EHS) mouse tumor cell-derived basement membrane proteins [42,43]. In pioneering studies, Bissell and collaborators [44,45] established a 3D breast cancer model in which normal and malignant breast epithelial cells were cultured on Matrigel™. These authors observed that malignant cells maintained their invasive phenotype and formed disorganized colonies, whereas normal cells differentiated into polarized hollow spherical monolayers (acini) that resembled the original breast tissue. Therefore, they provided basic tumor biology insights into the substantial role of the extracellular context, information that could not have been gained from monolayer cultures. Within PDT research, the group of Hasan [25,46,47] developed a 3D adherent ovarian cancer (OvCa) model to mimic the avascular metastatic tumors that coat surfaces of the peritoneal cavity. In brief, OvCa cells were cultured on the surface of Matrigel™ and grown until achieving a population of nodules larger than 200 μm in diameter, relevant dimensions for being subjected to mass transport limitations. They used this model as a powerful platform to screen drug candidates capable of breaking the protective microenvironment created by hypoxia. For this purpose, they developed two different strategies. Firstly, they proposed the use of PSs that could impart cytotoxicity across Type I and II phototoxicity mechanisms and, thus, were able to treat hypoxic regions through Type I mechanism [25]. On the other hand, they also demonstrated the synergic effect of combining PDT with chemotherapy drugs to target these hypoxic-resistant cells [47]. Furthermore, these authors developed co-culture models of tumor and stromal cells, which could shed more light on stroma contribution to disease progression and treatment response. For instance, they cultured OvCa cells with endothelial cells or normal fibroblasts micropatterned on Matrigel™ and characterized these 3D systems [46,48]. The advantages of natural scaffolds are their biocompatibility, easy accessibility and capacity to provide all the spectrum of chemical and physical cues that are needed to induce morphogenesis and function from many cells. However, the diversity of these cues could be a disadvantage when trying to isolate the effects of specific factors. Moreover, the same ECM component can be very variable in its composition and mechanical properties depending on its origin and specific batch, reducing the reliability and reproducibility of the particular assay [11,14,42].
To avoid these drawbacks, fully synthetic scaffolds are under continuous development. They can be custom tailored to mimic specific ECM properties, providing reproducible cellular environments [14]. Paradoxically, this advantage makes this class of biomaterials far more challenging because a wide range of factors have to be identified and precisely recreated, including porosity, stiffness, cell-adhesion sites and growth factors binding. At the moment, the group of Semino [49] worked with a 3D model based on the self-assembling peptide hydrogel RAD16-I (commercially available as BD™ PuraMatrix™). It is composed by a sequence of 16 amino acid chain AcN-(RADA)4-CONH2 (R arginine, A alanine and D aspartic acid). This hydrogel provides a non-instructive and defined microenvironment to cells, which was previously used to promote growth and proliferation of multiple cell types, including chondrocytes, hepatocytes and osteoblasts, as well as embryonic and somatic stem cells [50-53]. Hence, it enables the analysis of the intrinsic effects of the 3D architecture and molecular gradients in PDT outcome compared to 2D models, avoiding the influence of biochemical signals. Results revealed that PDT efficacy was dramatically lower in 3D than in 2D systems. Dynamic mass transfer effects accounted for this difference, since the intrinsic mechanism of action of the therapy was maintained in both systems. In fact, total death was only observed using 20-fold higher PS concentration or when a continuous flow of oxygen was maintained during PDT treatments, despite the demonstrated presence of free-drug and/or hypoxic cells that could not undergo PDT. This apparent paradox was explained by a death-signaling cascade that could be triggered to break the protective microenvironment created by oxygen and drug limitation, inducing neighboring cell death. Importantly, the production and decay of the cytotoxic species 1O2 could be observed for the first time in a 3D culture [49]. Next action should require the incorporation of the specific cell adhesion sites and growth factor binding that characterized the tumor to be modeled.
Future Perspectives: Optimizing 3D Models
A further step towards more realistic and predictive models could be the development of co-cultures models. It is largely demonstrated that the crosstalk between cancer and stroma cells contributes to the invasive growth and metastasis of the tumor [28]. Basically, stroma cells are responsible for the synthesis, deposition and modeling of much of the ECM. Moreover, they are a source of paracrine growth factors that induce cancer cell growth [33,54]. Therefore, these cells act as active participants in tumorogenesis rather than passive bystanders. For this reason, co-cultures models could be incorporated in PDT drug screening processes, providing a better predictive outcome for therapies [46,48,55].
