Research Article Open Access
Endoscopic Findings during the Early Induction Phase of Infliximab Therapy may Predict its Efficacy for Refractory Ulcerative Colitis
Masayuki Saruta1*, Nobuhiko Komoike1, Yoshinori Arai1, Daisuke Ide1, Tetsuyoshi Iwasaki1, Ryoichi Sawada1, Seiji Arihiro1, Mika Matsuoka1, Tomohiro Kato2and Hisao Tajiri1,21Division of Gastroenterology and Hepatology, Department of Internal Medicine, Japan
2Department of Endoscopy, The Jikei University School of Medicine, Tokyo, Japan
- *Corresponding Author:
- Masayuki Saruta
Division of Gastroenterology and Hepatology
Department of Internal Medicine
The Jikei University School of Medicine
3-25-8 Nishi-shinbashi, Minato-ku
Tokyo 105-0003, Japan
Tel: +81-3-3433-1111
E-mail: m-saruta@pg7.so-net.ne.jp
Received date: July 25, 2015, Accepted date: August 17, 2015, Published date: August 24, 2015
Citation: Saruta M, Komoike N, Arai Y, Ide D, Iwasaki T, et al. (2015) Endoscopic Findings during the Early Induction Phase of Infliximab Therapy may Predict its Efficacy for Refractory Ulcerative Colitis. J Gastrointest Dig Syst 5:324. doi:10.4172/2161-069X.1000324
Copyright: © 2015 Saruta M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Gastrointestinal & Digestive System
Abstract
Background and Aims: Infliximab (IFX) is one of the most potent and effective treatments for steroid- or immunomodulator-refractory ulcerative colitis (UC). We evaluated the early efficacy of IFX, based on endoscopic findings, and also attempted to define endoscopic findings predictive of IFX efficacy. Methods: Nine patients were treated with IFX induction therapy at weeks 0, 2, and 6. Early efficacy was evaluated, using endoscopic and clinical findings, at week 1 (n=9) and again at weeks 3 (n=3) or 7 (n=4). Efficacy was evaluated using the Mayo, Schroeder, and Rachmilewitz endoscopic (RES) scores. Results: At week 1, 8 of 9 (89%) patients showed a clinical response, and 11% (1 of 9) experienced clinical remission. The mean Mayo score was significantly decreased at week 1 (10 ± 1.2 at baseline vs. 5.6 ± 1.9 at week 1, p<0.001). By week 7, 63% of patients (5 of 8) achieved clinical remission and mucosal healing. We used the RESs at week 1 to evaluate the endoscopic findings and to detect marker(s) predictive of remission. We found that week 1 endoscopic findings of “vascular pattern,” “vulnerability of mucosa,” and “mucosal damage” were predictive. Additionally, C-reactive protein levels at weeks 1 and 6 were positive (>0.3 mg/dl) in the non-remission group, but were negative in the remission group.
Abstract
Background and Aims: Infliximab (IFX) is one of the most potent and effective treatments for steroid- or immunomodulator-refractory ulcerative colitis (UC). We evaluated the early efficacy of IFX, based on endoscopic findings, and also attempted to define endoscopic findings predictive of IFX efficacy.
Methods: Nine patients were treated with IFX induction therapy at weeks 0, 2, and 6. Early efficacy was evaluated, using endoscopic and clinical findings, at week 1 (n=9) and again at weeks 3 (n=3) or 7 (n=4). Efficacy was evaluated using the Mayo, Schroeder, and Rachmilewitz endoscopic (RES) scores.
Results: At week 1, 8 of 9 (89%) patients showed a clinical response, and 11% (1 of 9) experienced clinical remission. The mean Mayo score was significantly decreased at week 1 (10 ± 1.2 at baseline vs. 5.6 ± 1.9 at week 1, p<0.001). By week 7, 63% of patients (5 of 8) achieved clinical remission and mucosal healing. We used the RESs at week 1 to evaluate the endoscopic findings and to detect marker(s) predictive of remission. We found that week 1 endoscopic findings of “vascular pattern,” “vulnerability of mucosa,” and “mucosal damage” were predictive. Additionally, C-reactive protein levels at weeks 1 and 6 were positive (>0.3 mg/dl) in the non-remission group, but were negative in the remission group.
Conclusions: Week 1 endoscopic findings for “vascular pattern,” “vulnerability of mucosa,” and “mucosal damage” are very important for predicting IFX efficacy.
