Review Article Open Access
Adacolumn Therapeutic Leucocytapheresis for Ulcerative Colitis: Clinical and Endoscopic Features of Responders and Unresponders to this Nonpharmacologic Intervention
Tomotaka Tanaka1*, Hiroyuki Hanai H2 and Ingvar Bjarnason3
1Akitsu Prefectural Hospital, Akitsu-cho, Hiroshima, Japan
2Hamamatsu South Hospital, Hamamatsu, Japan
3Department of Gastroenterology, King’s College Hospital, London, UK
- *Corresponding Author:
- Tomotaka Tanaka MD
Akitsu Prefectural Hospital
4388 Akitsu-cho
Hiroshima 739-2402, Japan
Tel: 81 846450055
Fax 81 823740371
E-mail: tomotaka@c.do-up.com
Received date: October 13, 2011; Accepted date: November 30, 2011; Published date: December 02, 2011
Citation: Tanaka T, Hanai HH, Bjarnason I (2011) Adacolumn Therapeutic Leucocytapheresis for Ulcerative Colitis: Clinical and Endoscopic Features of Responders and Unresponders to this Non-pharmacologic Intervention. J Gastrointest Dig Syst 1:104. doi: 10.4172/2161-069X.1000104
Copyright: © 2011 Tanaka T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Gastrointestinal & Digestive System
Keywords
Ulcerative colitis; Neutrophils and monocytes; Mucosal biopsy; Loss of mucosal tissue; Granulocyte and monocyte apheresis; Neutrophil infiltration; Colonoscopy
Introduction
Ulcerative colitis (UC) is one of the two major phenotypes of the idiopathic inflammatory bowel diseases (IBD) of the intestine; the other major phenotype is Crohn’s disease (CD). UC and CD are both debilitating chronic disorders that afflict millions of individuals throughout the world with symptoms which impair function and quality of life. However, whereas UC is confined to the colon and the rectum, CD may affect any part of the gut from the mouth to the perianal [1,2]. A multitude of clinical manifestations represent the expressions of IBD. These include diarrhoea, rectal bleeding, abdominal discomfort, fever, anaemia, and weight loss. Both UC and CD tend to run a remitting-relapsing course affected by diverse environmental factors [2]. From here on, we shall focus on UC.
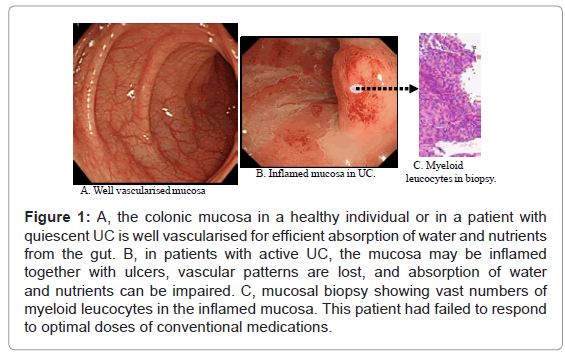
The severity of UC is often presented by clinical activity index (CAI). Another, but complementary parameter is endoscopic index (EI), both are described in (Table 1) for 120 patients with UC. In this manuscript, our endeavours were supported by the diagnostic power of colonoscopy to identify patients with an active flare of UC who were most likely to respond to selective, but therapeutic removal of circulating myeloid lineage leucocytes (granulocytes and monocytes/macrophages) by extracorporeal adsorption as a nonpharmacologic treatment intervention. This strategy is known as GMA, which stands for granulocyte and monocyte adsorption [3]. In (Figure 1) colonoscopic photographs from the colonic mucosa of a healthy human subject and from patients with UC are presented. The mucosa is the surface through, which nutrients and water from the food in the intestine are absorbed into the blood stream. Accordingly, healthy mucosa is typically well vascularised for adequate absorption. However, in patients with IBD, vascular patterns may be damaged by inflammation and ulcers as seen in (Figure 1).
| Demographic variable | Number of patients | Clinical response (%) | Clinical remission (%) | Endoscopic response (%) | Endoscopic remission (%) |
|---|---|---|---|---|---|
| Overall outcomes | 120 | 73.3 | 66.7 | 64.2 | 55.0 |
| Steroid naïve | 61 | 82.0 | 73.8 | 70.5 | 60.7 |
| Steroid dependent | 59 | 64.4 | 59.3 | 57.6 | 49.2 |
In this cohort of 120 patients, at entry and one week post last GMA session, Rachmilewitz’s clinical activity index (CAI) was applied to assess disease activity.9 Likewise, Rachmilewitz’s endoscopic index (EI) was used to assess mucosal disease activity
Table 1: Rates of clinical remission, clinical response, endoscopic remission and endoscopic response in 120 patients with ulcerative colitis (UC) that received adsorptive granulocyte/monocyte apheresis (GMA) as a non-pharmacologic treatment intervention. At entry, the average clinical activity index (CAI) and endoscopic index (EI) were 12.6±1.71 (range 10-16), and 10.3±1.34 (range 7-12), respectively (n=120). CAI ≤4 was defined as clinical remission, while EI ≤3 was defined as endoscopic remission. Similarly, clinical response was judged as a decrease of 4 points in the CAI score relative to baseline, while a decrease of 3 points in the EI score was judged as endoscopic response.
