Selected Proteins of the Complement System as Morphological Indicators of Early Myocardial Infarction
Received: 10-Apr-2016 / Accepted Date: 28-Apr-2016 / Published Date: 29-Apr-2016 DOI: 10.4172/2161-0681.1000273
Abstract
Background: Morphological diagnosis of advanced myocardial infarction is not difficult; however its early phases usually remain indistinguishable in a macroscopic examination, and are often invisible under the microscope or hard to interpret unequivocally. The aim of this paper is to determine the usefulness of immunohistochemical staining to detect C1q, C9 and C5b-9, in the morphological diagnosis of an early myocardial infarction. Data and methodology: The experimental group consisted of tissue samples with suspected myocardial infarction unconfirmed in a routine morphological diagnosis (n=22). The control group consisted of myocardium samples without salient pathologies (n=10). Immunohistochemical reactions were conducted using a detection system based on enzymatic peroxidase reaction. The IHC reaction assessment was conducted based on halfquantitative Immunoreactive Score scale. Results: Morphological analysis of preparations based on haematoxylin and eosin staining in the experimental group did not show important changes that would denote myocardial infarction. The immunohistochemical reaction for C1q and C9 was intense, whereas in case of C5b-9 it was weak in all examined myocardium samples of the experimental group. In the control group no positive immunohistochemical reaction was observed. Conclusion: The results can indicate that immunohistochemical reactions to detect C1q and C9 can be a morphological indicator of early myocardial infarction. Reactions to detect C5b-9 are not specific to early myocardial infarction.
Keywords: Myocardial infarction; Morphological diagnosis; Complement components
Introduction
Myocardial infarction (MI) is a serious clinical and social problem due to its high mortality rate. The clinical recognition of advanced myocardial infarction is based on detection of increase or decrease of cardiac biomarkers concentration (mainly troponins), in two measurements (at patient’s admission to the unit and after at least 6 hours), accompanied by at least one of the following features: clinical symptoms of ischemia, newly arisen pathological Q waves in the ECG or the demonstration of new impaired myocardial contractility or loss of viable myocardium in scan imaging [1,2]. The macro and microscopic diagnosis of advanced myocardial infarction is not difficult; however, its early phases are usually undistinguishable in the macroscopic examination, and are often invisible under the microscope or hard to interpret unequivocally. Routine staining with haematoxylin and eosin shows advanced changes, but it is insufficient in the assessment of the early changes.
Proof for complement system activation in myocardial ischemia on an animal model was provided by the work by Hill and Ward in 1970s [3,4]. Ever since, constant research has been conducted to clarify the intricacies and allow for understanding of this process. It was proven that reperfusion was a key factor in inflammatory system activation in myocardial infarction, whose role is the removal of cellular debris and fragments of necrotic tissue in the damaged area [5]. The positive correlation between complement activation and the scope of inflammatory response has been found [6]. On the other hand, factor inhibiting complement activation decrease the inflammation in the ischaemic region of the heart – these factors, such as endogenous C1 inhibitor are locally expressed in the myocardium following ischaemic episode [7].
Complement system is a group of a dozen or so proteins present in the plasma, as well as in other body fluids together with many functionally linked receptors and regulating proteins. The complement activation is a series of enzymatic and non-enzymatic cascade reactions. In case of complement activation, two important enzymes are created: a convertase of C3 and C5, which very strongly increases its effect duration. However, regardless of the activation method, the final stages of all these reactions are identical and lead to the formation of membrane attacking complex – MAC, which consists of C5b, C6, C7, C8 and polymeric C9 [8-10].
The key element in choosing the right diagnostic method is for it to be specific for the ischaemic injury of myocardial cells and useful in the assessment of posthumous material. Another problem is the inevitably progressing autolysis process of tissue material or the presence of posthumous bacterial flora, which considerably limits the possibilities of using some of the methods. A hypothesis posed by Laufer et al. deserves special attention because it refers to local (cardiac) synthesis of complement factors (from C1 to C9) [11]. The main source of plasma proteins is the liver. The confirmation of the above-mentioned hypothesis is an important element, and its meaning increases especially when stating that this phenomenon is specific for ischemia. This argument becomes even more important in the research of posthumous material at different autolysis level, whereas the methods using specific antibodies base on detecting contractile cell proteins, which can give a falsely positive result.