Keywords
Ulcerative colitis; Infliximab; Mayo score; Rachmilewitz endoscopic score; Schroeder score
Introduction
Crohn’s disease (CD) and ulcerative colitis (UC), collectively termed inflammatory bowel disease (IBD), are chronic relapsing intestinal inflammatory disorders. UC is characterized by the infiltration of the colonic mucosa with activated T-lymphocytes, neutrophils, and plasma cells [1]. Current evidence suggests that apoptosis-resistant activated T-lymphocytes in the intestinal mucosa are central to the pathogenesis of CD [2–4]. In addition, local mononuclear cell production of proinflammatory cytokines, such as interleukin (IL)-1, IL-6, and tumor necrosis factor (TNF)-α, are significantly increased in IBD patients [5–7]. Several IBD treatments, including azathioprine and 6-mercaptopurine; methotrexate; anti-TNF antibodies, such as infliximab (IFX) and adalimumab; and even sulfasalazine have been associated with induction of T-lymphocyte apoptosis in the lamina propria [8–11]. Medical management of UC that is refractory to corticosteroid therapy is limited to cyclosporine [12], IFX [13], and tacrolimus [14]; however, surgery, including total colectomy, is often required.
Two randomized, double-blind, placebo-controlled studies, the Active Ulcerative Colitis Trials 1 and 2 (ACT 1 and ACT 2, respectively), recently evaluated the efficacy of IFX induction and maintenance therapy in adults with UC [13]. In each study, patients with moderate-to-severe active UC, refractory to current medications, received intravenous IFX (5 mg or 10 mg/kg body weight) at weeks 0, 2, and 6, and then every 8 weeks. Patients receiving either 5 mg or 10 mg of IFX demonstrated more significant clinical responses at week 8 than did the placebo group patients (p<0.001 for both comparisons with placebo). In both studies, patients receiving IFX were also more likely to have a clinical response at week 30 (p<0.002 for all comparisons) [13]. In ACT 1, more patients receiving IFX had clinical responses at week 54 than did those receiving placebo (p<0.001 for both comparisons) [13]. IFX therapy also substantially improved the patients’ health-related quality of life (HRQL); maintenance IFX therapy sustained this benefit for 1 year [15]. In both the ACT 1 and ACT 2 studies, endoscopic findings showed improvements at week 8. Further, a recent report indicated that the IFX use prevents colectomy in two-thirds of corticosteroid-refractory UC patients in whom cyclosporine treatment fails [16]. Another study showed that cyclosporine was not more effective than IFX in patients with severe, acute UC refractory to intravenous steroids [17]. Therefore, IFX is considered one of the most potent and effective treatments for refractory UC.
In clinical practice, IFX demonstrates dramatic effects in some patients with UC after only 1 infusion. However, no reports have focused on the early efficacy of IFX infusion during the induction phase, especially efficacy results obtained using endoscopy. Therefore, we evaluated the early efficacy of IFX induction treatment in patients with UC, based on endoscopic findings, and also attempted to determine whether endoscopic findings predicted IFX efficacy.
Materials and Methods
Patients
Patients with moderate-to-severe active UC, refractory to their current medications, were selected for the study. All eligible patients had active disease, with Mayo scores of 6–12 points (range, 0–12, with high scores indicating increased disease severity) (Table 1) and moderate-to-severe active disease on colonoscopy (a Mayo endoscopic subscore [Schroeder score] of at least 2), despite treatment with corticosteroids alone or in combination with immunomodulators (azathioprine or tacrolimus) and medications containing 5-aminosalicylates.
| Stool frequency* 0 = Normal number of stools for this patient 1 = 1 to 2 stools more than normal 2 = 3 to 4 stools more than normal 3 = 5 or more stools more than normal Subscore, 0 to 3 |
| Rectal bleeding+ 0 = No blood seen 1 = Streaks of blood with stool less than half the time 2 = Obvious blood with stool most of the time 3 = Blood alone passes Subscore, 0 to 3 |
| Findings on endoscopy 0 = Normal or inactive disease 1 = Mild disease (erythema, decreased vascular pattern, and mild friability) 2 = Moderate disease (marked erythema, lack of vascular pattern, friability, and erosions) 3 = Severe disease (spontaneous bleeding and ulceration) Subscore, 0 to 3 |
| Physician’s global assessment ¶ 0 = Normal 1 = Mild disease 2 = Moderate disease 3 = Severe disease Subscore, 0 to 3 |
Table 1: Mayo score (17). The Mayo score ranges from 0 to 12, with high scores indicating increased disease severity. These data are presented from Schroeder et al. (18). * Each patient serves as his or her own control to establish the degree of abnormality of stool frequency. + Daily bleeding score represents the most severe bleeding experienced in the day. The physician’s global assessment includes the above 3 criteria, patient’s daily recollection of abdominal discomfort, and general sense of well-being and other observations, e.g. physical findings and patient’s performance status.