Figure 1: A, the colonic mucosa in a healthy individual or in a patient with quiescent UC is well vascularised for efficient absorption of water and nutrients from the gut. B, in patients with active UC, the mucosa may be inflamed together with ulcers, vascular patterns are lost, and absorption of water and nutrients can be impaired. C, mucosal biopsy showing vast numbers of myeloid leucocytes in the inflamed mucosa. This patient had failed to respond to optimal doses of conventional medications.
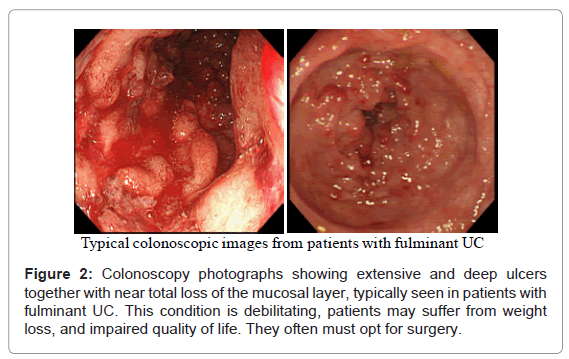
The symptoms of UC are due to the ulceration and the loss of the mucosal layer covering the inner wall of the large intestine (colon and the rectum). As the mucosal layer is involved in the absorption of nutrients and water from the gut, during severely active IBD, absorption of nutrients and water is seriously impaired. In (Figure 2), typical colonoscopy photographs from the surface of the colon or the rectum of patients with severe and fulminant UC are seen. Extensive and deep ulcers together with near total loss of the mucosal tissue are not uncommon in patients with severe UC even in the presence of conventional medications. This condition is debilitating, the patients may suffer from weight loss, and impaired quality of life. For example unabsorbed food and water will pass as watery diarrhoea, or bloody diarrhoea due to bleeding. Such patients are not likely to respond to any drug based medication or even to therapeutic depletion of myeloid leucocytes by GMA, they have fulminant UC (disease persists in the presence of optimal medication) and often must opt for colectomy. Needless to say that only an initial diagnostic colonoscopy can identify such patients as non-responders to drug based interventions so that the patient can opt for an alternative treatment at an early stage. This should significantly shorten morbidity time and save medical cost.
Therapeutic options for patients with ulcerative colitis
Despite the recognition of a genetic background together with environmental factors, which at present are thought to translate into an inappropriate inflammatory response in patients with UC [2,4-7], currently our understanding on the immunopathogenesis of UC is inadequate. Hence, up to now drug therapy of UC has been empirical rather than based on a sound understanding of disease aetiology. Accordingly, while drug therapy initially appears successful in the majority of patients, it comes at the cost of significant side effects [8,9]. Further, up to now, first line medications for exacerbation of UC include 5-aminosalicylic acid (5-ASA) or sulphasalazine in combination with a corticosteroid with consideration of azathioprine (or 6-mercaptopurine) and nutritional support for some patients [2,10- 14]. Treatment failure in patients with severe disease has often been an indication for colectomy in up to 40% of steroid refractory patients [2,15,16] although in recent years, cyclosporin A (CsA) has been introduced for corticosteroid refractory UC [14,17]. Despite being moderately effective in this clinical setting in reducing colectomy rate, there remain serious concerns over long-term efficacy and toxicity of CsA [18]. However, this is not to say that drugs have no place in the treatment of UC. In fact, no one can deny the role of medicines in the elimination of most diseases that our ancestors were left defenseless against.
Even in today’s era of modern medicine, it is essential to bear in mind that drug therapy by its very nature, involves adding a foreign substance to the body system and although initially effective, may lead to the disease becoming drug dependent or refractory. Additionally, many drugs are associated with toxic side effects, which can add to the disease complexity. Hence, a therapeutic strategy based on a nondrug intervention, a correction or support of body’s natural processes like GMA (which takes away from the body instead of adding to it), if effective, should have advantages over drugs, long term adverse side effects and refractoriness are unlikely [19,20].