An important matter in the use of immunohistochemical methods in the early myocardial infarction diagnosis is stating at what time from initiation of infarction it comes to complement activation, how quickly the activation products are detected and whether they are specific only in the case of ischemic myocardial injury. Studies on animal models suggest, that some components of the complement cascade might be useful as a marker of early MI, which is highly specific - for instance the deposition of C4d in the heart muscle of rat has been observed in MI independent from cardiac arrest itself [12].
Considering the data above, the aim of this work is to determine the usefulness of immunohistochemical staining to detect constituent proteins (C1q, C9) and membrane attacking complex complement system (C5b-9) in the morphological diagnosis of early myocardial infarction.
Data and Methodology
Tissue data
Research data consisted of tissue samples preserved in 4% formalin solution and embedded in paraffin, according to a routine procedure. Paraffin blocks came from the archive of Department of Pathology of Medical University of Warsaw, from years 2012-2013. The assessment of chosen proteins of the complement system was conducted on tissue samples of 22 people, who have undergone autopsy (within 2 days after death). A criterion that qualified the data to the experimental group was a suspicion of myocardial infarction, based on clinical data (ECG and troponin level examination), unconfirmed in a routine pathological diagnosis. Moreover, in histopathological examination neither tumours nor past myocardial infarction were observed. The control group consisted of muscle tissue without significant pathologies, collected from 10 people.
Conventional immunohistochemistry and light microscopy
Immunohistochemical reactions were conducted in paraffin samples, thickness 4 μm, using peroxidase activity in enzymatic reaction. The preparations, after deparaffinization and rehydration, underwent a process of epitope retrieval. In order to detect the examined proteins primary antibodies were used, such as Polyclonal Rabbit Anti-Human C1q Complement (A0136, Dako, Denmark); Human Complement Component C9 (NCL-CCC9, Novocastra, UK), Polyclonal Rabbit Anti-Human C5b-9 (ab55811, Abcam, UK). Samples were then transferred to 3% hydrogen peroxide solution in order to block the activity of endogenous peroxidase. 5% serum donkey solution was used to block non-specific antibody binding sites. Next, the samples were incubated in a primary antibody solution for 24 hours in 4ºC. In order to detect primary antibodies, secondary antibodies were used, directly conjugated with peroxidase particles (Vector). The reaction was presented using 3, 3’-diaminobenzidine (Dako) as chromogen. Cell nuclei were exposed in contrast staining with hematoxylin.
Result analysis
The assessment of immunohistochemical reaction was conducted on the basis of the IRS scale. The number of cells with positive reaction (PP) was assessed, as well as reaction intensity (SI) in 5 fields of vision of light microscope magnified at 20x. Product PP and SI constituted the final value of the reaction assessment. Final result was established based on medium values from 5 fields of vision and presented as contractual units. The obtained results, ranging from 0 to 12, were divided into 3 categories: 0-2 - low reaction; 3-5 - medium reaction; 6-12 - intense reaction.
Results
Table 1 summarizes the most important clinical and morphological features of the study group and control group.
| Study group n=25 | Control group n=10 | |
|---|---|---|
| Sex (F/M) | 7(28%)/18(72%) | 5(50%)/5(50%) |
| Age (years) | 65.02±14.65* | 54.80±16.96* |
| Inforact location: | ||
| LV | 4 (16%) | NA |
| LV + IVS | 5 (20%) | NA |
| LV + IVS + A | 16 (64%) | NA |
| cTnT1,3±0,9 ng/ml**Coronary atherosclerosis: | ||
| Low | 2 (8%) | 6 (60%) |
| Average | 9 (36%) | 3 (30%) |
| High | 14 (56%) | 0 (0%) |
| Coronary artery disease: | ||
| One Lessel | 11 (44%) | 3 (30%) |
| Two Lessel | 14 (56%) | 0 (0%) |
| Hypertension | 20 (80%) | 2 (20%) |
| Diabetes mellitus: | ||
| type I | 2 (8%) | 0 (0%) |
| type II | 6 (24%) | 0 (0%) |
| Obesity | 8 (32%) | 0 (0%) |
Table 1: Initial patient characteristics.
