Research Article Open Access
Association of Genetic Variants of VEGF gene with Gastric Carcinoma Risk
| Hong-Zhen Xia1, Qiang Wu1, Wei-Dong Du1,2*, Yi Liu3, Gang Chen4, Feng Yang1, Fu-Sheng Zhou4, Xian-Fa Tang4, Hua-Yang Tang4, Jian Ruan2, Yi-Lin Chen4, Liang-Dan Sun4 and Xue-Jun Zhang4 | |
| 1Department of Pathology, Anhui Medical University, Hefei, Anhui 230032,China | |
| 2Key Lab of Genome Research of Anhui Province, Anhui Medical University, Hefei, Anhui 230032, China | |
| 3Department of Surgery, The First Affiliated Hospital of Anhui Medical University, Hefei, Anhui 230032, China | |
| 4Key Laboratory of Dermatology, Anhui Medical University, Ministry of Education, China, Hefei, Anhui 230032, China | |
| Corresponding Author : | Dr. Wei-Dong Du Department of Pathology Anhui Medical University Meishan Road 81, Hefei Anhui 230032, China Tel: +86551 516 1002 Fax: +86 551 516 1016 E-mail: weidongdu@hotmail.com |
| Received September 26, 2010; Accepted November 11, 2011; Published November 22, 2011 | |
| Citation: Xia HZ, Wu Q, Du WD, Liu Y, Chen G, et al. (2011) Association of Genetic Variants of VEGF gene with Gastric Carcinoma Risk. J Biochip Tissue chip S1:005. doi:10.4172/2153-0777.S1-005 | |
| Copyright: © 2011 Xia HZ, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Bioengineering and Bioelectronics
Abstract
To date, few association analyses with regard to gene variants and expression of vascular endothelial growth factor (VEGF) in gastric carcinoma (GC) have been reported. We hypothesized the variants might also affect susceptibility to GC. To evaluate the correlation of VEGF variants with risk and clinicopathological characteristics of this disease in China, we detected fourteen single nucleotide polymorphisms (SNPs) of VEGF gene in 311 patients with gastric carcinoma and 425 age and gender-matched controls by using Sequenom iplex. We investigated expression of VEGF in combination with cyclooxygenase-2 (COX-2) in 238 tissues samples of the cases by tissue microarray (TMA) and immunohistochemistry (IHC). There were no significant differences in genotype, allele and haplotype distributions of the 14 VEGF SNPs between the cases and the controls. However, we found that there were significant clinicopathological correlations of the rs3024994C/T with gender, the rs3025021C/T with tumor size, the rs3025039C/T with tobacco smoking, the rs3025030G/C with tumor location, tobacco smoking and alcohol drinking (P<0.05, respectively). A/A genotype (P=0.050, OR=0.39, 95%CI=0.15-1.00) and A allele (P=0.024, OR=0.64, 95%CI=0.43-0.94) of the rs833052 significantly reduced the VEGF expression and T allele of the rs3025007 increased the VEGF expression (P=0.017, OR=1.70, 95%CI=1.10-2.61) when compared to the C allele of both the variants, respectively. The VEGF expression displayed a significant association with COX-2 (rs= 0.178, P=0.006). We concluded that none of the 14 SNPs of VEGF gene was significantly associated with the susceptibility to GC. Both VEGF and COX-2 showed close correlations with invasion and progression in advanced GC.