Another degree of complexity that can be incorporated in 3D models is vascularization. For this purpose, different strategies have been developed, including the use of perfusion bioreactors or the coculture with endothelial cells that are stimulated with the delivery of angiogenic factors as VEGF [56]. Up today, 3D models have been designed to partially recreate mass transport limitations that characterized tumor physiology (oxygenation and nutrition) by simple diffusion. For this reason, spheroids and scaffolds are always larger than 150-250 μm, which is the limit of simple diffusion [14,25].
Conclusions
3D models have the potential to become a fundamental research tool in PDT drug screening assays. Certainly, a big challenge in biophotonics will be the optimization and exploitation of this third dimension. These models are attractive platforms to (i) better predict the outcome of PDT; (ii) carefully study the molecular mechanisms underlying PDT and (iii) develop new drugs candidates capable of breaking the protective microenvironment triggered by drug and oxygen mass transport limitations. To fulfill all these needs, 3D models should have some essential properties. Basically, they should be customtailor depending on the tumor to be modeled and easy to apply and reproducible to enable high-throughput screening applications.
References
- Agostinis P, Berg K, Cengel KA, Foster TH, Girotti AW, et al. (2011) Photodynamic therapy of cancer: an update. CA Cancer J Clin 61: 250-281.
- Anand S, Ortel BJ, Pereira SP, Hasan T, Maytin EV (2012) Biomodulatory approaches to photodynamic therapy for solid tumors. Cancer Lett 326: 8-16.
- Sharman WM, Allen CM, van Lier JE (1999) Photodynamic therapeutics: basic principles and clinical applications. Drug Discov Today 4: 507-517.
- Castano AP, Demidova TN, Hamblin MR (2005) Mechanisms in photodynamic therapy: part two-cellular signaling, cell metabolism and modes of cell death. Photodiagn Photodyn Ther 2: 1-23.
- Ochsner M (1997) Photophysical and photobiological processes in the photodynamic therapy of tumours. J Photochem Photobiol B 39: 1-18.
- Garg AD, Nowis D, Golab J, Agostinis P (2010) Photodynamic therapy: illuminating the road from cell death towards anti-tumour immunity. Apoptosis 15: 1050-1071.
- Galluzzi L, Kepp O, Kroemer G (2012) Enlightening the impact of immunogenic cell death in photodynamic cancer therapy. EMBO J 31: 1055-1057.
- Brown SB, Brown EA, Walker I (2004) The present and future role of photodynamic therapy in cancer treatment. Lancet Oncol 5: 497-508.
- Stockert JC, Cañete M, Juarranz A, Villanueva A, Horobin RW, et al. (2007) Porphycenes: facts and prospects in photodynamic therapy of cancer. Curr Med Chem 14: 997-1026.
- Tejedor-Estrada R, Nonell S, Teixido J, Sagrista ML, Mora M, et al. (2012) An artificial neural network model for predicting the subcellular localization of photosensitisers for photodynamic therapy of solid tumours. Curr Med Chem 19: 2472-2482.
- Pampaloni F, Reynaud EG, Stelzer EH (2007) The third dimension bridges the gap between cell culture and live tissue. Nat Rev Mol Cell Biol 8: 839-845.
- Yamada KM, Cukierman E (2007) Modeling tissue morphogenesis and cancer in 3D. Cell 130: 601-610.
- Dutta RC, Dutta AK (2009) Cell-interactive 3D-scaffold; advances and applications. Biotechnol Adv 27: 334-339.
- Griffith LG, Swartz MA (2006) Capturing complex 3D tissue physiology in vitro. Nat Rev Mol Cell Biol 7: 211-224.
- Smalley KS, Lioni M, Herlyn M (2006) Life isn't flat: taking cancer biology to the next dimension. In Vitro Cell Dev Biol Anim 42: 242-247.