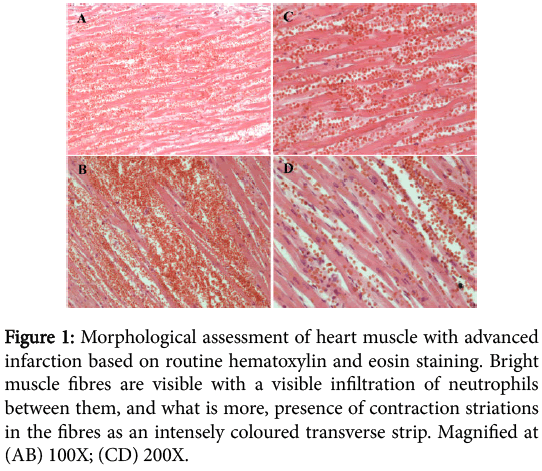
The result of hematoxylin and eosin staining of advanced myocardial infarction indicated necrosis on various stages of development. In the performed myocardial infarction muscle fibres are bright and eosinophilic due to the denaturing necrosis, and between fibres we can observe a visible neutrophilic infiltration. Necrotically altered muscle cells lack nuclei and transverse striations (Figure 1).
Figure 1: Morphological assessment of heart muscle with advanced infarction based on routine hematoxylin and eosin staining. Bright muscle fibres are visible with a visible infiltration of neutrophils between them, and what is more, presence of contraction striations in the fibres as an intensely coloured transverse strip. Magnified at (AB) 100X; (CD) 200X.
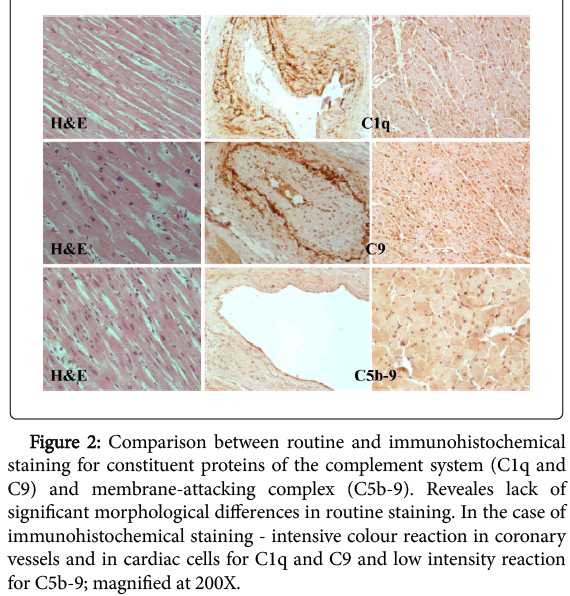
The morphological analysis of the preparations in the experimental group through the use of hematoxylin and eosin staining did not show significant alterations indicating myocardial infarction (Figure 2).
Figure 2: Comparison between routine and immunohistochemical staining for constituent proteins of the complement system (C1q and C9) and membrane-attacking complex (C5b-9). Reveales lack of significant morphological differences in routine staining. In the case of immunohistochemical staining - intensive colour reaction in coronary vessels and in cardiac cells for C1q and C9 and low intensity reaction for C5b-9; magnified at 200X.
Immunohistochemical staining was performed in the same samples to detect constituent proteins of the complement system (C1q, C9) and membrane-attacking complex (C5b-9).
Immunoreactivity of constituent proteins C1q and C9 of the complement system was presented in all analysed heart muscle samples of the experimental group. These proteins were located primarily on the entire surface of coronary vessels endothelium, and the staining was omnipresent. In the area of muscle cells their location was cytoplasmatic. Most of the analysed samples stained in a uniform way, and the reaction was intense, and medium immunohistochemical reaction in singular heart muscle cells were observed only in a few cases.
Immunohistochemical staining to detect membrane-attacking complex (C5b-9) has indicated its location in coronary vessels as well as in muscle cells. The immunohistochemical reaction had a homogeneous character, and most of the analysed samples coloured evenly. The coloured reaction had low intensity - only a small percentage of analyzed preparations presented medium reaction intensity (Figure 2).