| Keywords |
| Gastric carcinoma (GC); Vascular endothelial growth factor(VEGF); Single nucleotide polymorphism (SNP) |
| Abbreviations |
| VEGF: Vascular Endothelial Growth Factor; GC: Gastric Carcinoma; SNPs: Single Nucleotide Polymorphisms; COX-2: Cyclooxygenase-2; TMA: Tissue Microarray; IHC: Immunohistochemistry; Ors: Odds ratios; CIs: Confidence Intervals |
| Introduction |
| Angiogenesis, denoting the formation of new capillaries from existing blood vessels, is an essential process for the growth, development and metastasis of solid tumors, including gastric carcinoma (GC) [1-4]. Vascular endothelial growth factor (VEGF, OMIM +192240) is one of the important angiogenic factors and a potent endothelial cell-specific mitogen, which acts as a modulator of changes in vascular permeability [3]. The gene encoding VEGF is located at chromosome 6p12-21 and comprises eight exons separated by seven introns. The correlations of either VEGF gene or its protein to gastric carcinoma have been investigated [5,6]. Experiments in vitro and in vivo have shown that increased VEGF expression is associated with tumor growth and metastasis [2,5]. VEGF mRNA and VEGF expression are found to differentially differ in intestinal metaplasia, dysplasia [7,8] and gastric carcinomas [9], revealing a trend of increasing immunohistochemical staining density during the progress from intestinal metaplasia, dysplasia to carcinomas. However, precise role of VEGF expression in tissues in association with biological characteristics of GC remains to be argued. Kösem et al found that the expression of VEGF gene was selectively correlated with the degree of differentiation and lymphatic metastasis, but not with depth of cancer invasion, size of tumor, age or sex [2]. Instead, the data from Japanese population indicated that VEGF expression was correlated with depth of invasion and metastatic lymph node [5]. It is acceptable that VEGF is proposed as a prognostic marker in GC [5]. Blood or tissue VEGF levels could reflect response to therapy and disease progression [10,11]. Inhibition of VEGF expression results in suppression of tumor growth and tumor induced neoangiogenesis [12]. Significant benefits from VEGF targeted therapy for GC are achieved in clinical trials recently, which open a potential application prospect in treatment of the entity [13]. Previous study indicated that genetic polymorphisms and environmental risk factors may play a concurrent role in the development of GC [14]. The human VEGF gene is highly polymorphic; more than 20 single nucleotide polymorphisms (SNPs) have been identified [15-21]. It has been reported that a few of the VEGF gene polymorphisms would be involved in the development of GC and other solid tumors[16,17,20,21], of which the +405 polymorphism in the VEGF gene affected the expression of the gene [22]. Four potentially functional variants: -2578C/A (rs699947), -460C/T (rs833061), +405C/G (rs2010963), and +936C/T (rs3025039), were associated with the production levels of VEGF [23,24] and usually studied in the risk of GC [16,17,20]. However, few studies demonstrated that roles of VEGF SNPs in risk predisposition of GC, in particular, limited recruited patients from various ethnic populations in these studies were analyzed to validate the prognostic value of the VEGF gene polymorphisms [15,20,25,26]. To better understand the role of VEGF gene variants in tumor angiogenesis and correlation between tumor biological characteristics, an overall association analysis between VEGF variants and GC risk needs to be performed further. It is reasonable to hypothesize that VEGF might be a good candidate for determining the risk of developing this entity. Therefore, we assumed that there might be an association between genetic variants and expression of VEGF, and both of them might correlate with GC susceptibility or biological behavior. To reach this hypothesis, we performed genotyping analyses for 14 SNPs of VEGF gene in a case-control study of 311 GC and 425 age and gender-matched controls of China and investigated the VEGF expression by tissue microarray (TMA) and immunohistochemistry (IHC) in cases. Furthermore, we also addressed the expression of cyclooxygenase-2 (COX-2) for its possible correlation with VEGF [5]. |