Patients were treated with intravenous IFX (5 mg/kg body weight) at weeks 0, 2, and 6. Prior to IFX therapy, all patients were screened using tuberculin skin tests involving a purified protein derivative, chest radiographs, computed tomography (CT), whole blood tuberculosis infection diagnostic tests (QuantiFERON-TB Gold® [Qiagen, Venlo, Netherlands], based on the quantitative measurement of gamma-interferon), and hepatitis B core antibody assays.
Follow-up and safety and efficacy evaluations
Colonoscopy was performed, during the induction phase, on 9 patients with moderate-to-severe UC. We obtained informed consent from each patient prior to each colonoscopy evaluation. Colonoscopy was not performed in the absence of consent or if the patient demonstrated severe, active disease. IFX administration continued every 8 weeks following the induction at weeks 0, 2, and 6. To determine the early IFX efficacy, endoscopic and clinical findings were evaluated at week 1, (1 week after the first infusion), and at weeks 3 (1 week after the second infusion) or 7 (1 week after the third infusion). Overall clinical responses were evaluated using the Mayo score [18]; a clinical response was defined as a total Mayo score decrease of at least 30% (3 points) from baseline, with an accompanying decrease in the subscore for rectal bleeding of at least 1 point or an absolute subscore of 0 or 1. Clinical remission was defined as a total Mayo score of ≤2 points, with no individual subscore exceeding 1 point [18]. Endoscopic findings were evaluated using the Schroeder endoscopic index (Table 2) [19], which is part of the Mayo score. Mucosal healing was defined as an absolute endoscopic subscore of 0 or 1. In addition, detailed endoscopic findings were evaluated using the Rachmilewitz endoscopic score (RES; scale, 0–12, with higher scores indicating increased disease severity) (Table 3) [19] to focus on mucosal changes in greater detail. Mucosal healing was defined as a total RES of ≤4 points.
| Score | Findings of Endoscopy |
| 0 | Normal or inactive disease |
| 1 | Mild disease (erythema, decreased vascular pattern, mild friability) |
| 2 | Moderate disease (marked erythema, lack of vascular pattern, friability, erosions) |
| 3 | Severe disease (spontaneous bleeding, ulceration) |
Table 2: Schroeder endoscopic index (18). Subscore, 0 to 3.
| Granulation scattering reflected light | No | 0 |
| Yes | 2 | |
| Vascular pattern | Normal | 0 |
| Faded/disturbed | 1 | |
| Absent | 2 | |
| Vulnerability of mucosa | None | 0 |
| Contact bleeding | 2 | |
| Spontaneous bleeding | 4 | |
| Mucosal damage (mucus, fibrin, erosion, ulcer) | None | 0 |
| Slight | 2 | |
| Pronounced | 4 |
Table 3: Rachmilewitz Endoscopic Score (19). Rachmilewitz endoscopic score (RES) can range from 0 to 12, with higher scores indicating increased disease severity. Mucosal healing is defined as a total RES of ≤4 points.
All patients received 200 mg of hydrocortisone, by drip infusion, immediately before IFX to prevent infusion reactions. Adverse events and concomitant medications were recorded at each visit, in all cases.
Statistical Analysis
The primary endpoint was mucosal healing and a clinical response or remission at week 1. Secondary endpoints were clinical responses or clinical remission at week 7, and mucosal healing at weeks 3 and 7.
Differences between the mean values for groups were analyzed using Student’s t-test; a p-value < 0.05 was considered significant.
Results
We evaluated 9 patients with moderate-to-severe, active UC that was resistant to prednisolone and/or immunosuppressants. All patients treated with IFX underwent colonoscopy at week 1.
