Passive Treatment Technologies for the Treatment of AMD From Abandoned Coal Mines, eMalahleni, South Africa-Column Experiments
Received: 01-Feb-2018 / Accepted Date: 08-Feb-2018 / Published Date: 15-Feb-2018
Abstract
Acid mine drainage (AMD) production from abandoned coal mines is a world-wide environmental problem. Characteristics of AMD includes low pH (<4), high sulfate (SO4) concentrations, high acidity levels and potentially hazardous metals such as Al, Fe and Mn. Passive treatment technologies for AMD remediation can function in remote areas with low costs of operation, monitoring and maintenance and therefore are practical for setting up on abandoned mine sites. Even though such systems have been used to treat acid mine water efficiently, limitations such as coating and clogging as a result of Al3+ and Fe3+ oxyhydroxide precipitates have been reported.
For solving the clogging problems associated with most of the passive treatments, dispersed alkaline substrate (DAS) was introduced in Spain by Rotting, et al. A DAS is a system composed of coarse matrix mixed with a fine grained alkaline material. The main aim of the study was to investigate the effectiveness of the DAS system in treating AMD from an abandoned coal mine of eMalahleni, South Africa and compare it with the traditional reducing and alkalinity producing system (RAPS).
The column experiments remediated acid water successfully for 21 weeks after which the DAS system clogged while RAPS was continuing to treat AMD successfully. For assessment of the treatment systems water parameters such as pH, Redox, total dissolved solids (TDS), concentrations of metals and metalloids were analysed weekly. Both treatment systems were able to raise the pH from an average of 3 to 8. Contaminants such as Fe, Al, and Zn were completely removed. Mn concentrations were reduced but were still above the South African water quality standards.
Keywords: Passive treatment technologies; Acid mine drainage; Dispersed alkaline substrate; Reducing and alkalinity producing system
Introduction
Acid mine drainage (AMD) from old and abandoned coal mines is a world-wide environmental disaster. In South Africa, the state is legally responsible for rehabilitation of such environmental complications since most of such mines operated before implementation of any legislation such as Minerals and Petroleum Resources Development Act (MPRDA), No.28 of 2002, which puts emphasis on sustainable development and environmental protection [1].
AMD is produced when sulfide minerals such as Pyrite (FeS2) undergo oxidation in the presence of water and oxygen forming sulfates and Ferrous Iron (Fe2+) (Equation 1) [2-4].
2FeS2(s)+7O2(g)+2H2O(l)=2Fe2+(aq)+4SO42−(aq)+4H+(aq) (1)
Characteristics of AMD include low pH (<4), High electrical conductivity (EC) and total dissolved solids (TDS), high sulphate (SO4) concentrations and high acidity levels leaching potentially toxic metals such as aluminium (Al), iron (Fe), and manganese (Mn) in streams/rivers threatening the limited water resources of South Africa. An example is comprehended in the study done in the Loskop Dam Nature reserve, downstream of Olifants River Catchment in Mpumalanga where AMD from abandoned coal mines was linked to the death of fish and crocodiles in the dam [5-8].
Passive treatment technologies of AMD are more practical for setting up on abandoned mine sites since some removal of acid and toxic metals will benefit the receiving environment and due to the association with low cost of implementing, operation and maintenance [9-11]. Such conventional treatments have been tried and tested in many parts of the world with their performance and effectiveness in acid water remediation differing in longevity and success from one place to another.
Reducing and alkalinity producing system (RAPS) which is the combination of anoxic limestone drains and a compost wetlands is one of the traditional passive treatment options for net-acidic mine water treatment [12,13]. Dispersed alkaline substrate (DAS) is a passive system introduced in Spain by Rotting et al. with the intention of solving the clogging problems associated with most of the passive treatments [14]. DAS is a system composed of coarse matrix (e.g. wood shavings) mixed with a fine grained alkaline material (e.g. limestone). The woodchips/wood shavings are supposed to provide high permeability while the limestone provides a bulk reactive surface area, where it will dissolve and react with AMD before it is coated [14,15].
Aims and Objectives
The general aim of the research was to contribute towards mine water treatment solutions in SA by investigating different passive treatment systems in remediating AMD from coal mines. The study investigated a DAS system in treating AMD from an abandoned coal mine in eMalahleni, Mpumalanga Province and compared it with RAPS which have been investigated and implemented in many parts of the world.