- Kunz-Schughart LA, Freyer JP, Hofstaedter F, Ebner R (2004) The use of 3-D cultures for high-throughput screening: the multicellular spheroid model. J Biomol Screen 9: 273-285.
- Goodman TT, Ng CP, Pun SH (2008) 3-D tissue culture systems for the evaluation and optimization of nanoparticle-based drug carriers. Bioconjug Chem 19: 1951-1959.
- Jiang X, Xin H, Gu J, Xu X, Xia W, et al. (2013) Solid tumor penetration by integrin-mediated pegylated poly(trimethylene carbonate) nanoparticles loaded with paclitaxel. Biomaterials 34: 1739-1746.
- Minchinton AI, Tannock IF (2006) Drug penetration in solid tumours. Nat Rev Cancer 6: 583-592.
- Ridky TW, Chow JM, Wong DJ, Khavari PA (2010) Invasive three-dimensional organotypic neoplasia from multiple normal human epithelia. Nat Med 16: 1450-1455.
- Frantz C, Stewart KM, Weaver VM (2010) The extracellular matrix at a glance. J Cell Sci 123: 4195-4200.
- Ng MR, Brugge JS (2009) A stiff blow from the stroma: collagen crosslinking drives tumor progression. Cancer Cell 16: 455-457.
- Rohwer N, Cramer T (2011) Hypoxia-mediated drug resistance: novel insights on the functional interaction of HIFs and cell death pathways. Drug Resist Updat 14: 191-201.
- Fukumura D, Jain RK (2007) Tumor microvasculature and microenvironment: targets for anti-angiogenesis and normalization. Microvasc Res 74: 72-84.
- Evans CL, Abu-Yousif AO, Park YJ, Klein OJ, Celli JP, et al. (2011) Killing hypoxic cell populations in a 3D tumor model with EtNBS-PDT. PLoS One 6: e23434.
- Shannon AM, Bouchier-Hayes DJ, Condron CM, Toomey D (2003) Tumour hypoxia, chemotherapeutic resistance and hypoxia-related therapies. Cancer Treat Rev 29: 297-307.
- Fischbach C, Chen R, Matsumoto T, Schmelzle T, Brugge JS, et al. (2007) Engineering tumors with 3D scaffolds. Nat Methods 4: 855-860.
- Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144: 646-674.
- Bissell MJ, Radisky D (2001) Putting tumours in context. Nat Rev Cancer 1: 46-54.
- Friedrich J, Seidel C, Ebner R, Kunz-Schughart LA (2009) Spheroid-based drug screen: considerations and practical approach. Nat Protoc 4: 309-324.
- Desoize B, Jardillier J (2000) Multicellular resistance: a paradigm for clinical resistance? Crit Rev Oncol Hematol 36: 193-207.
- Vinci M, Gowan S, Boxall F, Patterson L, Zimmermann M, et al. (2012) Advances in establishment and analysis of three-dimensional tumor spheroid-based functional assays for target validation and drug evaluation. BMC Biol 10: 29.
- Kim JB (2005) Three-dimensional tissue culture models in cancer biology. Semin Cancer Biol 15: 365-377.
- Mehta G, Hsiao AY, Ingram M, Luker GD, Takayama S (2012) Opportunities and challenges for use of tumor spheroids as models to test drug delivery and efficacy. J Control Release 164: 192-204.
- Sutherland RM, McCredie JA, Inch WR (1971) Growth of multicell spheroids in tissue culture as a model of nodular carcinomas. J Natl Cancer Inst 46: 113-120.
- Sutherland RM, Sordat B, Bamat J, Gabbert H, Bourrat B, et al. (1986) Oxygenation and differentiation in multicellular spheroids of human colon carcinoma. Cancer Res 46: 5320-5329.
- Huygens A, Huyghe D, Bormans G, Verbruggen A, Kamuhabwa AR, et al. (2003) Accumulation and photocytotoxicity of hypericin and analogs in two- and three-dimensional cultures of transitional cell carcinoma cells. Photochem Photobiol 78: 607-614.
- Roelants M, Van Cleynenbreugel B, Lerut E, Van Poppel H, de Witte PA (2011) Human serum albumin as key mediator of the differential accumulation of hypericin in normal urothelial cell spheroids versus urothelial cell carcinoma spheroids. Photochem Photobiol Sci 10: 151-159.