Colonoscopy was again performed on 2 of these patients at week 3, on 1 at week 4 (the colonoscopy was scheduled for week 3, but the patient elected to receive it at week 4), on 3 at week 7, and on 1 patient at both weeks 3 and 7. The patients’ baseline characteristics (Table 4) show a mean baseline Mayo score of 10 points (range, 7–11). One patient (Case 9) discontinued IFX treatment after the first dose because of a persistent high fever.
| Variable | Results |
| Male, n (%) | 7 (78%) |
| Median age at entry, years (range) | 49 (19–65) |
| Median duration of UC, years (range) | 11 (0–40) |
| Disease site: Left-sided | 1 (11%) |
| Pan-colonic | 8 (89%) |
| Mayo score, median (range) | 10 (7–11) |
| Median C-reactive protein concentration (mg/dl) | 3.0 (0.2–8.4) |
| Concomitant medications, n (%) | |
| Corticosteroids (Oral or Enema(1) | 6 (67%) |
| Azathioprine (2) | 2 (22%) |
| Tacrolimus (3) | 2 (22%) |
| Oral 5-aminosalicylates a | 8 (89%) |
| LCAP | 1 (11%) |
| 1 and 2 | 2 (22%) |
| 1 and 3 | 2 (22%) |
Table 4: Baseline characteristics of the 9 ulcerative colitis patients treated with IFX. a Mesalamine and sulfasalazine.
Clinical and endoscopic effectiveness
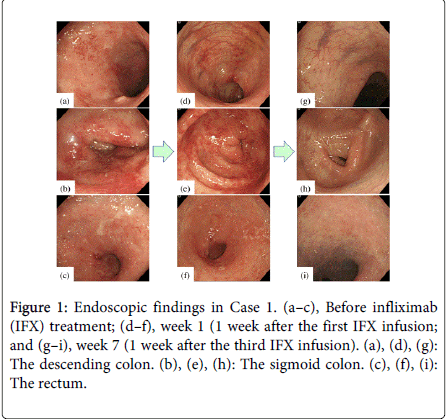
At week 1, 89% of the patients (8 of 9) showed a clinical response, and 11% (1 of 9) experienced clinical remission, based on the Mayo score (Table 5a); the mean Mayo score had markedly decreased by week 1 (10 ± 1.2 at baseline vs. 5.6 ± 1.9 at week 1, p < 0.001). Three of the 9 patients (33%) demonstrated mucosal healing, based on the Schroeder score, at week 1; these 3 patients had endoscopic subscores of 1 (Table 5b). Using the RES criteria, one patient (11%) experienced mucosal healing at week 1, with the scores in the remaining 8 (89%) patients having decreased more than 30% from the baseline (range, 30–63%). The “vascular pattern,” “mucosal vulnerability,” and “mucosal damage,” based on the RES, were markedly improved in this group of 8 patients, but “granulation scattering” persisted at week 1 (Figures 1–3). The endoscopic findings of “vascular pattern,” “vulnerability of mucosa,” and “mucosal damage” were significantly improved (p = 0.004, p = 0.008, and p = 0.004, respectively) (Table 6). Figure 1 illustrates the marked improvements in mucosal edema and erythema observed at week 1; erosions and mucosal bleeding were also absent.
| Case | Age, Sex | Concomitant medications | Disease extension | Mayo score | Mayo score | Mayo score | Mayo score Week 7 |
| Baseline | Week 1 | Week 3 (or 4) | |||||
| 1 | 63y.o., M | 5-ASA, AZA, PSL | total | 10 | 4 | - | 1 |
| 2 | 62y.o., F | 5-ASA | total | 7 | 2 | - | 1 |
| 3 | 30y.o., M | 5-ASA, TAC, CsA | total | 11 | 5 | - | 2 |
| 4 | 32y.o., M | 5-ASA, AZA, LCAP | left-sided | 11 | 7 | 2 | - |
| 5 | 66y.o., F | PSL | total | 10 | 6 | 2 (Week 4) | - |
| 6 | 21y.o., M | 5-ASA, PSL | total | 9 | 6 | - | - |
| 7 | 62y.o., M | 5-ASA | total | 11 | 7 | 4 | 3 |
| 8 | 62y.o., M | 5-ASA, PSL | total | 10 | 4 | - | - |
| 9 | 54y.o., M | 5-ASA, TAC | total | 11 | 9 | canceled | canceled |
| Mean ± SD | 10 ± 1.2 | 5.6 ± 1.9 | 2.7 ± 0.9 n=3 | 1.8 ± 0.8 n=4 | |||
Table 5a: Mayo Score. PSL: Prednisolone; CsA: Cyclosporine; TAC: Tacrolimus; LCAP: Leukocytapheresis.