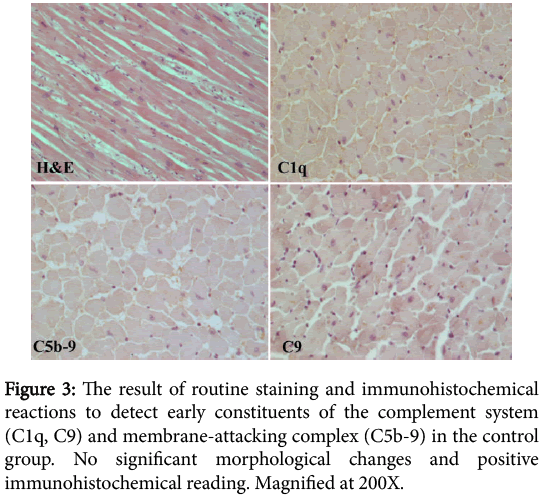
In the analysed microscopic preparations of the control group there was no sign of significant morphological changes in routine staining and no positive immunohistochemical reading of initial constituents of the complement system (C1q, C9) and the membrane-attacking complex (C5b-9) (Figure 3).
Averaged results of half-quantitative immunoreactivity assessment of the analyzed proteins are presented (Table 2).
| C1q | C9 | C5b-9 | |
|---|---|---|---|
| Experimental group | 8,91±2,01 | 9,46±1,91 | 2,38±0,72 |
| Control group | 0 | 0 | 0 |
Table 2: Results of half-quantitative immunoreactivity assessment of early constituents of the complement system membrane-attacking complex in the experimental and control groups (medium values +/- SD).
Discussion
In the available bibliographical data only a few reports treat of the possibility of using immunohistochemical methods in early myocardial infarction diagnosis [13-15]. This research indicated increased immunoreactivity of early constituents of the complement system such as C1q and C9 and low immunoreactivity of the membrane-attacking complex (C5b-9) in the early myocardial infarction. It should be mentioned that posthumous material with varying degree of autolysis was used for the analysis. The above results are compatible with the research by Hohmeister et al. [16] on an animal model and Thomsen et al. [17] on human cells. Both teams researched the use of membraneattacking complex (C5b-9) in histopathological diagnosis. Hohmeister et al. [16] marked the presence of C5b-9 with immunohistochemical methods on a rabbit model of myocardial infarction. The research had two stages: the immunohistochemical reaction was performed after 3 hours and 10 days after the infarction. In contrast, Thomsen et al. [17] immunohistochemically marked the presence of C5b-9 in the tissue material deriving from 12 people with advanced autolysis signs. Neither of the teams confirmed the specificity of the immunohistochemical reaction using the membrane-attacking complex (C5b-9) in posthumous material with autolysis features.
The pathomorphological diagnosis of myocardial infarction is a posthumous diagnosis and demands from a given method to fulfil specific conditions. The material is always characterized with a lower or higher level of autolysis. It is important to state whether the alterations are vital or posthumous. An important aspect, which determines the usefulness of a given method in pathomorphological diagnosis, is the possibility to distinguish between the artifacts likely to arise at the moment of undertaking and conducting basic and advanced life support for a longer period of time. The above issues were dealt with by several research teams - among others, under the supervision of Edston [18] and Ortmann [19]. Observations of both teams suggest ambiguous conclusions. On the basis of the analysed tissue material deriving from 75 forensic medical autopsies, Edston et al. said that immunohistochemical marking of complement component C9 is invaluable in the analysis of the autolysed material, even at significant change development. What is more, the immunohistochemical reaction, as one of the verifying methods, is taken into consideration in distinguishing between posthumous and vital alterations. Analogical observations were also made by Fechner et al. [20], as well as Edston et al. [21]. Different conclusions were formed by research teams lead by Ortmann. The researchers sceptically referred to the above method, in the research matter of the autolyzed material in significant degree, and stated that the obtained results can be falsely positive. In the tissue material analysis deriving from 54 autopsies with varying degrees of autolysis, in 35% of the cases the researchers obtained a falsely positive result. When it comes to the use of the discussed method to distinguish between posthumous and vital changes in the tissue material of negligible autolysis the authors confirmed in their work the results of Edston et al. [19]. In another research, Ortmann et al. verified the usefulness of the immunohistochemical method using a specific antibody against complement component C9 and the complex C5b-9 in the heart muscle deriving from 16 autopsies with minimal autolysis features and obtained from 6 exhumed organs after burial, ranging from 10 to 60 days. Problem raised by the authors related to the interpretation of the staining results. The results obtained in immunohistochemical staining allow for some inference, with high probability, ambiguous or impossible diagnosis of early myocardial infarction [22]. It remains fundamental to notice here that posthumous diagnosis