Myeloid leucocytes, cytokines and ulcerative colitis
It is now known that UC is exacerbated by inflammatory cytokines like tumour necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6, IL-8 and others [21]. Accordingly, anti-cytokine antibodies, notably anti-TNF antibodies like infliximab (IFX) are being used and new antibodies are being developed for the treatment of IBD [22]. Indeed, the efficacy of IFX in patients with CD [23] as well as in UC [22] has validated the role of this cytokine in the immunopathogenesis of IBD. However, the enthusiasm towards biologicals is increasingly being dampened by concerns about their long-term efficacy and in particular, the safety profiles of biologics [24,25]. Patients with active IBD harbour elevated and activated myeloid lineage leucocytes (granulocytes and monocytes) in the presence of compromised lymphocytes [1,26-29]. Further, histologic examinations of mucosal biopsies from patients with active IBD reveals a spectrum of pathologic manifestations among which an abundance of neutrophils accounts not only for the morphologic lesions in IBD, but also for the prevailing patterns of mucosal inflammation [2,30,31]. When activated, myeloid leucocytes produce an array of pleiotropic cytokines like TNF-α, IL-1β, IL-6, IL-12, IL- 23, which are strongly pro-inflammatory [21,32]. Therefore, targeting leucocytes as key players in the exacerbation of IBD is what lies behind GMA with the Adacolumn [3]. Likewise, neutrophils in patients with IBD show activation behaviour [26] and prolonged survival time [33]. Factors that are known to promote neutrophil survival in IBD include inflammatory cytokines [34] and paradoxically corticosteroids [35], which are commonly used to treat IBD patients. Myeloid leucocytes, like the CD14(+)CD16(+) monocytes are major sources of TNF-α [36,37], and it could be valid to say that selective depletion of myeloid leucocytes by GMA should alleviate inflammation and promote remission or at least enhance the efficacy of pharmacologics. However, clinical studies in patients with UC have reported unmatched efficacy outcomes, ranging from an 85% [38] to a statistically insignificant level [39], indicating that certain subpopulations of patients respond to GMA while others not so, suggesting that patients’ baseline demographic variables might determine clinical response to this non-pharmacologic mode of therapy [19,20].
Therapeutic leucocytapheresis in ulcerative colitis – logics and mechanisms
For an extracorporeal intervention to be a novel non-drug therapeutic option, it should be able to selectively deplete leucocytes, which in patients with UC are thought to contribute to the disease pathogenesis. For example, patients with active IBD are found to have compromised lymphocytes [27-29]. With this in mind, certain sub-populations of lymphocytes like the CD4(+)CD25(+) phenotype, known as the regulatory T cells (Treg) have essential immunoregulatory roles and therefore, are indispensable to the host [40-45]. Based on these understandings, the Adacolumn leucocytapheresis system (Figure 3) is designed to spare lymphocytes. It is filled with specially designed cellulose acetate beads of 2mm in diameter as the column leucocytapheresis carriers that are bathed in physiologic saline [46]. The carriers remove from blood in the column most of the granulocytes, monocytes/macrophages together with some platelets [3,47]. Surprisingly, the procedure has been associated with a sustained increase in absolute lymphocyte counts in the post treatment phase [28,29,46] including the regulatory phenotype, CD4(+)CD25(+) Treg [3,45,46]. The mechanisms for sparing lymphocytes are briefly described here. Patients with immune dysfunction may have immune complexes (IC) in their plasma [3,47,48]. Cellulose acetate adsorbs immunoglobulin G (IgG) and IC from the plasma [48,49]. Upon adsorption, the binding sites on IgG and IC become available for the Fcγ receptors (FcγRs) on myelocytes [3,47-49]. Further, cellulose acetate with adsorbed IgG and IC generates complement activation fragments including C3a and C5a [3,48,49]. The opsonins C3b/C3bi and others derived from the activation fragments also adsorb onto the carriers and serve as binding sites for the leucocyte complement receptors, CR1, CR2, CR3 (Mac-1, CD11b/CD18). Hence, leucocyte adsorption to the GMA carriers in the Adacolumn is governed by the opsonins, FcγRs and the leucocytes complement receptors [3,49]. The expressions of these sets of receptors are common features of myeloid lineage leucocytes. Lymphocytes are not known to express complement receptors except on small subsets of B, T and natural killer (NK) cells. Similarly, FcγRs are not widely expressed on lymphocytes except on small populations of CD19+B cells and CD56+NK cells [3,47]. These basic phenomena proceed well on the carriers and lend the Adacolumn GMA selectivity.