| Case | Age, Sex | Concomitant medications | Disease extension | Schroeder Baseline | Schroeder Week 1 | Schroeder Week 3 (or 4) | Schroeder Week 7 |
| 1 | 63y.o., M | 5-ASA, AZA, PSL | total | 2 | 1 | - | 0 |
| 2 | 62y.o., F | 5-ASA | total | 2 | 1 | - | 0 |
| 3 | 30y.o., M | 5-ASA, TAC, CsA | total | 3 | 2 | - | 0 |
| 4 | 32y.o., M | 5-ASA, AZA, LCAP | total | 3 | 2 | 1 | - |
| 5 | 66y.o., F | PSL | total | 3 | 2 | 1 (Week 4) | - |
| 6 | 21y.o., M | 5-ASA, PSL | left-sided | 2 | 1 | - | - |
| 7 | 62y.o., M | 5-ASA | total | 3 | 2 | 2 | 2 |
| 8 | 62y.o., M | 5-ASA, PSL | total | 3 | 2 | - | - |
| 9 | 54y.o., M | 5-ASA, TAC | total | 3 | 3 | canceled | canceled |
| Mean ± SD | 2.7 ± 0.5 | 1.9 ± 0.7 | 1.3 ± 0.5 n=3 | 0.5 ± 0.9 n=4 | |||
Table 5b: The Schroeder score. PSL: Prednisolone; CsA: Cyclosporine; TAC: Tacrolimus; LCAP: Leukocytapheresis.
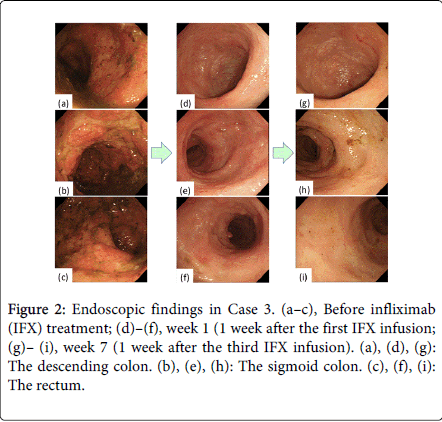
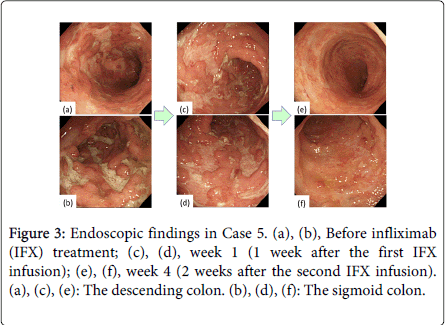
Although large, deep ulcerations were detected before the initial IFX treatment, almost all lesions showed improvement at week 1 (Figure 2). The deep, extensive ulcerations and diffuse mucosal edema were markedly improved 1 week after the first IFX infusion (Figure 3). Regenerating epithelium was also broadly detected at week 1 (Figures 3c and 3d).
Two additional patients demonstrated clinical remission and mucosal healing, based on their Mayo and Schroeder scores, after the second IFX infusion (weeks 3 and 4). Thus, 5 patients demonstrated mucosal healing (Schroeder score) by week 3/4. Two additional patients (a total of 3 patients) experienced mucosal healing by week 3/4, based on their RES. In the patient examined at week 4, almost all of the extensive ulcerations had improved and the vascular patterns were reconstructed (Figure 3e and 3f).
An additional 2 patients showed clinical remission (based on their Mayo scores) at week 7. Based on the Schroeder index, 63% of the patients (5 of 8 patients; one patient did not undergo endoscopy at week 7) demonstrated mucosal healing by week 7. Based on the RES, 67% of the patients (6 of 9) showed mucosal healing by week 7. In contrast, 2 (22%) of the 9 patients, with week 1 endoscopy scores of 3, failed to show clinical responses or mucosal healing by week 7.
At week 7, the endoscopic findings (RES) were compared between patients in the remission (Cases 1–5) and non-remission (Cases 7–9; Case 6 did not undergo endoscopy at week 7) groups. The “vascular pattern”, “mucosal vulnerability”, and “mucosal damage” scores tended to be improved at week 1 in the remission group but not in the non-remission group (Table 6).