| Materials and Methods |
| Study population |
| Three hundred and eleven cases with GC and 425 cancer-free controls were investigated in this study. Patients with GC were collected from the First Affiliated Hospital of Anhui Medical University between March 2008 and July 2009 and received no chemotherapy or radiotherapy before surgical gastrectomy. The patients were comprised of 246 men and 65 women with an average age of 60.4±10.4 years (range 24 to 83 years). Of which, 238 cases had whole clinical pathological data, including 119 men and 47 women with an average age of 61.0±9.4 years (range 26 to 83 years). Another 30 samples of adjacent noncancerous tissues from the patients were used as IHC controls. Clinical diagnosis and staging of GC were assessed by two pathologists according to the World Health Organization classifications and tumor-node-metastasis (TNM) classifications issued in 2006. Unrelated matched donors were enrolled from the same area, including 336 men and 89 women with an average age of 60.6±8.4 years (range 30 to 86 years). Informed consents were obtained from all the participants; this study was approved by the ethics committee for genome research of the Anhui Medical University of China. |
| Extraction of peripheral blood DNA |
| Blood sample was collected from each subject in EDTA and stored at -80oC until analysis. Genomic DNA was extracted from peripheral blood by using QIAamp DNA Blood Midi Kit (Qiagen Inc., Germany) according to the manufacturer’s protocol. The quantitative concentration of DNA was measured by the Nanodrop Spectrophotometer (ND-1000, USA) of full wavelength and standardized to 50ng/μl. |
| SNP selection and sequenom assay |
| We selected 14 SNPs of VEGF gene according to previous research [18].Genotyping analysis of the SNPs for fast-track validation analysis was performed using the Sequenom Mass Array system. Fifteen ng of genomic DNA was standardized for genotyping of each sample. Locusspecific PCR and detection primers were designed using the Mass ARRAY Assay Design 3.0 software (Sequenom, San Diego, California, USA) following the manufacturer’s instructions. The DNA samples were amplified by multiplex PCR reactions. The PCR products were then used for locus-specific single-base extension reactions. The resulting products were desalted and transferred to a 384-element SpectroCHIP array. Allele detection was performed using MALDI-TOF MS. The mass spectrograms were analyzed by the Mass ARRAY Typer software (Sequenom). |
| TMA block preparation |
| Typical areas of cancers on HE-stained section slides were selected under microscope. Circles were drawn on the slides around the representative areas of the section. Using the slides as guides, core samples (0.6 mm in diameter) were punched out from each corresponding paraffin-embedded block using a tissue microarray tool. Duplicated cores were applied in order to avoid shedding. Fifty-four cores were embedded in a TMA block with 8x7 alignments. Thus, a total of nine TMA blocks were produced creating a panel of TMA for the 238 tumor samples. |
| Immunohistochemical staining (IHC) |
| Histological sections (4μm) from the 9 TMA blocks were used for the study. The TMA sections were deparaffinized in xylene and rehydrated through descending concentrations of ethanol. Antigen retrieval was achieved by microwave treatment in 0.01 mol/L citrate buffer (pH 6.0) for 2 min, followed by cooling for 2 h. After washing in phosphatebuffered saline (PBS) and exposure to normal goat serum to reduce non-specific binding, the slides were incubated with rabbit monoclonal antibody against human of VEGF and COX-2 (Zymed, USA, clone ID: SP28 and SP21), respectively, at 4°C overnight. After PBS washing, slides were then incubated with substrate diaminobenzidine and hydrogen peroxide for 10 min. Finally, the sections were counterstained with hematoxylin. All reagents purchased from Beijing Zhongshan Biotechnology Co., Ltd. (Beijing, P.R. China). |