of sudden cardiac death is basically confronting the microscopic analysis results (including immunohistochemical analysis) with all the information of the analyzed case, including both the macroscopic analysis and the interview regarding lesions and symptoms prior to death. Nevertheless, considering the results of this study, it is stated truly that immunohistochemical and immunofluorescent verification of the assessed component proteins of the complement system in confrontation with clinical data is a key argument in posthumous diagnosis of early myocardial infarction. While it has been suggested earlier, that C9 might be a useful factor in the diagnosis of the early MI in autopsy samples [23], in our study the novel factor, C1q has been found, which might be suitable for the analysis. Taking into account all the difficulties associated with such studies, it might be suggested that more than one staining should be considered to confirm or deny the diagnosis of MI with desired level of specificity and sensitivity. Thence the need to identify as many complement components as possible as far their usefulness for such assessment is concerned.
We should furthermore keep in mind that our study was conceived as an important contribution for pathological praxis – thence the choice of methods for analysis of immunohistochemical staining. The use of scores is definitely closer to routine assessment by pathologists than optometric analyses. Although most modern pathology departments have relevant software and imaging cameras at their disposal, their implementation require much time and effort. The assessment of routine histochemical staining, such as in the case of HER in breast cancer is based on scores (negative, 1+, 2+, 3+) given subjectively by the pathologist [24].
In reference to the global definition of myocardial infarction [1,2], already cited in the introduction, a particular fact draws attention – the recent publication of several papers concerning an increased level of troponins in patients with kidney insufficiency, pulmonary embolism or after chemotherapy [25-35]. It is worth noticing that Jacobs et al., who within a period of six months thrice marked the troponin T level using a high sensitivity test (hs-TnT) and a standard test (cTnT) with over 200 patients suffering from end stage kidney insufficiency without symptoms of heart diseases. All three samples revealed increased troponin T intensity in all examined patients, both in the standard and high sensitivity tests [36,37]. In turn, another research by Wu et al. on a group of 75 patients revealed that after the introduction high sensitivity tests for troponine marking I (hs-TnI) to routine diagnosis, this marker was often increased in patients with end stage kidney insufficiency without heart disease symptoms (over 60% of researched population) [38]. On the other hand, Freola et al. [33] in a prospective examination of 52 patients with breast cancer and without heart disease symptoms, subject to chemotherapy, observed statistically significant increase in troponin I level just after a month of treatment (p<0,01). In one year and two years after the ending of the treatment neither did they notice further increase of troponin I level nor lesions in the circulatory system.
Conclusion
Posthumous morphological diagnosis of advanced myocardial infarction is not difficult. The problem concerns early myocardial infarction – the initial phases are indistinguishable macroscopically and invisible under a microscope or difficult to interpret unequivocally. Moreover, the tissue material is characterised with a smaller or greater autolysis level. In the light of the above reports, an increased troponin level demand for the obtained results to be considered carefully, which significantly impedes clinical and morphological diagnosis of early myocardial infarction, especially in the population of patients who suffer from end stage kidney insufficiency or are oncologically treated. Currently, the most reasonable method of a proper diagnosis of early myocardial infarction is to extend range tests with immunohistochemical reading. In questionable cases it would be appropriate to use several antibodies against different antigens.
References
- Thygesen K, Alpert JS, White HD, Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction (2007) Universal definition of myocardial infarction. Eur Heart J 28: 2525-2538.
- Mendis S, Thygesen K, Kuulasmaa K, Writing Group on behalf of the participating experts of the WHO consultation for revision of WHO definition of myocardial infarction (2011) World Health Organization definition of myocardial infarction: 2008-09 revision. Int J Epidemiol 40: 139-146.