| Case | Age, Sex | Granulation scattering | Vascular Pattern | Vulnerability of mucosa | Mucosal damage | |||||
| Pre | Week 1 | Pre | Week 1 | Pre | Week 1 | Pre | Week 1 | |||
| Remission group | 1 | 63y.o., M | 2 | 0 | 2 | 1 | 2 | 2 | 4 | 2 |
| 2 | 62y.o., F | 2 | 2 | 2 | 1 | 2 | 0 | 2 | 2 | |
| 3 | 30y.o., M | 2 | 2 | 2 | 1 | 4 | 2 | 4 | 2 | |
| 4 | 32y.o., M | 2 | 2 | 2 | 1 | 2 | 2 | 4 | 2 | |
| 5 | 66y.o., F | 2 | 2 | 2 | 2 | 4 | 0 | 4 | 4 | |
| Mean ± SD | 2.0 ± 0 | 1.6 ± 0.8 | 2.0 ± 0 | 1.2 ± 0.4 | 2.8 ± 1.0 | 1.2 ± 1.0 | 3.6 ± 0.8 | 2.4 ± 0.8 | ||
| Non-Remission group | 7 | 62y.o., M | 2 | 2 | 2 | 2 | 4 | 2 | 4 | 4 |
| 8 | 62y.o., M | 2 | 2 | 2 | 1 | 4 | 2 | 4 | 2 | |
| 9 | 54y.o., M | 2 | 2 | 2 | 2 | 2 | 2 | 4 | 2 | |
| Mean ± SD | 2.0 ± 0 | 2.0 ± 0 | 2 ± 0 | 1.7 ± 0.5 | 3.3 ± 0.9 | 2.0 ± 0 | 4.0 ± 0 | 2.7 ± 0.9 | ||
| Mean ± SD (n=8) | 2 ± 0 | 1.8 ± 0.6 | 2 ± 0 | 1.4 ± 0.5 | 3.0 ± 1.0 | 1.5 ± 0.9 | 3.8 ± 0.7 | 2.5 ± 0.9 | ||
| t-TEST (n=8) | p=0.35 | p=0.01 | p=0.02 | p=0.01 | ||||||
Table 6: Endoscopic findings in RES* at week 1. * RES: Rachmilewitz endoscopic score.
The C-reactive protein (CRP) levels were positive (>0.3mg/dl)in the non-remission group (Case 7–9) at weeks 1 (2.3 ± 1.7 mg/dL) and 6 (3.8 ± 1.8 mg/dL), and negative in the remission group (Cases 1–5) at weeks 1 (0.1 ± 0.12 mg/dL) and 6 (0.27 ± 0.38 mg/dL) (Table 7).
| Case | Age, Sex | Pre | Week 1 | Week 6 | |
| Remission group | 1 | 63y.o., M | 0.31 | 0.34 | 0.04 |
| 2 | 62y.o., F | 0.23 | 0.04 | 0.04 | |
| 3 | 30y.o., M | 0.98 | 0.04 | 0.04 | |
| 4 | 32y.o., M | 0.29 | 0.06 | 1.02 | |
| 5 | 66y.o., F | 1.32 | 0.07 | 0.22 | |
| Mean ± SD | 0.63 ± 0.44 | 0.1 ± 0.12 | 0.27 ± 0.38 | ||
| Non-Remission group | 7 | 62y.o., M | 5.02 | 2.66 | 5.41 |
| 8 | 62y.o., M | 1.27 | 0.09 | 1.35 | |
| 9 | 54y.o., M | 5.21 | 4.18 | 4.62 | |
| Mean ± SD | 3.8 ± 1.8 | 2.3 ± 1.7 | 3.8 ± 1.8 | ||
Table 7: CRP during clinical course.
Safety
One patient had a persistent fever (>39°C), without any symptoms of infection, for 10 days following the first IFX infusion. Since a delayed infusion reaction to IFX could not be excluded, this patient did not receive a second IFX infusion. The other patients received IFX without any specific adverse effects during the induction and maintenance phases.
Discussion
In this study, patients with moderate-to-severe, active UC that was refractory to initial treatment showed clinical responses or remission, based on their Mayo scores, within 1 week of starting IFX induction therapy. Endoscopic remission rates indicated that IFX efficacy was prompt and required only 1 IFX infusion for most patients. Similar to the ACT 1 and 2 studies (13), our data also showed that clinical remission was evident in 56% of the patients by week 7.
Our previous experience indicated that a single IFX infusion worked extremely well for UC patients; therefore, this study focused on the endoscopic findings during the early phase of treatment. Specifically, the first post-treatment colonoscopy, 1 week after the first IFX infusion, was performed to evaluate the detailed endoscopic changes that had occurred, i.e., the extent of damaged colonic mucosal recovery. The endoscopic findings describing the RESs for “vascular pattern”, “mucosal vulnerability”, and “mucosal damage” demonstrated significant improvement within the first week. Because a single IFX infusion resulted in these dramatic endoscopic findings, IFX is considered one of the most potent induction therapies for UC, as it is for CD. A single infusion, however, appeared to be insufficient to result in “granulation scattering” improvement, which did not show recovery until after the second or third infusion. Laharie et al. showed that the initial IFX infusion works as well as intravenous cyclosporine therapy within the first week [17]. Our data also showed that IFX efficacy can be evaluated, using colonoscopy, 1 week after the first IFX infusion.