By ensuring that the main aim is met the following specific objectives were looked at:
• Reaching neutral pH (6~9)
• Lower concentrations of metals, e.g. Fe<1 mg/L, Al<0.15 mg/L and Mn<1 mg/L
• Lower sulfate levels (<200 mg/L)
Materials and Methods
Feedstock
AMD treated for this study was collected from a discharge collecting point of an abandoned mine situated about 25 Km South East of eMalahleni. A 25 L container was used to carry the acid water from the site to the laboratory. The container was rinsed many times (about 5 times) before filling it with the AMD. This acid water is characterized by pH levels that are approximately 2.7, EC values that are extremely high (~1672 mS/m), high sulfate levels and high concentrations of metals (Fe, Al and Mn) exceeding industrial water standards as set by DWAF (Table 1).
| Parameters | Average Douglas discharge | Target water quality range (DWAF)-Industrial standards |
|---|---|---|
| pH | 3 | 6~9 |
| EC (µS/m) | 1672 | 70 |
| Al (mg/L) | 115 | 0.15 |
| Fe (mg/L) | 180 | 0.1 |
| SO42-(mg/L) | 6091 | 200 |
| Mg (mg/L) | 20 | 30 |
| Mn (mg/L) | 5 | 0.05 |
Table 1: Average water results AMD and the target water quality range (DWAF Standards).
Cow manure that was used comprised pH of approximately 6.8, EC was about 280 mS/cm and moisture content was measured to be 68.9%. Limestone used in both treatment systems contained about 93% of calcium carbonate in the form of calcite (CaCO3) and dolomite (CaMg(CO3)2. The particle sizes of the limestone used in the DAS system were <0.106 mm while the limestone used in the RAPS system were >7 mm.
Experimental setup
Two passive treatments, i.e. DAS and RAPS, were staged in the laboratory for the passive treatment of AMD.
DAS: Acid water was pumped with a peristaltic pump at a constant flow rate in an upward flow movement into the first column containing a mixture of 25% (v/v) limestone and 75% (v/v) wood shavings. The residence time in the treatment system was set to 24 hours so that there can be enough effluent for sampling resulting in the acid water being treated to be in contact with the material in one column for 12 hours.
RAPS: Acid water was pumped with a peristaltic pump through a tube at a constant flow rate into the first column containing a layer of manure underlain by limestone. Manure substrate occupied 30% (v/v) of the column, while the limestone occupied 50% (v/v). In a downward movement, the treated water from the first column flows into the second column which is the same material contained in the first column. The residence time of 24 hours was set for the acid water to be treated in the passive system with 12 hour contact time of the treated water in each column.
Analytical methods
XRD and XRF analysis were used for the reactive material (limestone) for mineral and elements (traces and major) identification and concentrations before and after use respectively. Tests for water pH, redox (pE), EC, TDS and dissolved oxygen (DO) were done in the laboratory to evaluate the quality of the treated water using pH and EC meter, also called multi-meter. The multi-meter was calibrated every time before use. For the conductivity probe, EC solution was used for calibration and pH calibration solutions, 4.0, 7.0 and 10.0, were used for the pH probe.
The water samples were collected weekly following WRC 2000 procedure and submitted for the IC and ICP-MS (inductively coupled mass spectrometry) analysis to identify concentrations of metals and metalloids. Alkalinity and acidity tests were also conducted to check whether the water is acidic or alkaline.
Geochemical method
PHREEQC was used to evaluate the behavior of the selected treatment methods in order to predict its efficiency in treating acid mine water [16]. PHREEQC uses a solubility method to detect thermodynamically probable solid phases using the Activity and Massaction equation (Equation 2), where SI is saturation index, IAP is ion activity product and KS is solid solubility product.