- Hill JH, Ward PA (1971) The phlogistic role of C3 leukotactic fragments in myocardial infarcts of rats. J Exp Med 133:885-900.
- Maroko PR, Carpenter CB, Chiariello M, Fishbein MC, Radvany P, et al. (1978) Reduction by cobra venom factor of myocardial necrosis after coronary artery occlusion. J Clin Invest 61: 661-670.
- Anzai T (2013) Post-infarction inflammation and left ventricular remodeling: a double-edged sword. Circ J 77: 580-587.
- Maroko PR, Carpenter CB, Chiariello M, Fishbein MC, Radvany P, et al. (1978) Reduction by cobra venom factor of myocardial necrosis after coronary artery occlusion. J Clin Invest 61: 661-670.
- Emmens RW, Baylan U, Juffermans LJ, Karia RV, Ylstra B, et al. (2016) Endogenous C1-inhibitor production and expression in the heart after acute myocardial infarction. CardiovascPathol 25: 33-39.
- Golab J, Jakbisiak M, Lasek W (2011): Immunologia, WydawnictwoNaukowe PWN, Warszawa, 66-77.
- Chapel H, Haeney M, Misbah S (2009) Immunologiakliniczna, Wydanie I polskie pod redakcjaGrzegorzaSenatorskiego, WydawnictwoCzelaj, Lublin.
- terWeeme M, Vonk ABA, Kupreishvili K, van Ham M, Zeerleder S (2010) Activated complement ismore extensively present in diseased aortic valves than naturally occurring complement inhibitors: a sign of ongoing inflammation. Eur J Clin Invest 40: 4-10.
- Laufer J, Katz Y, Passwell JH (2001) Extrahepatic synthesis of complement proteins in inflammation. MolImmunol 38: 221-229.
- Vuohelainen V, Paavonen T, Hamalainen M, Moilanen E, Mennander AA (2015) C4d Deposition Reveals Myocardial Infarction After Cardiac Arrest--Experimental Study. AdvClinExp Med 24: 393-399.
- Brinkmann B, Sepulchre MA, Fechner G (1993) The application of selected histochemical and immunohistochemical markers and procedures to the diagnosis of early myocardial damage. Int J Legal Med 106: 135-141.
- Doran JP, Howie AJ, Townend JN, Bonser RS (1996) Detection of myocardial infarction by immunohistological staining for C9 on formalin fixed, paraffin wax embedded sections. J ClinPathol 49: 34-37.
- Ribeiro-Silva A, S Martin CC, Rossi MA (2002) Is immunohistochemistry a useful tool in the postmortem recognition of myocardial hypoxia in human tissue with no morphological evidence of necrosis? Am J Forensic Med Pathol 23: 72-77.
- Homeister JW, Satoh P, Lucchesi BR (1992) Effects of complement activation in the isolated heart. Role of the terminal complement components. Circ Res 71: 303-319.
- Thomsen H, Held H (1994) Susceptibility of C5b-9(m) to postmortem changes. Int J Legal Med 106: 291-293.
- Edston E (1997) Evaluation of agonalartifacts in the myocardium using a combination of histological stains and immunohistochemistry. Am J Forensic Med Pathol 18: 163-167.
- Ortmann C, Pfeiffer H, Brinkmann B (2001) Immunohistochemical alterations after intravital and post-mortem traumatic myocardial damage. Int J Legal Med 115: 23-28.
- Fechner G, Bajanowski T, Brinkmann B (1993) Immunohistochemical alterations after muscle trauma. Int J Legal Med 105: 203-207.
- Edston E (1997) Evaluation of agonalartifacts in the myocardium using a combination of histological stains and immunohistochemistry. Am J Forensic Med Pathol 18: 163-167.
- Ortmann C, Pfeiffer H, Brinkmann B (2000) Demonstration of myocardial necrosis in the presence of advanced putrefaction. Int J Legal Med 114: 50-55.
- Jenkins CP, Cardona DM, Bowers JN, Oliai BR, Allan RW, et al. (2010) The utility of C4d, C9, and troponin T immunohistochemistry in acute myocardial infarction. Arch Pathol Lab Med 134: 256-263.