Figure 3: This figure shows the Adacolumn medical device. The column is filled with cellulose acetate beads of 2 mm in diameter as adsorptive carriers. The Adacolumn is the first and the only adsorptive type leucocytapheresis medical device in clinical use. Also seen in Figure 3 are scanning electron photomicrographs of leucocytes adsorbed onto an Adacolumn carrier. The high magnification views show that no lymphocyte was adsorbed to the carrier. Further, as seen, adsorbed leucocytes undergo extensive release reaction. Up to now, IL-1receptor antagonist, hepatocyte growth factor, IL-10 and soluble TNF receptors have been measured (all have anti-inflammatory effects, reviewed in ref. 49). Accordingly, during GMA, the blood, which returns to patients from the column outflow may be likened to a biologic cocktail containing a vast number of soluble substances released by adherent leucocytes.
Usually, each patient receives one or two GMA sessions per week, up to 10 sessions [19,28,30,51]. Each session is 60 minutes at 30ml per minute.
Colonoscopic features of typical responders to GMA
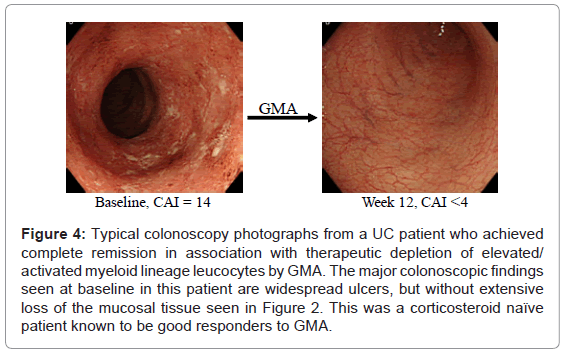
In Figure 4, colonoscopic images from a typical GMA responder patient are presented. Clinical experience has shown that GMA in patients with steroid dependent or steroid refractory UC was associated with significant efficacy as assessed by measuring the fall in UC clinical activity index (CAI) and tapering or discontinuation of steroids, while in steroid naïve patients, GMA spared patients from exposure to steroids [19,20]. Therefore, published data [19,20,28,30,51] suggest that steroid naive patients respond particularly well. Characteristically they respond faster with fewer GMA sessions and have a high cumulative rate of remission. Thus, the remission rate in steroid naïve patients reported by Suzuki et al. [28] was an 85%. Similarly, Tanaka et al. [30] treated a cohort of 45 patients, 26 steroid naive and 19 steroid dependent. Each patient could receive up to a maximum of 11 GMA sessions (or until CAI decreased to 4 or less). At week 12, the response rate (CAI ≤4) in steroid naïve subgroup was 22 of 26 patients (84.6%) and in steroid dependent sub-group was 11 of 19 (57.9%. Colonoscopy revealed that most non-responders in both groups had deep colonic ulcers and extensive loss of the mucosal tissue. Further, this is the only study that looked at the impact of GMA on leucocytes level in the colonic mucosa. Biopsies taken during colonoscopy revealed massive infiltration of the colonic mucosa by neutrophils and GMA was associated with a striking reduction of neutrophils in the mucosa (Figure 5). Tanaka’s colonoscopic observations [19,30] echo those of Suzuki et al. [28] a few years earlier , who also reported that the only 3 non-responders in their cohort of 20 steroid naïve patients had deep colonic ulcers. In a very thorough study by Suzuki et al. [52], the authors aimed at determining the responders to GMA. Their major findings are as follows. Seven days after the last GMA session, 20 of 28 patients (71.4%) achieved clinical remission including all 8 patients who had their first UC episode. The mean duration of UC in the 8 first episode cases was just 3.4 months compared with 40.2 months for all 28 patients and 65.4 months for another 8 patients who did not respond. The response to GMA seemed to be independent of baseline CAI. The authors concluded that first UC episode and short disease duration might be good predictors of response to GMA in that clinical setting. Further, they stated that GMA could be an effective first line medication for steroid naïve patients [19,28,52].
Figure 4: Typical colonoscopy photographs from a UC patient who achieved complete remission in association with therapeutic depletion of elevated/ activated myeloid lineage leucocytes by GMA. The major colonoscopic findings seen at baseline in this patient are widespread ulcers, but without extensive loss of the mucosal tissue seen in Figure 2. This was a corticosteroid naïve patient known to be good responders to GMA.