SI=Log IAP/KS (2)
Results and Discussion
pH
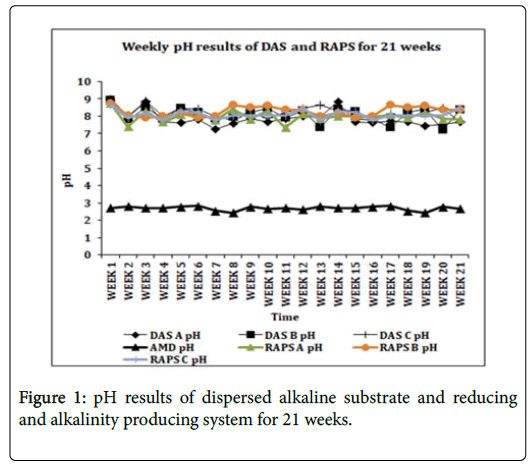
Increment of the pH was achieved by both passive treatment systems (Figure 1) to be within the water quality standard of 6~9 as set by DWAF [17]. The average pH value of the treated water from both DAS and RAPS system increased to 8. For the DAS system, this pH value obtained was higher compared to the DAS treatment systems that were since introduced and tested by Caraballo et al. that indicated an average pH of 6.4 [18,19]. RAPS system also showed a higher average pH compared to the RAPS pilot systems by Nairn & Mercer and Matthies et al. which presented a pH of 6 and 5 respectively [20,21].
The pH increase was primarily due to the calcite dissolution (Equation 3 and 4), releasing Ca2+ into the solution resulting in generation of alkalinity in the systems to be increased from 0 mg/L in the acid mine water to an average of 462 mg/L as CaCO3 in the DAS system and 272 mg/L as CaCO3 in the RAPS system.
CaCO3+2H+ →Ca2++H2O+CO2 (3)
CaCO3+H2CO3 →Ca2++2HCO3 (4)
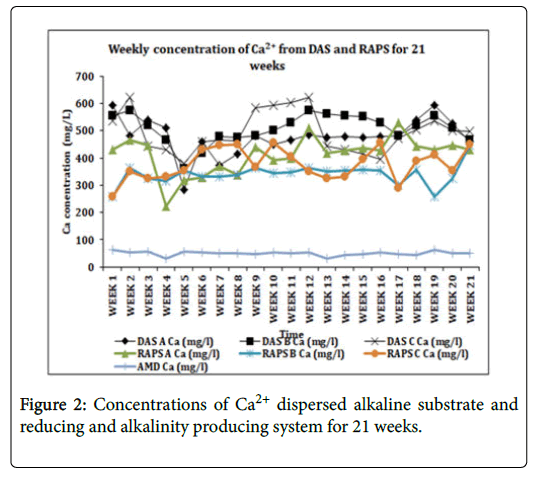
The increase in the Ca2+ concentration was witnessed in the DAS (~493 mg/L) and RAPS (~376 mg/L) systems compared with the AMD Ca2+ concentration which was very low with an average of ~49 mg/L (Figure 2). This was however expected with the pH increment and alkalinity increase, as the limestone reacted with the acidic water.
Metals: Fe, Al and Mn
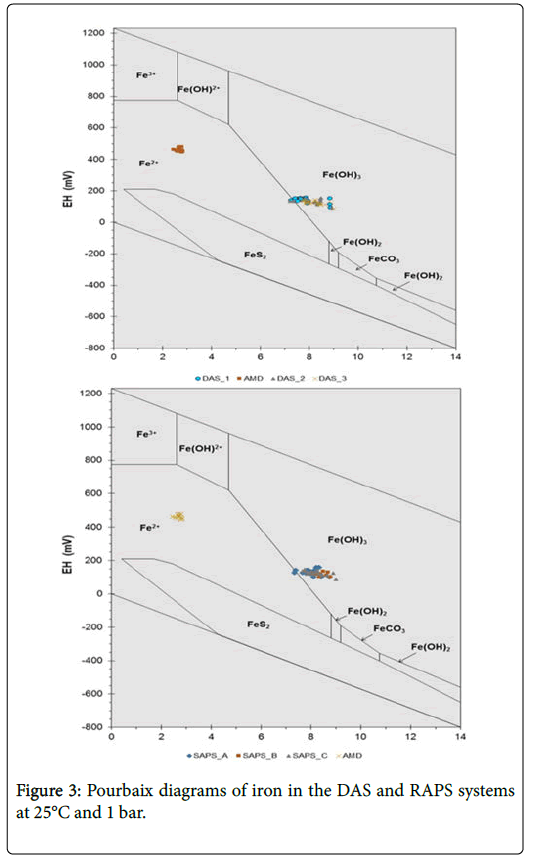
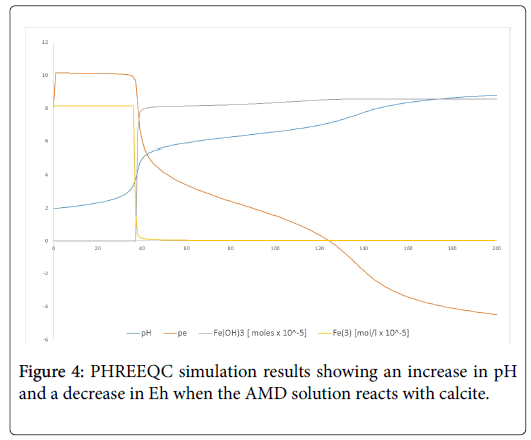
