Make the best use of Scientific Research and information from our 700+ peer reviewed, Open Access Journals that operates with the help of 50,000+ Editorial Board Members and esteemed reviewers and 1000+ Scientific associations in Medical, Clinical, Pharmaceutical, Engineering, Technology and Management Fields.
Meet Inspiring Speakers and Experts at our 3000+ Global Conferenceseries Events with over 600+ Conferences, 1200+ Symposiums and 1200+ Workshops on Medical, Pharma, Engineering, Science, Technology and Business
Research Article Open Access
Patch-Clamp Measurements on a Chip with Full Control over the Oxygen Content
| A. Alrifaiy1,3, N. Bitaraf1,3, M. Druzin2, O. Lindahl1,3 and K. Ramser1,3* | |
| 1Department of Computer Science, Electrical and Space Engineering, Luleå University of Technology, SE-971 87, Luleå, Sweden | |
| 2Department of Integrative Medical Biology, Section for Physiology, Umeå University, SE-901 87 Umeå, Sweden | |
| 3CMTF, Centre for Biomedical Engineering and Physics, Luleå University of Technology and Umeå University, Luleå and Umeå, Sweden | |
| Corresponding Author : | Kerstin Ramser Associate Professor in Biomedical Engineering Department of Computer Science Electrical and Space Engineering LuleåUniversity of Technology SE-971 87 Luleå, Sweden E-mail: kerstin.ramser@ltu.se |
| Received January 10, 2011; Accepted February 07, 2012; Published April 21,2012 | |
| Citation: Alrifaiy A, Bitaraf N, Druzin M, Lindahl O, Ramser K (2012) Patch-Clamp Measurements on a Chip with Full Control over the Oxygen Content. J Biochip Tissue chip 2:102.doi:10.4172/2153-0777.1000102 | |
| Copyright: © 2012 Alrifaiy A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Bioengineering and Bioelectronics
| Keywords |
| Microfluidics; Patch-Clamp; Optical Trapping and Nerve Cells |
| Introduction |
| Interruption of the blood supply to brain tissue leads to stroke. The affliction is usually caused by clots that block the blood flow or by the bursting of a blood vessel to or inside the brain. The disease is mainly a middle and high income country disease and females are more representative than males [1]. Strokes caused by blockage of a blood vessel to the brain are called ischemic and can be either thrombotic or embolic. A thrombotic stroke is caused by a cloth formed locally in the blood vessel to the brain while embolic strokes are caused by cloths formed elsewhere in the body. 15 million people suffer from strokes yearly and out of these 5 million people lose their lives and 5 million are permanently disabled [2]. |
| To better understand the intrinsic neuroprotective functions against stroke the electrophysiological response of cells to oxygen deprivation may be assessed by the patch-clamp technique [3]. In combination with other techniques that can assess the biochemical actions. Patch-clamp experiments on neurons in reduced oxygen environments have been performed previously and the response to hypoxia is heterogenous even for neurons within the same nucleus [4]. But the definition of hypoxic or anoxic solution and the control over the oxygen content in cell surroundings vary considerably. |
| Oxygen levels in hypoxic and anoxic solutions in studies vary from approximately 1 to 4% (10-30 mm Hg) [5-9]. In some cases the solutions are simply purged with N2 or CO2 gases for certain amounts of time varying from 10 minutes [10] up to one [11] or two [12] hours. Hypoxic environments are also achieved by so called chemical anoxia by inhibition of the oxidative phosphorylation with Cyanide [13,14]. |
| The need of a tool to enable measurements of electrophysiological activity in combination of other techniques of single cells with total control of the oxygen content in cell surroundings is clear. Chemical anoxia inhibits the oxidative phosphorylation but does not inhibit other effects that oxygen may have on the endogenous defense mechanisms against stroke. For example, neuroglobin is a protein with a neuroprotective function against damage caused by stroke [15]. One theory of its mechanism is that it works by inhibition of apoptosis [16]. The activation of this protein and other defense mechanisms against stoke may be related to the oxygen content around cells. Therefore there is a clear need for the possibility to perform patch-clamp experiments with control over the gaseous and biochemical surroundings of cells while the bio chemical action of the protein is assessed. Here we present a new approach for a combination of lab-on-a-chip techniques and patch-clamp. Lab-on-a-chip technique provides a promising opportunity to perform the patch clamp measurement under well controllable environment of the investigated cells. |