The impact of GMA on mucosal leucocytes
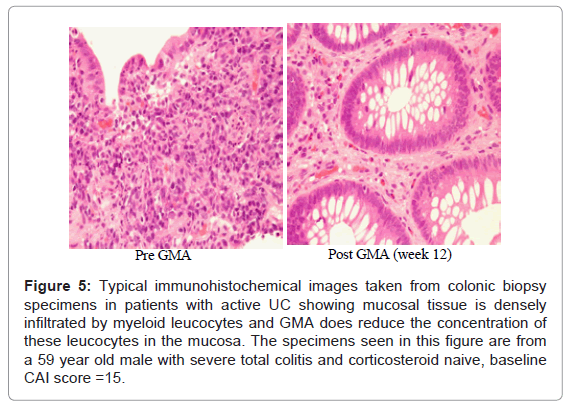
It is of particular interest to see if GMA, in fact does impact the mucosal level of infiltrating myeloid leucocytes. As stated above, colonic biopsies were taken from active disease sites before and after GMA induced remission in patients with active UC. (Figure 5) shows representative histology photographs from a GMA responder patient. The specimen taken at baseline shows the colonic mucosa is infiltrated by a vast number of inflammatory leucocytes, primarily granulocytes and monocytes/macrophages; the density of the infiltrating cells was strongest in or around the glandular lumen (crypt abscesses). The specimen taken when the patient had achieved remission shows very striking reduction in inflammatory cell infiltrate. Surprisingly, the density of leucocytes was reported to be stronger in steroid naïve patients vs patients on steroids, suggesting that corticosteroids have inhibitory effects on neutrophils [19].
Figure 5: Typical immunohistochemical images taken from colonic biopsy specimens in patients with active UC showing mucosal tissue is densely infiltrated by myeloid leucocytes and GMA does reduce the concentration of these leucocytes in the mucosa. The specimens seen in this figure are from a 59 year old male with severe total colitis and corticosteroid naive, baseline CAI score =15.
Colonoscopic features of non-responders to GMA
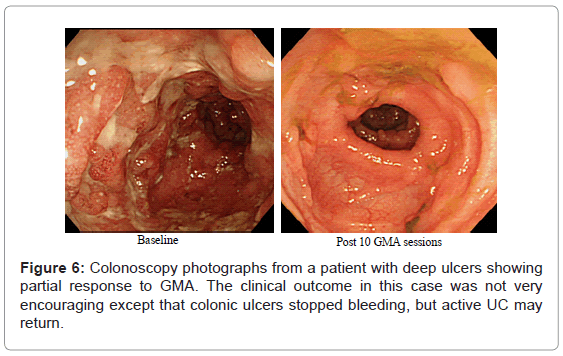
As reviewed above, several studies have reported that any patient with a fair level of intact colonic mucosa is a potential responder to GMA. With this in mind, (Figure 2) (above) shows deep and extensive colonic lesions with virtually no mucosal tissue left at the lesion sites in two typical GMA non-responder patients. Such patients are unlikely to respond to any medication. Additionally, patients with the loss of the mucosal layer are at an increased risk of viral infection, notably, cytomegalovirus and therefore, should be tested and receive antiviral medication if positive. Even patients with a near equal CAI score had very different mucosal damage status, indicating that CAI per se does not reflect the full extent of mucosal damage in patients with UC. (Figure 6) shows colonoscopic images from the colonic mucosa of a steroid dependent patient who showed partial response to GMA. We tried to treat a few such patients because there are patients who do not wish to have colectomy like young ladies for social reasons or they fear colectomy may reduce their chance of conceiving a child. At baseline, the major colonoscopic findings seen are deep ulcers with multiple polyp-like protrusions. Following a course of GMA therapy, inflammation has alleviated, suggesting a fair level of mucosal tissue was left prior to the initiation of GMA therapy. Based on CAI, this patient might be in clinical remission, but has not achieved endoscopic remission, and the UC may become active again.
A small minority of patients without deep colonic lesions or extensive loss of the mucosal tissue do not respond to GMA as well. Colonoscopy photographs from one such patient is presented in (Figure 1), showing strong inflammation, but without extensive ulcers (entry CAI, 15, while on conventional medication). These cases are likely to have a long history of exposure to multiple conventional drugs. However, no patient with the entry colonoscopy features seen in (Figure 2) did show any significant fall in CAI score.
Clinical and colonoscopic features of patients who are most likely to respond to GMA
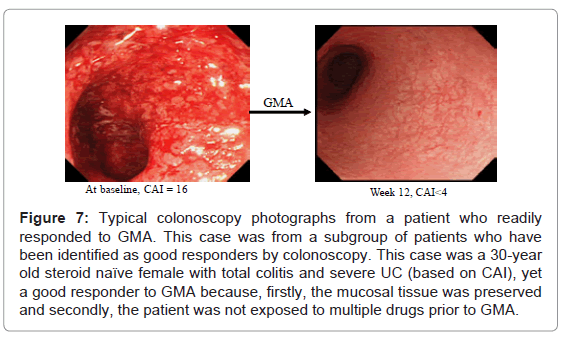
As stated above, drug naïve patients without deep lesions, usually first episode cases are the best responders to GMA, respond soon after a few GMA sessions and can be spared from multiple drug therapy. Typical colonoscopic features in these patients are seen in (Figure 7). Accordingly, GMA should have maximum therapeutic impact if applied immediately after a flare up, and be most effective in first episode cases.