The ICP-MS results revealed that the total Fe was reduced from an average of 136 mg/L in the AMD to 1 mg/L in DAS and 3 mg/L in RAPS but increasing again after exposure to oxygen in both the treatment systems. The concentration of total Fe in AMD according to the spectrophotometer used, was all Fe2+. The Pourbaix diagrams (Figure 3), was also used to predict the different ions of iron at different values of the pH and pE. The decrease in the pE values were also observed during the respective experimental runs, which is due to the buffering effect of Fe(OH)3, as complimented by the PHREEQC simulation on Figure 4. It is also observed from the same figure that pE (pE=Eh/0.059 V), decreases as the pH is being increased by neutralization reaction.
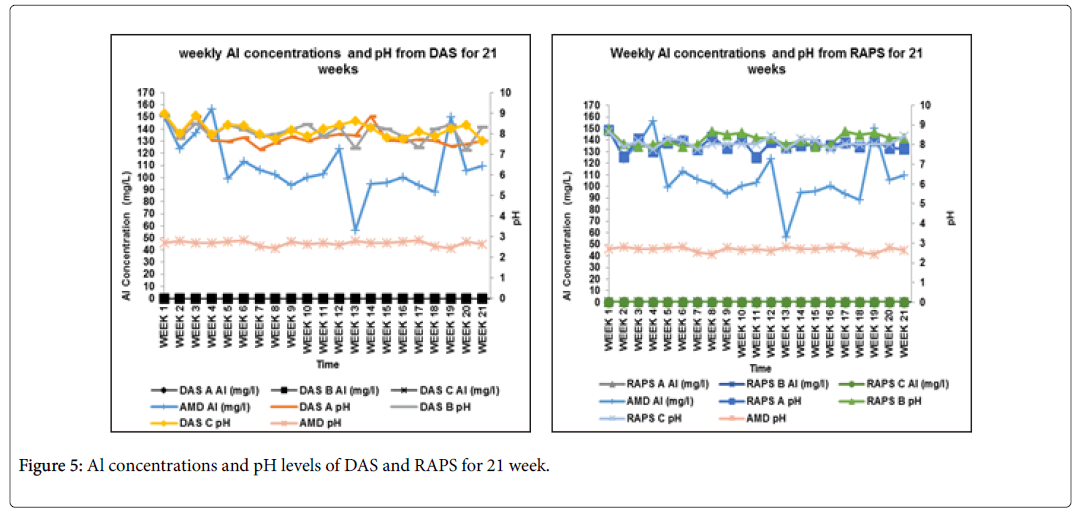
According to the ICP-MS results Al was also decreased in the passive systems from an average of 110 mg/L in AMD to 0 mg/L in the DAS and RAPS passive systems (Figure 5). The removal of Al was an objective met, to reduce the Al concentration to the acceptable water quality standard of <1.5 mg/L as set by DWAF [17]. The pH increase led to the insolubility of the Al concentrations as Al hydrolysis occurs resulting in low concentration levels.
Mn concentrations were reduced by the two passive treatment systems even though the concentrations were still above the water quality range of the domestic water standards according to DWAF [17]. Mn was reduced from an average of 7 mg/L to 3.2 mg/L in the DAS and 3.7 mg/L in the RAPS. According to the study by Thomas & Romanek, Mn needs a very high pH of greater than 8 to precipitate out [22]. PHREEQC simulations suggested that the decrease in Mn concentration in the treated water was likely precipitated out as MnCO3.