| Evaluation IHC staining |
| Immunoreactivity was evaluated according to both of the proportion of stained cells and their intensity. Staining intensity was scored as 0=negative, 1=weak, 2=medium, and 3=strong. Extent of staining was scored semiquantitatively as follows: 0=negative; 1=1- 25% of cells; 2=26-50% of cells; 3=51-75% of cells; and 4=76-100% of cells were stained. The sum of the intensity and extent score was used as the final staining score. A score of ≥4 was considered as a positive expression. |
| Data analysis |
| The differences in frequency distributions of genotypes and alleles of VEGF between the cases and the controls were tested using χ2 test. Both age and sex-adjusted odds ratios (ORs) and 95% confidence intervals (95%CIs) were calculated using logistic regression analysis. The correlation between the clinicopathological parameters and protein expression of VEGF and COX-2 and SNPs of VEGF was analyzed using χ2 test and further logistic regression analysis. The Hardy-Weinberg equilibrium, linkage disequilibria (LD) and the haplotype analysis were calculated with SHEsis, available online http://analysis.bio-x.cn [27], Shanghai, China. |
| Results |
| VEGF polymorphisms and risk of GC |
| We investigated 14 VEGF SNPs in this study: rs1547651A/T, rs699947C/A, rs2010963G/C, rs1005230C/T, rs735286G/A, rs833052C/A, rs833068G/A, rs833070G/A, rs3024994C/T, rs3025007C/T, rs3025021C/T, rs3025030G/C, rs3025035C/T, and rs3025039C/T, all of which were coherent with the assumption of Hardy Weinberg equilibrium (P>0.01, data not shown). The distributions of genotypes and alleles in this study were similar to those reported by other Chinese groups. Genotype and allele distributions of the fourteen VEGF SNPs were summarized Table 1. There were no significant differences of genotype and allele distributions of all the VEGF SNPs between the cases and the controls. However, a borderline genotype distributions between the patients group and the controls in the rs3025039C/T was observed (P=0.064). Further logistic regression analysis revealed that the T/T homozygous genotype of the rs3025039 tended to increase the disease’s risk (P=0.060, adjusted OR=2.61, 95%CI=0.96-7.10). |
| VEGF haplotypes and risk of GC |
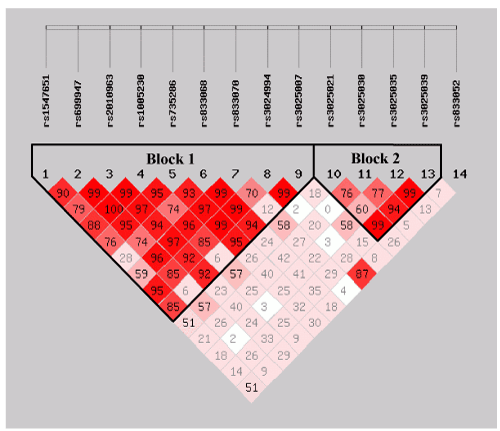
| We estimated the LDs and haplotypes based on the 14 VEGF SNPs. As a result, the two haplotypes were produced according to the scoring of bolds in (Figure 1) the block 1 included 9 SNPs and the block 2 consisted of 4 variant alleles, all minor allele frequencies were all higher than 5%. However, the frequency distributions of these haplotypes between the cases and the controls were not significantly different (P>0.05, Table 2), indicated that they were not associated with risk of GC. |
| Correlation of VEGF variants, VEGF expression and clinicopathological characteristics of GC |
| The tumor prognostic factors were analyzed in the 238 patients who had resected tumor tissues available for IHC. We compared the relationship of VEGF polymorphisms, VEGF expression in tumor tissues and risk factors of GC (Supplementary Table 1). The results indicated that there were significant correlations between the rs3024994C/T genotype and male (P=0.009, OR=0.35, 95%CI=0.16- 0.76), the rs3025021C/T and tumor size (P=0.025), the rs3025039CT genotype and tobacco smoking (P=0.026, OR=1.82, 95%CI=1.07- 3.09), the rs3025030G/C and tumor location (P=0.023), the CG genotype of rs3025030G/C and both tobacco smoking (P=0.001, OR=2.45, 95%CI=1.45-4.14) and alcohol drinking (P=0.006, OR=2.04, 95%CI= 1.22-3.39), respectively. As shown in Table 3, several VEGF polymorphisms significantly affected the VEGF expression. When compared with the C/C genotype, the rs833052 A/A genotype reduced the VEGF protein expression (P=0.050, OR=0.39, 95%CI=0.15-1.00). Further alleles analysis suggested the alleles of the rs833052 and rs3025007 correlated VEGF expression significantly. Allele A of the rs833052 significantly decreased (P=0.024, OR=0.64, 95%CI=0.43- 0.94) and allele T of the rs3025007 increased the VEGF expression (P=0.017, OR=1.70, 95%CI=1.10-2.61), respectively, when compared to the C allele (data not shown). |