- Huygens A, Crnolatac I, Develter J, Van Cleynenbreugel B, Van der Kwast T, et al. (2008) Differential accumulation of hypericin in spheroids composed of T-24 transitional cell carcinoma cells expressing different levels of E-cadherin. J Urol 179: 2014-2019.
- Huygens A, Kamuhabwa AR, Roskams T, VAN Cleynenbreugel B, VAN Poppel H, et al. (2005) Permeation of hypericin in spheroids composed of different grade transitional cell carcinoma cell lines and normal human urothelial cells. J Urol 174: 69-72.
- Albrecht DR, Underhill GH, Wassermann TB, Sah RL, Bhatia SN (2006) Probing the role of multicellular organization in three-dimensional microenvironments. Nat Methods 3: 369-375.
- Lee J, Cuddihy MJ, Kotov NA (2008) Three-dimensional cell culture matrices: state of the art. Tissue Eng Part B Rev 14: 61-86.
- Breslin S, O'Driscoll L (2013) Three-dimensional cell culture: the missing link in drug discovery. Drug Discov Today 18: 240-249.
- Petersen OW, Rønnov-Jessen L, Howlett AR, Bissell MJ (1992) Interaction with basement membrane serves to rapidly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells. Proc Natl Acad Sci U S A 89: 9064-9068.
- Lee GY, Kenny PA, Lee EH, Bissell MJ (2007) Three-dimensional culture models of normal and malignant breast epithelial cells. Nat Methods 4: 359-365.
- Rizvi I, Anbil S, Celli JP, Alagic N, Massodi I, et al. (2013) Modeling stromal determinants of 3D tumor growth to inform PDT-mediated combination treatments. SPIE Proceedings 8568.
- Rizvi I, Celli JP, Evans CL, Abu-Yousif AO, Muzikansky A, et al. (2010) Synergistic enhancement of carboplatin efficacy with photodynamic therapy in a three-dimensional model for micrometastatic ovarian cancer. Cancer Res 70: 9319-9328.
- Xu F, Celli J, Rizvi I, Moon S, Hasan T, et al. (2011) A three-dimensional in vitro ovarian cancer coculture model using a high-throughput cell patterning platform. Biotechnol J 6: 204-212.
- Alemany-Ribes M, García-Díaz M, Busom M, Nonell S, Semino CE (2013) Toward a 3D Cellular Model for Studying In Vitro the Outcome of Photodynamic Treatments: Accounting for the Effects of Tissue Complexity. Tissue Eng Part A.
- Kisiday J, Jin M, Kurz B, Hung H, Semino C, et al. (2002) Self-assembling peptide hydrogel fosters chondrocyte extracellular matrix production and cell division: implications for cartilage tissue repair. Proc Natl Acad Sci U S A 99: 9996-10001.
- Garreta E, Genové E, Borrós S, Semino CE (2006) Osteogenic differentiation of mouse embryonic stem cells and mouse embryonic fibroblasts in a three-dimensional self-assembling peptide scaffold. Tissue Eng 12: 2215-2227.
- Genové E, Schmitmeier S, Sala A, Borrós S, Bader A, et al. (2009) Functionalized self-assembling peptide hydrogel enhance maintenance of hepatocyte activity in vitro. J Cell Mol Med 13: 3387-3397.
- Marí-Buyé N, Luque T, Navajas D, Semino CE (2013) Development of a three-dimensional bone-like construct in a soft self-assembling peptide matrix. Tissue Eng Part A 19: 870-881.
- Bhowmick NA, Neilson EG, Moses HL (2004) Stromal fibroblasts in cancer initiation and progression. Nature 432: 332-337.
- Celli JP (2012) Stromal interactions as regulators of tumor growth and therapeutic response: A potential target for photodynamic therapy? Isr J Chem 52: 757-766.
- Lovett M, Lee K, Edwards A, Kaplan DL (2009) Vascularization strategies for tissue engineering. Tissue Eng Part B Rev 15: 353-370.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 14145
- [From(publication date):
specialissue-2013 - Sep 23, 2024] - Breakdown by view type
- HTML page views : 9679
- PDF downloads : 4466