No sulfate reduction was observed in both the treatment systems. In fact, an increase in sulfate average concentrations was observed in all the samples. The same trend of not being able to decrease the sulfate concentration was also experienced in the study done by Nairn & Mercer [20]. This may be due to various reasons such as that the organic substrate in both the treatment systems does not contain any sulfate reducing bacteria (SRB) which are important microorganisms in reducing sulfates to sulfites. Measures of analyzing the organic substrate for biological activities were not conducted and therefore it is not known whether sulfate reducing bacteria were present or not.
Conclusions
The laboratory scale experimental work was done to compare the effectiveness of the DAS system in treating AMD from an abandoned coal mine in eMalahleni, Mpumalanga Province, with the traditional RAPS which have been investigated and implemented in many parts of the world for net-acidic mine water treatment. DAS was introduced in Spain by Rotting, et al. to address the problem of choking that is associated with most reported passive treatments [14]. From the experiment, the DAS system with the setup explained in this study blocked after 21 weeks of operation while the traditional treatment system that was being compared with, RAPS, was still able to treat acid water efficiently without any signs of clogging.
Before DAS system choked, both technologies (DAS and RAPS) were able to take most contaminants to the accepted water quality standards of South Africa as set by DWAF [17]. Most of such contaminants are mentioned below:
• pH was raised from an average of 3 in AMD to 8, which is neutral conditions in the two systems solely from limestone dissolution.
• Acidity was completely removed and alkalinity was generated from 0 mg/L in the AMD to an average of 462 in the DAS and 272 in the RAPS system.
• Low redox potential which concluded the presence of reducing conditions in the systems and therefore oxidation process is slowed down or stopped completely in the system.
• High concentrations of contaminants such as Fe and Al were completely removed from an average of 132 mg/L and 110 mg/L respectively.
• Mn concentrations were also reduced but remained above the water quality standards due to the maximum pH of 8 in both DAS and RAPS. The decrease was from an average of 7 mg/L to 3.2 mg/L in the DAS and 3.7 mg/L in RAPS.
Some of the contaminants that were equal or increased by the two systems are mentioned below:
• Increase in Ca2+ concentration was observed from an average of 49 mg/L in the AMD to 376 mg/L and 493 mg/L in the RAPS and DAS systems respectively.
• Average sulfate concentrations increased in all instances.
It is concluded that treatment of AMD by passive treatment systems is possible looking at the discussion above. Such technologies are mostly short term solutions depending on the design and materials used. From the experiment, the DAS system with the setup explained in this study blocked after 21 weeks of operation while the traditional treatment system that was being compared with, RAPS, was still able to treat acid water efficiently without any signs of clogging. In the columns containing the DAS system in the laboratory, observation of an orange-yellow precipitate was made which started at the bottom where the treated water enters the column, running upwards, clogging the system to a point where the water was not able to pass through the column.
More research on different designs, setups and materials to use for acid mine drainage is still needed to address:
• The issue of longevity as a result of coating and clogging from Al3+ and Fe3+ oxy-hydroxide precipitates resulting in the passivation of the alkaline substrate leading to failure of the system.
• Material that can increase the pH to accommodate Mn removal to the accepted water quality standards.
• Material that would allow for SO42- reduction.
Acknowledgment
This research work was supported by the Council for Geoscience (CGS). All laboratory analyses were done at the CGS laboratories. Koena Ramasenya is acknowledged for his great assistance. Maphuti Kwata and Juad Sathekge are acknowledged for assisting during field work. Acknowledgement to Mr. Nicolaus Van Zweel for his supervision throughout the project. Thanks to Obed Novhe for being a good mentor.
References
- Mineral and Petroleum Resources Development Act (MPRDA) No.28. (2002) Government gazette, Cape Town, Republic of South Africa. 448: 1-122.
- Younger PL, Banwart SA, Hedin RS (2002) Mine water: Hydrology, pollution, and remediation. Kluwer Academic Publishers, Dordrecht, Netherlands. 5: 998-999.
- Mine Environment Neutral Drainage Program (MEND) (2008) Acid rock drainage prediction manual: A manual of chemical evaluation procedures for the prediction of acid generation from mine wastes. Department of energy, mines and resources, Canada. 1-77.
- Jacobs JA, Lehr JH, Stephen MT (2014) Acid mine drainage, rock drainage, and acid sulfate soils: Causes, assessment, prediction, prevention and remediation (1st edn). John Wiley & Sons Inc. 1-520.