However, 22% of the treated patients, with a week 1 Schroeder endoscopy score of 3, did not exhibit clinical responses or mucosal healing at week 7. Although some of the patients had a clinical response at week 1, they had not achieved clinical remission by week 7. In the remission group, 5 of the 6 patients achieved “vascular pattern” improvements at week 1, but only 1 of 3 patients achieved this finding in the non-remission group. Therefore, we believe that if “vascular pattern”, “mucosal vulnerability”, and “mucosal damage” improvements can be determined using the RES (with a particular focus on “vascular pattern”) at week 1, the patients may have a greater likelihood of achieving clinical remission during the induction phase.
Identifying a predictive marker for patients who experience a significant positive response to IFX remains difficult. Our results suggest that endoscopic findings at week 1 may be reliable predictors of an IFX response in patients with UC. CRP levels are generally recognized as useful predictive markers of CD and UC activity [20–22]. In this study, CRP was positive at weeks 1 and 6 in the non-remission group and negative (CRP ≤ 0.3) at weeks 1 and 6 in the remission group. Since CRP was negative when IFX infusion resulted in remission, CRP levels at weeks 1 and 6 may also be reliable predictive markers of IFX efficacy. Recently, Brandse et al. reported that IFX can be detected in the feces of patients with severe IBD and that the highest concentrations were measured on the day after initiation of therapy (Brandse JF et al., Digestive Disease Week, May 18–21, 2013, Orlando, FL, USA). Three of our patients did not respond to IFX during the induction phase. Cases 7 and 9 had high CRP (Table 7) and low albumin concentrations at week 6 (3.0 g/dL and 2.6 g/dL, respectively); therefore, these patients might have lost too much IFX and albumin in their feces. Measuring serum IFX concentrations at weeks 1 and 6 may become a useful predictive marker of IFX efficacy, but further study will be required to confirm its utility.
Limitation of Study and Recommendations for Future Research
A limitation of the current study is the small number of the patients. This was the first trial to evaluate the early endoscopic improvements resulting from IFX infusion in patients with steroid- or immunomodulator-resistant UC. The small number of patients reflects the few who were willing to undergo frequent colonoscopies. The endoscopic improvements observed during the early phase of IFX treatment may predict overall IFX efficacy; however, additional multicenter studies are required to clearly determine the significance of the present observations.
Conclusions
We demonstrated dramatic endoscopic improvements following IFX infusion in steroid- or immunomodulator-resistant UC patients. Specifically, we demonstrated endoscopic improvements in “vascular pattern”, “mucosal vulnerability”, and “mucosal damage” within 1 week after the first IFX infusion. We believe that these observations may be important for predicting IFX treatment efficacy.
References
- Geboes K, De Hertogh G (2003) Indeterminate colitis. Inflamm Bowel Dis 9: 324-331.
- Ina K, Itoh J, Fukushima K, Kusugami K, Yamaguchi T, et al. (1999) Resistance of Crohn's disease T cells to multiple apoptotic signals is associated with a Bcl-2/Bax mucosal imbalance. J Immunol 163: 1081-1090.
- Bu P, Keshavarzian A, Stone DD, Liu J, Le PT, et al. (2001) Apoptosis: one of the mechanisms that maintains unresponsiveness of the intestinal mucosal immune system. J Immunol 166: 6399-6403.
- Sturm A1, Mohr S, Fiocchi C (2002) Critical role of caspases in the regulation of apoptosis and proliferation of mucosal T cells. Gastroenterology 122: 1334-1345.
- Mahida YR, Wu K, Jewell DP (1989) Enhanced production of interleukin 1-beta by mononuclear cells isolated from mucosa with active ulcerative colitis of Crohn's disease. Gut 30: 835-838.
- Mitsuyama K, Sata M, Tanikawa K (1991) Significance of interleukin-6 in patients with inflammatory bowel disease. Gastroenterol Jpn 26: 20-28.
- Akazawa A, Sakaida I, Higaki S, Kubo Y, Uchida K, et al. (2002) Increased expression of tumor necrosis factor-alpha messenger RNA in the intestinal mucosa of inflammatory bowel disease, particularly in patients with disease in the inactive phase. J Gastroenterol 37: 345–353.