Figure 7: Typical colonoscopy photographs from a patient who readily responded to GMA. This case was from a subgroup of patients who have been identified as good responders by colonoscopy. This case was a 30-year old steroid naïve female with total colitis and severe UC (based on CAI), yet a good responder to GMA because, firstly, the mucosal tissue was preserved and secondly, the patient was not exposed to multiple drugs prior to GMA.
Conclusion
IBD, in particular UC is a very debilitating health disorder. UC patients present with diverse clinical and endoscopic disease severity levels, and therefore, their clinical response to medical interventions can be complete remission, partial response or no response. Further, patients with UC have activated myeloid lineage leucocytes, which infiltrate the mucosa in vast numbers, potentially a pathologic factor. Accordingly, selective depletion of myeloid leucocytes by GMA should have therapeutic effect in patients with UC. In spite of this view, efficacy outcomes from cohorts who received GMA are both encouraging as well as disappointing, reflecting different demographic variables marking patients as responders or otherwise as unresponders. By acquiring experience over a decade in patients with UC, we have learnt that all patients with the first UC episode and short duration of disease readily respond to GMA and can be spared from multiple drug therapy. Similarly, most steroid naïve or dependent patients who have a fair level of intact mucosal tissue are potential responders to GMA. Patients with extensive loss of the mucosal tissue and those with a long history of exposure to multiple drugs like corticosteroids are unlikely to respond to GMA. Further, one of the most favoured features of Adacolumn GMA is its safety profile. Serious side effects are very rare. This is in sharp contrast to multiple severe side effects associated with most conventional pharmacologics and new biologics. Our view is that in patients with UC, there is an evolving scope for therapeutic opportunity based on taking away the sources of inflammatory cytokines.
Acknowledgements
The authors received no external funding in connection with the work described in this manuscript. Likewise, the authors have no conflict of interest in relation to the publication of this work.
References
- Selby W (1997) The natural history of ulcerative colitis. Baillieres Clin Gastroenterol 11: 53-64.
- Allison MC, Dhillon AP, Lewis WG, Pounder RE (1998) Inflammatory Bowel Disease. London: Mosby.
- Saniabadi AR, Hanai H, Fukunaga K, Sawada K, Bjarnason I, et al (2007) Therapeutic leucocytapheresis for inflammatory bowel disease. Transfus Apher Sci 37: 191-200.
- Sartor RB (1997) Pathogenesis and immune mechanisms of chronic inflammatory bowel diseases. Am J Gastroenterol 92 (Suppl): 5S-11S.
- Fiocchi C (1998) Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology 115: 182-205.
- Podolsky DK (2002) Inflammatory bowel disease. N Engl J Med 347: 417-429.
- Taffet SL, Das KM (1983) Sulphasalazine-adverse effects and desensitization. Dig Dis Sci 28: 833-842.
- Present DH (2000) How to do without steroids in inflammatory bowel disease. Inflamm Bowel Dis 6: 48-57.
- Rachmilewitz D (1989) Coated mesalazine (5-aminosalicylic acid) versus sulphasalazine in the treatment of active ulcerative colitis: a randomized trial. BMJ 298: 82-86.
- Kornbluth A, Marion JF, Salomon P, Janowitz HD (1995) How effective is current medical therapy for severe ulcerative colitis? An analytic review of selected trials. J Clin Gastroenterol 20: 280-284.
- Truelove SC, Jewell DP (1974) Intensive intravenous regimens for severe attacks of ulcerative colitis. Lancet 1: 1067-1070.
- Jarnerot G, Rolny P, Sandberg-Gertzen H (1985) Intensive intravenous treatment of ulcerative colitis. Gastroenterology 89: 1005-1013.
- Hanauer SB (2004) Medical therapy for ulcerative colitis 2004. Gastroenterology 126: 1582-1592.
- O'Keefe SJ (1996) Nutrition and gastrointestinal disease. Scand J Gastroenterol 31: 52-59.
- Kornbluth A, Marion JF, Salomon P, Janowitz HD (1995) How effective is current medical therapy for severe ulcerative and crohn's colitis? An analytic review of selected trials. J Clin Gastroenterol 20: 280-284.
- Hyde GM, Thillainayagam AV, Jewell DP (1998) Intravenous cyclosporin as rescue therapy in severe ulcerative colitis: time for a reappraisal? Eur J Gastroenterol Hepatol 10: 411-413.
- Hanauer SB (2001) Can Cyclosporine go it alone in severe ulcerative colitis. Curr Gastroenterol Rep 3: 455-456.