- Rapson LA (2004) The development of a risk-based computer database to prioritise the environmental rehabilitation of defunct and abandoned collieries in the witbank area of South Africa. 1-153.
- Munnik V (2010) The social and environmental consequences of coal mining in South Africa-A case study. Environmental Monitoring Group. 1-24.
- Colvin C, Burns A, Schachtschneider K, Mahery A, Charmier J, et al. (2011) Coal and water futures in South Africa: The case for protecting headwaters in the Enkangala grasslands. World Wide Fund (WWF), Cape Town, South Africa. 1-82.
- Lai J (2013) Impact of anthropogenic pollution on selected biota in Loskop Dam. 1-135.
- Hedin RS, Nairn RW, Kleinmann RLP (1994) Passive treatment of coal mine drainage. Bereau of Mines, Unite State Department of Interior,Information Circular 9389. 1-44.
- Hedin RS, Watzlaf GR, Nairn RW (1994) Passive Treatment of Acid-Mine Drainage with Limestone. J Environ Qual 23: 1338-1345.
- Watzlaf GR, Schroeder KT, Kleinmann RLP, Kairies CL, Nair RW (2004) The passive treatment of coal mine drainage. U.S. Department of Energy. Washington DC, USA.
- Riefler RG, Krojn J, Stuart B, Scotch C (2008) Role of sulfur-reducing bacteria in a wetland system treating acid mine drainage. Sci Total Environ 394: 222-229.
- Kepler DA, McCleary EC (1994) Successive alkalinity producing system (SAPS) for the treatment of acidic mine drainage. In proceedings of the international land reclamation and mine drainage conference and the 3rd international conference on abatement of acidic drainage, Pittsburgh, PA. 195-205.
- Rotting TS, Caraballo MA, Serrano JA, Ayora C, Carrera J (2008) Field application of calcite dispersed alkaline substrate (calcite-DAS) for passive treatment of acid mine drainage with high Al and metal concentrations. Appl Geochem 23: 1660-1674.
- Macias F, Caraballo MA, Nieto JM, Rotting TS, Ayora C (2012) Natural pretreatment and passive remediation of highly polluted acid mine drainage. J Environ Manage, 104: 93-100.
- Parkhurst BDL, Appelo CAJ (2013) Description of input and examples for PHREEQC version 3-A computer program for speciation, batch-reaction, one dimensional transport, and inverse geochemical calculations: U.S. Geological survey techniques and methods. pp. 497.
- Department of Water Affairs and Forestry (DWAF) (1996) South African water quality guidelines: Recreational water. The Government printer, Republic of South Africa, Pretoria. 2: 1-74.
- Caraballo MA, Macias F, Nieto JM, Ayora C (2007) Treatment of high metal concentration AMD using dispersed alkaline substrate (DAS), a novel passive treatment system. InGoldschmidt Conference Abstracts, Geochem Cosmochem Acta.
- Caraballo MA, Macias F, Nieto JM, Castillo J, Quispe D, et al. (2011) Hydrochemical performance and mineralogical evolution of a dispersed alkaline substrate (DAS) remediating the highly polluted acid mine drainage in the full-scale passive treatment of Mina Esperanza (SW Spain). Am Mineral 96: 1270-1277.
- Nairn RW, Mercer MN (2000) Alkalinity generation and metals retention in a successive alkalinity producing system. Mine Water Environ 19: 124-133.
- Matthies R, Jarvis AP, Aplin AC (2009) Performance evaluation of two reducing and alkalinity producing systems for coal mine drainage remediation after 4 Years of operation. Sci Total Environ 531-538.
- Thomas RC, Romanek CS (2002) Passive treatment of low-ph, ferric iron-dominated acid rock drainage in a vertical flow wetland II: Metal removal. Paper presented at the National meeting of the American society of mining and reclamation, Lexington KY. 752-775.
Citation: Dube GM, Novhe O, Ramasenya K, Zweel NV (2018) Passive Treatment Technologies for the Treatment of AMD From Abandoned Coal Mines, eMalahleni, South Africa-Column Experiments. J Ecol Toxicol 2: 110.
Copyright: © 2018 Dube GM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 5196
- [From(publication date): 0-2018 - Nov 16, 2025]
- Breakdown by view type
- HTML page views: 4240
- PDF downloads: 956