| Association of Expression of VEGF and COX-2 in GC |
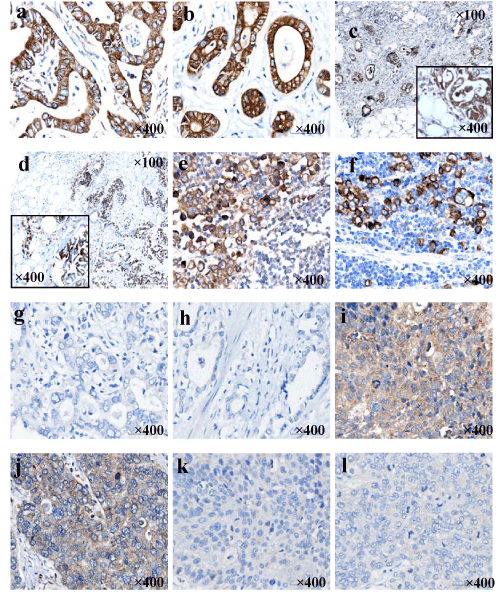
| We detected expression of VEGF and COX-2 in GC by means of TMA and IHC. The specific location of immunostaining of VEGF was observed in cytoplasmic, while COX-2 was mainly expressed in cytoplasmic and/or membranous. Positive immunoreactivities of both VEGF and COX-2 were primarily found in adenocarcinomas (Figure 2a and b) and invasive adenocarcinoma cells in the serosa (Figure 2c and d) and in the metastatic lymph nodes (Figure 2e and f). However, part of adenocarcinomas did not express the proteins of VEGF and COX-2 (Figure 2g and h). Additionally, VEGF appeared weakly positive in all 3 squamous cell carcinomas (Figure 2i) and one of 2 neuroendocrine carcinomas tested (Figure 2j). Differently, COX-2 negative expression occurred in squamous-cell carcinomas (Figure 2k) and neuroendocrine cancers (Figure 2l). The frequencies of VEGF in the primary tumor and noncancerous tissue were 62.6% and 26.7% (P=0.000), respectively. Similarly, the COX-2 immunoreactions was significantly more predominant in the GC (61.8%) than in the noncancerous mucosa (36.7%, P=0.008). As showed in Table 4, protein expression of VEGF and COX2 significantly correlated with tumor size (P=0.013 and P=0.050), serosa invasion (P=0.023 and P=0.001), clinical stage 10 (P=0.001 with only VEGF), TNM stage (P=0.014 and P=0.000), and lymph node metastasis (P=0.014 and P=0.002). In addition, The VEGF expression displayed a significant association with COX-2 (P=0.006) with an acceptable rs of 0.178. |
| Discussion |
| Increasing evidences demonstrate that genetic variations or disease susceptibility may play a crucial role in GC pathogenesis [15,20,25,26].Several studies have investigated the associations of VEGF polymorphisms with risk of GC [15,17,19,20], but the conclusions remains quite controversial. A case-control study revealed that the heterozygous-634CG (rs2010963) and the combined -634C/ G+C/C carriers increased the risk of GC in a USA population [16]. Bae et al. found the VEGF +936C/T polymorphism, especially the T allele, was susceptible to GC in Korean population [19]. Instead, in a Chinese cohort study [15], none of the four potentially functional SNPs (-2578C/A, -1498T/C, -634G/C, and +936C/T) of the VEGF gene or their haplotypes achieved a significant difference in their distributions between GC cases and controls. A report from Omani population indicated that the three VEGF polymorphisms (+405 G/C, -460 T/C, and +936 C/T) were rather prognostic values than risk predispositions of GC [20]. In this study, our data revealed neither significant association between the risk of GC and the three VEGF SNPs, -2578C/ A (rs699947), +405C/G (rs2010963) and +936C/T (rs3025039), nor correlation between the VEGF protein expression or the tumor biological characteristics and these VEGF variants. However, we found that the homozygous rs3025039TT genotype seemed to have a trend to increase the risk of GC (P=0.060, adjusted OR=2.61, 95%CI=0.96- 7.10) compared to the CC genotype. In addition, we found a significant relation between the rs3025039 CT genotype and tobacco smoking (P=0.026, OR=1.82, 95%CI=1.07-3.09). In view of the facts that the rs3025039 T/T genotype was associated with increase of the plasma levels of VEGF [28] and smoking brought high risk to patients with GC [29], therefore, we assumed that this genotype might be a potential risk factor of GC, at least in Anhui province of China. Interestingly, although the two VEGF SNPs, rs3025007 and rs833052, presented no obvious association to either GC risk or clinicopathological connection, they were