| The field of microfluidics deals with the smart design of microfluidics to achieve precise control of the amount and the dynamics of fluids. The devices usually consist of transparent materials [17] with microsized channels, reservoirs and sealed inlets and outlets. The big impact is to enable precise control of the fluid flow within the channels during rapid environmental changes [18,19]. |
| Microfluidic systems have been used widely for various biological investigations combined with other techniques [20]. There are many techniques used in fabrication of microfluidic devices such as photolithography, soft lithography, and CNC (Computer Numerical Control) micromachining. They are produced of diverse types of polymeric or non polymeric materials such as PDMS (Polydimethylsiloxane), PMMA (Poly-methyl methacrylate), silicon or glass, and can easily be combined with optical techniques on most types of optical microscopes. |
| If biological cells are investigated in closed lab-on-a-chips, it is essential to control the individual cells in 3D within the micro-channels. This has been achieved by flow control using ultra-sound techniques [21]. Acoustic forces induce low mechanical stress on the cells. Therefore, ultrasonic standing wave manipulation allows long-term acoustic field exposure without displaying any cell damage. However, the spatial control is not sufficient for 3D manipulation to a precise position. Another promising approach is to manipulate the cells optically by using optical trapping [22], which provide an excellent way to trap and control the biological cells. Optical tweezers use light radiation to induce forces acting on dielectric particles in three dimensions. Experimentally, a stable optical trap is achieved by focusing a laser beam strongly through a high-numerical aperture (NA) microscope objective onto the sample. The trapped object is usually manipulated by moving the trap and/or the sample stage. |
| The combinations of microfluidic systems with optical trapping technique have been used in a range of applications involve single cell investigations [23]. Furthermore, the integration of optical tweezers and lab-on-a-chip with patch-clamp technique could be advantageous for many applications. For example, electrophysiological investigations using the conventional patch-clamp are based on a micropipette steered in three dimensions to get in close contact to a biological cell. Accordingly the possibility to patch a cell in a closed microfluidic system by the pipette is unfeasible without innovative design and modification. |
| Here we present a closed microfluidic chip based on PMMA including an integrated patch-clamp micro-pipette where optically trapped biological cells were manipulated within the microfluidic channels to enable patch-clamp measurements within a controlled environment. |
| Materials and Methods |
| Preparation of neurons and solutions |
| Ethical approval of the procedures described was given by the regional ethics committees for animal research (“Umeå djurförsöksetiska nämnd”, approval No. A13-08 and A18-11). |
| Nerve cells where prepared from the brains of Sprague Dawley rats (3-6 weeks old) that were decapitated without anesthetics. The brains were rapidly removed and placed in pre-oxygenated ice-cold (≤ 4 °C) incubation solution containing (in mM) 150 NaCl, 5 KCl, 2 CaCl2 (×2H2O), 10 HEPES, 10 glucose, 4.93 Trizma-base, pH 7.4 which was also used throughout the entire slicing procedure. |
| The slicing procedure was performed by a vibratome (Vibroslicer 752 M, Campden Instruments, Leicestershire, UK) to cut 200 - 300 mm thick coronal slices which were allowed to recover for at least 45 minutes in Incubation solution at temperature 27-28°C. |
| The acute dissociation of the nerve cells was performed by a glass rod (tip diameter of ~0.5 mm) mounted on a piezo-electric bimorph crystal, to apply mechanical vibration at sites on slices composed mainly of grey matter [24]. Dissociated cells were then inserted to a gravity fed perfusion system with very short tubing connected to the anoxic chamber to reduce mechanical damage to the cells during the loading. |
| Recording solutions |
| Extracellular solution (EC) containing (in mM) 137 NaCl, 5.0 KCl,1.0 CaCl2 (×2H2O), 1.2 MgCl2 (×6H2O), 10 HEPES, 10 glucose, 3 μM glycine, pH 7.4 (NaOH) was used for flushing the cells. |