- Jonjic N, Mustac E, Tomic S, Razumovic JJ, Sarcevic B, et al. (2015) INTERLABORATORY CONCORDANCE IN HER-2 POSITIVE BREAST CANCER. ActaClin Croat 54: 479-485.
- Omland T, de Lemos JA, Sabatine MS, Christophi CA, Rice MM, et al. (2009) A sensitive cardiac troponin T assay in stable coronary artery disease. N Engl J Med 361: 2538-2547.
- Otsuka T, Kawada T, Ibuki C, Seino Y (2010) Association between highsensitivity cardiac troponin T levels and the predicted cardiovascular risk in middle-aged men without overt cardiovascular disease. Am Heart J 159: 972-997.
- Apple F, Murakami M, Pearce L, Herzog CA (2004) Multi-biomarker risk stratification of N-terminal pro-B-type natriuretic peptide, high-sensitivity C-reactive protein, and cardiac troponin T and I in end-stage renal disease for all-cause death. ClinChem 50: 2279-2285.
- Duman D, Tokay S, Toprak A, Duman D, Oktay A, et al. (2005) Elevated cardiac troponin T is associated with increased left ventricular mass index and predicts mortality in continuous ambulatory peritoneal dialysis patients. Nephrol Dial Transplant 20: 962-967.
- deFilippi C, Wasserman S, Rosanio S, Tiblier E, Sperger H, et al. (2003) Cardiac troponin T and C-reactive protein for predicting prognosis, coronary atherosclerosis, and cardiomyopathy in patients undergoing long-term hemodialysis. JAMA 290: 353-359.
- Hayashi T, Obi Y, Kimura K (2008) Cardiac troponin T predicts occult coronary artery stenosis in patients with chronic kidney disease at the start of renal replacement therapy. Nephrol Dial Transplant 23: 2936-2942.
- Jaroszynski AJ, Zaluska W, Bober E, Ksiazek A (2005) [Factors producing increase of QRS complex amplitude during hemodialysis]. PrzeglLek 62: 270-273.
- Feola M, Garrone O, Occelli M, Francini A, Biggi A, et al. (2011) Cardiotoxicity after anthracycline chemotherapy in breast carcinoma: effects on left ventricular ejection fraction, troponin I and brain natriuretic peptide. Int J Cardiol 148: 194-198.
- Kilickap S, Barista I, Akgul E, Aytemir K, Aksoyek S, et al. (2005) cTnT can be a useful marker for early detection of anthracyclinecardiotoxicity. Ann Oncol 16: 798-804.
- Seino Y, Tomita Y, Nagai Y (1993) Cardioprotective effects of ace-inhibitor (Cilazapril) on adriamycincardiotoxicity in spontaneously hypertensive rats. Circulation 88: I633 [abstrakt].
- Lipshultz SE, Rifai N, Sallan SE, Lipsitz SR, Dalton V, et al. (1997) Predictive value of cardiac troponin T in pediatric patients at risk for myocardial injury. Circulation 96: 2641-2648.
- Jacobs L, van de Kerkhof J, Mingels A, Kleijnen VW, van der Sande FM (2009) Haemodialysis patients longitudinally assessed by highly sensitive cardiac troponin T and commercial cardiac troponin T and cardiac troponin I assays. Annals of Clinical Biochemistry 46:283-290.
- Mingels A, Jacobs L, Michielsen E, Swaanenburg J, Wodzig W (2009) Reference population and marathon runner sera assessed by highly sensitive cardiac troponin T and commercial cardiac troponin T and I assays. ClinChem 55:101-108.
- Wu AH, Feng YJ (1998) Biochemical differences between cTnT and cTnI and their significance for diagnosis of acute coronary syndromes. Eur Heart J 19 Suppl N: N25-29.
Citation: Ilczuk T, Szparecki G, Wasiutynski A, Wolinska E, Gabzdyl N, et al. (2016) Selected Proteins of the Complement System as Morphological Indicators of Early Myocardial Infarction. j Clin Exp Pathol 6:273. Doi: 10.4172/2161-0681.1000273
Copyright: © 2016 Ilczuk T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 11447
- [From(publication date): 4-2016 - Apr 27, 2024]
- Breakdown by view type
- HTML page views: 10756
- PDF downloads: 691