- ten Hove T, van Montfrans C, Peppelenbosch MP, van Deventer SJ (2002) Infliximab treatment induces apoptosis of lamina propria T lymphocytes in Crohn's disease. Gut 50: 206-211.
- Van den Brande JM1, Braat H, van den Brink GR, Versteeg HH, Bauer CA, et al. (2003) Infliximab but not etanercept induces apoptosis in lamina propria T-lymphocytes from patients with Crohn's disease. Gastroenterology 124: 1774-1785.
- Doering J, Begue B, Lentze MJ, Rieux-Laucat F, Goulet O, et al. (2004) Induction of T lymphocyte apoptosis by sulphasalazine in patients with Crohn's disease. Gut 53: 1632-1638.
- Tiede I, Fritz G, Strand S, Poppe D, Dvorsky R, et al. (2003) CD28-dependent Rac1 activation is the molecular target of azathioprine in primary human CD4+ T lymphocytes. J Clin Invest 111: 1133-1145.
- Lichtiger S, Present DH, Kornbluth A, Gelernt I, Bauer J, et al. (1994) Cyclosporine in severe ulcerative colitis refractory to steroid therapy. N Engl J Med 330: 1841-1845.
- Rutgeerts P, Sandborn WJ, Feagan BG, Reinisch W, Olson A, et al. (2005) Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med 353: 2462-2476.
- Ogata H, Matsui T, Nakamura M, Iida M, Takazoe M, et al. (2006) A randomised dose finding study of oral tacrolimus (FK506) therapy in refractory ulcerative colitis. Gut 55: 1255-1262.
- Feagan BG, Reinisch W, Rutgeerts P, Sandborn WJ, Yan S, et al. (2007) The effects of infliximab therapy on health-related quality of life in ulcerative colitis patients. Am J Gastroenterol 102: 794-802.
- Chaparro M, Burgueño P, Iglesias E, Panés J, Muñoz F, et al. (2012) Infliximab salvage therapy after failure of ciclosporin in corticosteroid-refractory ulcerative colitis: a multicentre study. Aliment Pharmacol Ther 35: 275-283.
- Laharie D, Bourreille A, Branche J, Allez M, Bouhnik Y, et al. (2012) Ciclosporin versus infliximab in patients with severe ulcerative colitis refractory to intravenous steroids: a parallel, open-label randomised controlled trial. Lancet 380: 1909–1915.
- Schroeder KW, Tremaine WJ, Ilstrup DM (1987) Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med 317: 1625-1629.
- Rachmilewitz D (1989) Coated mesalazine (5-aminosalicylic acid) versus sulphasalazine in the treatment of active ulcerative colitis: a randomised trial. BMJ 298: 82-86.
- Karoui S, Laz S, Serghini M, Bibani N, Boubaker J, et al. (2011) Correlation of C-reactive protein with clinical and endoscopic activity in patients with ulcerative colitis. Dig Dis Sci 56: 1801-1805.
- Schoepfer AM, Beglinger C, Straumann A, Trummler M, Renzulli P, et al. (2009) Ulcerative colitis: correlation of the Rachmilewitz endoscopic activity index with fecal calprotectin, clinical activity, C-reactive protein, and blood leukocytes. Inflamm Bowel Dis 15: 1851–1858.
- Karoui S, Ouerdiane S, Serghini M, Jomni T, Kallel L, et al. (2007) Correlation between levels of C-reactive protein and clinical activity in Crohn's disease. Dig Liver Dis 39: 1006-1010.
Relevant Topics
- Constipation
- Digestive Enzymes
- Endoscopy
- Epigastric Pain
- Gall Bladder
- Gastric Cancer
- Gastrointestinal Bleeding
- Gastrointestinal Hormones
- Gastrointestinal Infections
- Gastrointestinal Inflammation
- Gastrointestinal Pathology
- Gastrointestinal Pharmacology
- Gastrointestinal Radiology
- Gastrointestinal Surgery
- Gastrointestinal Tuberculosis
- GIST Sarcoma
- Intestinal Blockage
- Pancreas
- Salivary Glands
- Stomach Bloating
- Stomach Cramps
- Stomach Disorders
- Stomach Ulcer
Recommended Journals
Article Tools
Article Usage
- Total views: 15091
- [From(publication date):
August-2015 - Sep 22, 2024] - Breakdown by view type
- HTML page views : 10734
- PDF downloads : 4357