| Intracellular solution (IC) contining 140 Cesium-acetate, 3.0 NaCl, 1.2 MgCl2 (×6H2O), 10 HEPES, 10 EGTA, pH 7.2 (CsOH)) was used as pipette-filling solution. |
| Patch-clamp technique |
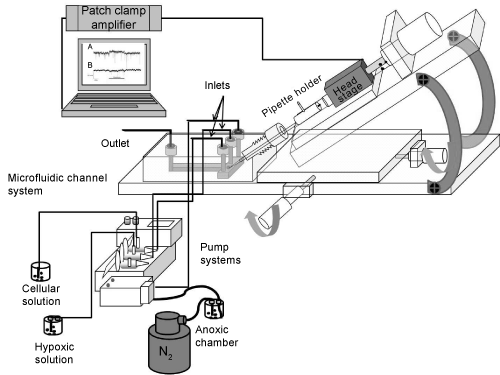
| Patch-clamp was used [3] to register the electrical signals across the plasma membrane of single neurons. Electric signal recordings were made between an electrode placed in the outlet of the lab-on-a-chip mounted on an inverted Zeiss Axiovert 25 CFL microscope (Carl Zeiss, Germany) and a recording electrode placed in the micro-pipette that was pulled from borosilicate glass (GC150, Harvard Apparatus Ltd., UK). The recording electrode within the recording pipette was attached to a nerve cell. Pipettes with a resistance of 3-5 MΩ was filled with IC and inserted into the EC-filled micro-channel. Signals were recorded using an Axopatch 200 A amplifier, a Digidata 1200 interface and software program pClamp 7 (all from Axon Instruments, Union City, CA, USA). Recordings were made in current clamp and voltage clamp conditions. |
| Oxygen measurement |
| The oxygen level was controlled with a fiber optic oxygen sensor probe, (FOXY AL300), connected to a MultiFrequency Phase Fluorometer (Ocean Optics, Dunedin, Florida, USA). |
| Optical tweezers |
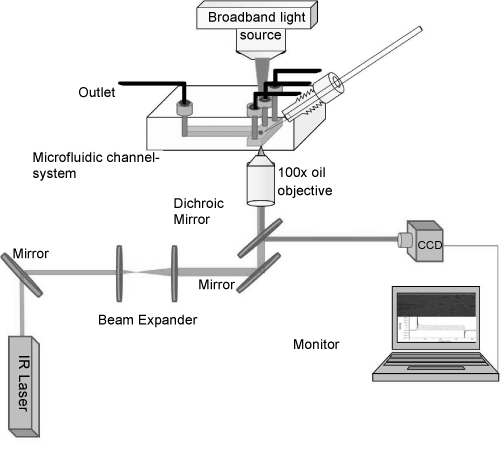
| The setup of optical tweezers was constructed on an inverted optical microscope (Zeiss Axiovert-25 USA) used for patch-clamp, as seen in (Figure 1). A NIR diode laser beam (IQ1A, Power Technology, USA) with power of 200 mW and wavelength of 808 nm was used. The wavelength was selected to minimize the effects of heating and photodamage of the sample [25]. The beam was guided through a mirror and expanded by a 1:5 beam expander of two positive lenses of 50 and 250 mm, mounted on two XYZ-translation stages. The beam expander act simultaneously as beam steering where moving one lens in three dimensions will result in translation of the laser spot on the sample in 3D. The expanded beam is then steered by mirrors (Thorlabs, USA) to the sample plane throughout a dichroic mirror (750-dcspxr, Chroma Technology, USA) and oil immersion objective (100x, 1.4 NA, Olympus, Japan) to obtain good trap stiffness. The position of the optical trap was fixed by the beam steering so that the laser spot was positioned in the center of the field of view. The position of the optically trapped cell was controlled by the nanometer precision translation stage of the microscope, i.e., the whole sample but not the position of the optical trap was moved. |
| Closed microfluidic system |
| The microfluidic chip was fabricated by PMMA material using CNC micromachining technology (Figure 2) [26]. The fabrication was prepared in two steps. First, holes with different diameters and shapes were drilled to function as inlets, outlet and a channel for the insertion of the pipette. The next step was to transfer the desired micro channels onto the PMMA sheet by CNC micromachining. |
| The procedure of integrating a patch-clamp micropipette started by CNC drilling of a hole that extended at 45 degrees from the upper edge of the microfluidic chip to the specific-zone within the microfluidic channel. The upper edge of the hole was threaded for integration of a fine hollow screw (JR-5508-5, VICI Jour, Switzerland). The micropipette was moved precisely through the hollow screw and the drilled hole into the microfluidic chamber, whereas the tip of the pipette was monitored to be found within the microfluidic channel. To ensure a sufficient air-seal surrounding the drilled hole, the diameter of the hole was prepared to fit with the outer diameter of the micropipette. Additionally, a gas-tight fitting (PEEK, JR-55003-5, VICI Jour, Switzerland) was used to make an air-seal between the hollow screw and the hole. The hollow screw was used to fine-position the tip of the micropipette into the micro channel. |
| Next step of fabrication is to design micro channels by a software program (QCAD) to be transferred onto the pre-drilled slab of PMMA by the CNC machine. The 100 x 100 channels diameter were then sealed by cover slip using an UV-curable adhesive material (EPO-TEK OG603, Epoxy Technology, USA) with properties of low viscosity and high optical transparency. To assure that the epoxy was not blocking the micro channels, positive air-pressure was applied into the channel during the curing of the epoxy. This had the advantage to coat the walls of the channels to produce higher optical transparency. The epoxy was classified to USP Class VI biocompatibility standards that meet the requirements for medical application. The microfluidic chamber with inlets and outlet adjacent to the micro channels were connected by gastight PEEK tubing (ScanTec, Sweden) to the pump system (neMESYS, Cetoni, Germany). The pump system was used for the infusion of cells and solutions with varying oxygen content. |