correlated with VEGF expression: T allele of the rs3025007 significantly increased the VEGF expression (P=0.017, OR=1.70, 95%CI= 1.10-2.61) compared to the C allele, which may suggest that T allele of the rs3025007 affect the progression of this disease; Whereas the rs833052 significantly reduced the VEGF protein by both AA genotype and A allele (P=0.050, OR=0.39, 95%CI=0.15- 1.00; P=0.024, OR=0.64, 95%CI =0.43-0.94, respectively). We assumed that this SNP might be a protective index to GC. However, the same genotype to increase the risk of bladder cancer was reported in the Spanish population (P=0.036, OR=2.52, 95%CI=1.06-5.97), the reason remained unclear [18]. Besides, we found significant correlations between the rs3024994CT genotype and male (P=0.009, OR=0.35, 95%CI=0.16-0.76), and the rs3025021C/T polymorphism and tumor size (P=0.025), separately. Moreover, there were significant correlations between the rs3025030G/C polymorphisms and tumor location (P=0.031), the CG genotype and tobacco smoking (P=0.001, OR=2.45, 95%CI=1.45-4.14) and alcohol drinking (P=0.006, OR=2.04, 95%CI =1.22-3.39), respectively, indicating that VEGF variants might result in altered tumor progression and aggravation of biological behaviors of GC. To further address potential combined effects of VEGF SNPs, we analyzed haplotypes of VEGF variants. We finally confirmed two groups of haplotypes from the 14 SNPs. We found neither of the haplotypes any significant susceptibility to GC. Only the TAGTGAGCC of haplotype 1 tended to being a risk combination to GC in this population (P=0.090, OR=1.77, 95%CI=0.91-3.45). Furthermore, our results confirmed these conclusions that neither the haplotypes of the rs699947, rs2010963, rs833061 and rs3025039 in Chinese population [15], nor those of the rs2010963, rs833061 and rs3025039 in the USA [17] or Omani [20] populations were associated with the risk of GC. VEGF, as a key mediator of angiogenesis, may be involved in multiple important cellular processes, triggering tumor angiogenesis. VEGF SNPs may effect on clinical outcomes of patients with GC [30]. In this study, we tested 238 samples of GC in Anhui province of China by IHC. Our data showed that VEGF positive expression was significantly correlated with the progressive characteristics of tumor, such as size of >5cm (69.8%, P=0.013), serosa invasion(66.7%, P=0.023) , aggressive stage (65.6%, P=0.001) , III+IV of TNM stage (69.4%, P=0.014) and local lymph node metastasis (68.4%, P=0.014), indicating that VEGF played a crucial important role in aggravation and metastasis of GC and would be used a valuable predictive and prognostic marker for the disease. COX- 2 unregulated matrix metalloproteins and inhibited antiangiogenic cytokine interleukin-12 [31]. Expression of COX-2 in tumors stimulates angiogenesis by promoting the production of VEGF [32,33] and products of arachidonic acid, enabling tumor vascular invasion and progression. However, the association of COX-2 and VEGF expression in GC tissues quite differed [5,31]. In our study, we found that VEGF variants did not effect expression of COX-2 in tumor tissues. COX-2 immunoreaction was significantly more predominant in the GC than in the normal mucosa. The COX-2 expression was significantly correlated with the serosa invasion, the metastasis in the local lymph nodes and the TNM stage. Moreover, our results showed a tight correlation between the immunohistochemical expressions of VEGF and COX-2 in GC (rs = 0.178, P =0.006), consistent with the previous study [5]. The positive VEGF carcinomas have associated COX-2 immunoreactivity in 42.8% of the cases. Interaction combination of VEGF and COX-2 would promote progression of GC. |
| References |
|
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 | Table 4 |
Figures at a glance
 |
 |
| Figure 1 | Figure 2 |
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 13626
- [From(publication date):
specialissue-2011 - Sep 23, 2024] - Breakdown by view type
- HTML page views : 9187
- PDF downloads : 4439