- Serkova NJ, Christians U, Benet LZ (2004) Biochemical mechanisms of cyclosporine neurotoxicity. Mol Interv 4: 97-107.
- Tanaka T, Okanobu H, Kuga Y, Yoshifuku Y, Fujino H, et al. (2010) Clinical and endoscopic features of responders and non-responders to adsorptive leucocytapheresis: a report based on 120 patients with active ulcerative colitis. Gastroenterol Clin Biol 34: 687-695.
- Saniabadi AR, Hanai H (2010) Therapeutic apheresis from the early civilizations to the twenty-first century. Gastroenterol Clin Biol 34: 645-648.
- Papadakis KA, Targan SR (2000) Role of cytokines in the pathogenesis of inflammatory bowel disease. Annu Rev Med 51: 289-298.
- Rutgeerts P, Sandborn WJ, Feagan BG, Reinisch W, Olson A, et al. (2005) Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med 353: 2462-2476.
- van Dullemen HM, van Deventer SJ, Hommes DW, Bijl HA, Jansen J, et al. (1995) Treatment of Crohn's disease with anti-tumour necrosis factor chimeric monoclonal antibody (cA2). Gastroenterology 109: 129-135.
- Brown SL, Greene MH, Gershon SK, Edwards ET, Braun MM (2002) Tumor necrosis factor antagonist therapy and lymphoma development: twenty-six cases reported to the Food and Drug Administration. Arthritis Rheum 46: 3151- 3158.
- Atzeni A, Ardizzone S, Sarzi-Puttini P, Colombo E, Maconi G, et al. (2005) Autoantibody profile during short-term infliximab treatment for Crohn's disease: a prospective cohort study. Aliment Pharmacol Ther 22: 453-461.
- McCarthy DA, Rampton DS, Liu YC (1991) Peripheral blood neutrophils in inflammatory bowel disease: morphological evidence of in vivo activation in active disease. Clin Exp Immunol 86: 489-493.
- Heimann TM, Aufses AH Jr (1985) The role of peripheral lymphocytes in the prediction of recurrence in Crohn's disease. Surg Gynecol Obstet 160: 295- 298.
- Suzuki Y, Yoshimura N, Saniabadi AR, Saito Y (2004) Selective neutrophil and monocyte adsorptive apheresis as a first line treatment for steroid naïve patients with active ulcerative colitis: a prospective uncontrolled study. Dig Dis Sci 49: 565-571.
- Aoki H, Nakamura K, Yoshimatsu Y, Tsuda Y, Suzuki Y, et al. (2007) Adacolumn selective leukocyte adsorption apheresis in patients with active ulcerative colitis: clinical efficacy, effects on plasma IL-8 and the expression of toll like receptor 2 on granulocytes. Dig Dis Sci 52: 1427-1433.
- Tanaka T, Okanobu H, Yoshimi S, Murakami E, Kogame A, et al. (2008) In patients with ulcerative colitis, adsorptive depletion of granulocytes and monocytes impacts mucosal level of neutrophils and clinically is most effective in steroid naive patients. Dig Liver Dis 40: 731-736.
- Tibble JA, Sigthorsson G, Bridger D, Fagerhol MK, Bjarnason I (2000) Surrogate markers of intestinal inflammation are predictive of relapse in patients with inflammatory bowel disease. Gastroenterology 119: 15-22.
- Schreiber S, Nikolaus S, Hampe J, Hämling J, Koop I, et al. (1999) Tumour necrosis factor alpha and interleukin 1beta in relapse of Crohn's disease. Lancet 353: 459-461.
- Brannigan AE, O'Connell PR, Hurley H O'Neill A, Brady HR, et al. (2000) Neutrophil apoptosis is delayed in patients with inflammatory bowel disease. Shock 13: 361-366.
- Lee A, Whyte M, Haslett C (1993) Inhibition of apoptosis and prolongation of neutrophil functional longevity by inflammatory mediators. J Leukoc Biol 54: 283-288.
- Meagher LC, Cousin JM, Seckl JR, Haslett C (1996) Opposing effects of glucocorticoids on the rate of appoptosis in neutrophilic and eosinophilic granulocytes. J Immunol 156: 4422-4428.
- Belge KU, Dayyani F, Horelt A, Siedlar M, Frankenberger M, et al. (2002) The proinflammatory CD14+CD16+ monocytes are a major source of TNF. J Immunol 168: 3536-3542.
- Hanai H, Iida T, Takeuchi K, Watanabe F, Yamada M, et al. (2008) Adsorptive depletion of elevated proinflammatory CD14+CD16+DR++ monocytes in patients with inflammatory bowel disease. Am J Gastroenterol 103: 1210-1216.
- Cohen RD (2005) Treating ulcerative colitis without medications - "Look Mom, No Drugs!" Gastroenterology 128: 235-236.