| The Experimental Results and Discussions |
| First the oxygen content within the lab-on-a-chip was measured with the fiber optic oxygen sensor probe. The normoxic condition was approximately 19-21% O2 (atmospheric oxygen concentration) while the anoxic conditions were achieved by purging solutions with N2 gas until an oxygen content of approximately 0.5-1.5%O2 was achieved. The measurements showed that a reliable control of the oxygen level was established. The time required for the fluid variation within the micro-channel was measured to approximately 3s with a fluid flow rate of 0.1 μl/s. |
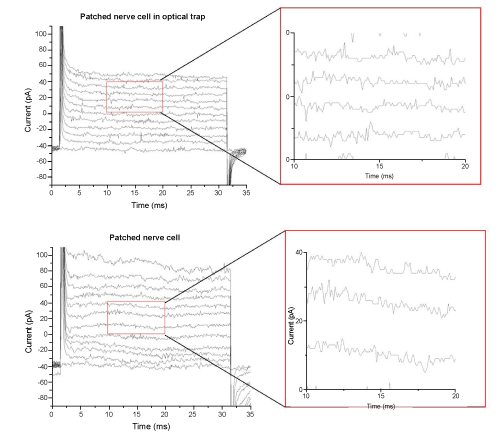
| To assure that the infrared laser of the optical trap did not interfere with the patch-clamp measurements a nerve cell was caught in the optical trap of the tweezers and brought to the recording micropipette of the patch-clamp setup. After successful patch was reached several recordings were made. A protocol of 12 voltage steps from -74 mV to +56 mV with a delta level of 10 mV was applied to evoke voltage gated currents. (Figure 3) shows the protocol applied to a nerve cell in the optical trap and to a nerve cell by conventional patch-clamp experiments. No voltage gated components could be observed in any of these cells. Magnified parts of both recordings are displayed to show that the noise level of the signal does not increase when measuring on cells within an optical trap. |
| Gapfree measurements were recorded in voltage-clamp and current- clamp mode, see (Figure 4). No synaptic events could be detected in any of these recordings. The lack of voltage gated components and synaptic events implied that the nerve cell was not a neuron but a glial cell. |
| The results showed that patching a cell by bringing it to the patchclamp micro-pipette by means of optical tweezers was feasible. Next, the lab-on-a-chip was tested. The closed microfluidic chamber with the integrated micropipette was placed on the microscope stage and connected to the pump systems for the insertion of cells and for the variation of the oxygenation state of the solution. The micropipette was carefully inserted through the hollow screw into the intersection-zone within the microfluidic channel and monitored visually. The cells were introduced to the microfluidic channel system in a low flow rate (0.1 μl/s) to enable the selection of a healthy cell for experiments. Several nerve cells were brought in contact with the micropipette and seals up to 90 MΩ were achieved. However, the viability of the cells was weak and no GΩ seals could be reached. |
| Conclusions |
| We have presented a concept to combine a microfluidic system, patch-clamp and optical techniques for single cell investigations under optimal control of the oxygen content. The viability and the transport of the nerve cells within the lab-on-a-chip need further investigations though, before full understanding. Improvements may include shorter transport of the cells with enhanced optimized flow or by modifying the same microfluidic chamber to act as a Petri dish for direct dissociation of the cells within the chamber. |
| The developed closed microfluidic chamber offered the possibility to analyze individual cells under environmental control with a minimal oxygen-diffusion into the micro channels. In the future, the system will be refined to perform complete electrophysiological investigations with simultaneous monitoring of the biochemical composition of the sample by optical spectroscopy. The simplicity and stability of the system has excellent potential to enable measurements on the function of Ngb upon the electrophysiological functioning of neurons. In the future, this setup will also be combined with optical spectroscopy to assess the biochemical functioning of biological cells. |
| Acknowledgements |
| The authors would like to thank EU Structural fund, Objective 2, Norra Norrland, the Swedish Research Council, and the Kempe Foundation for supporting this work. |
| References |
|
Figures at a glance
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
Post your comment
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 14072
- [From(publication date):
April-2012 - Sep 25, 2024] - Breakdown by view type
- HTML page views : 9683
- PDF downloads : 4389