- Sands BE, Sandborn WJ, Feagan B, Löfberg R, Hibi T et al. (2008) A randomized, double-blind, sham-controlled study of granulocyte/monocyte apheresis for active ulcerative colitis. Gastroenterology 135: 400-409.
- Powrie F, Read S, Mottet C, Uhlig H, Maloy K (2003) Control of immune pathology by regulatory T cells. Novartis Found Symp 252: 92-98.
- Maul J, Loddenkemper C, Mundt P, Berg E, Giese T, et al. (2005) Peripheral and intestinal regulatory CD4+CD25(high) T cells in inflammatory bowel disease. Gastroenterology 128: 1868-1878.
- Uhlig HH, Powrie F (2005) The role of mucosal T lymphocytes in regulating intestinal inflammation. Springer Semin Immunopathol 27: 167-180.
- Liu H, Hu B, Xu D, Liew FY (2003) CD4+CD25+ regulatory T cells cure murine colitis: the role of IL-10, TGF-beta, and CTLA4. J Immunol 171: 5012-5017.
- Huber S, Schramm C, Lehr HA, Mann A, Schmitt S, (2004) Cutting edge: TGFbeta signaling is required for the in vivo expansion and immunosuppressive capacity of regulatory CD4+CD25+ T cells. J Immunol 173: 6526-6531.
- Yokoyama Y, Fukunaga K, Fukuda Y, Tozawa K, Kamikozuru K, et al. (2007) Demonstration of Low-Regulatory CD25(High+)CD4(+) and high-proinflammatory CD28(-)CD4(+) T-cell subsets in patients with ulcerative colitis: modified by selective granulocyte and monocyte adsorption apheresis. Dig Dis Sci 52: 2725-2731.
- Saniabadi AR, Hanai H, Takeuchi K, Bjarnason I, Lofberg R (2003) Adacolumn, an adsorptive carrier based granulocyte and monocyte apheresis device for the treatment of inflammatory and refractory diseases associated with leukocytes. Ther Apher Dial 7: 48-59.
- Saniabadi AR, Hanai H, Suzuki Y, Ohmori T, Sawada K, et al. (2005) Adacolumn for selective leukocytapheresis as a non-pharmacological treatment for patients with disorders of the immune system: an adjunct or an alternative to drug therapy? J Clin Apher 20: 171-184.
- D'Arrigo C, Candal-Couto JJ, Greer M, Veale DJ, Woof JM (1993) Human neutrophil Fc receptor-mediated adhesion under flow: a hallow fibre model of intravascular arrest. Clin Exp Immunol 100: 173-179.
- Hiraishi K, Takeda Y, Shiobara N, Shibusawa H, Jimma F, et al. (2003) Studies on the mechanisms of leukocyte adhesion to cellulose acetate beads: an in vitro model to assess the efficacy of cellulose acetate carrier-based granulocyte and monocyte adsorptive apheresis. Ther Apher Dial 7: 334-340.
- Hanai H, Takeda Y, Eberhardson M, Gruber R, Saniabadi AR, et al. (2011) The mode of actions of the Adacolumn therapeutic leucocytapheresis in patients with inflammatory bowel disease: a concise review. Clin Exp Immunol 163: 50-58.
- Hanai H, Watanabe F, Takeuchi K, Iida T, Yamada M, et al. (2003) Leukcocyte adsorptive apheresis for the treatment of active ulcerative colitis: a prospective, uncontrolled, pilot study. Clin Gastroenterol Hepatol 1: 28-35.
- Suzuki Y, Yoshimura N, Fukuda K, Shirai K, Saito Y, et al. (2006) A retrospective search for predictors of clinical response to selective granulocyte and monocyte apheresis in patients with ulcerative colitis. Dig Dis Sci 51: 2031-2038.
Relevant Topics
- Constipation
- Digestive Enzymes
- Endoscopy
- Epigastric Pain
- Gall Bladder
- Gastric Cancer
- Gastrointestinal Bleeding
- Gastrointestinal Hormones
- Gastrointestinal Infections
- Gastrointestinal Inflammation
- Gastrointestinal Pathology
- Gastrointestinal Pharmacology
- Gastrointestinal Radiology
- Gastrointestinal Surgery
- Gastrointestinal Tuberculosis
- GIST Sarcoma
- Intestinal Blockage
- Pancreas
- Salivary Glands
- Stomach Bloating
- Stomach Cramps
- Stomach Disorders
- Stomach Ulcer
Recommended Journals
Article Tools
Article Usage
- Total views: 19902
- [From(publication date):
December-2011 - Sep 22, 2024] - Breakdown by view type
- HTML page views : 15471
- PDF downloads : 4431