Research Article Open Access
Six Months Results of the Duodenal-Jejunal Bypass Liner for the Treatment of Obesity and Type 2 Diabetes
| Eduardo Guimarães Hourneaux De Moura1*, Bruno Da Costa Martins1, Guilherme Sauniti Lopes1, Ivan Roberto Bonotto Orso1, Suzana Lopes De Oliveira1, Marcio C. Mancini1, Manoel Passos Galvão Neto2, Marco Aurélio Santo3, Paulo Sakai1 and Ivan Cecconello3 | |
| 1Department of Gastroenterology, Gastrointestinal Endoscopy Unit, University of São Paulo Medical School, São Paulo, Brazil | |
| 2Department of Gastro Obeso Center, University of São Paulo Medical School, São Paulo, Brazil | |
| 3Department of Gastroenterology - University of São Paulo Medical School, São Paulo, Brazil | |
| Corresponding Author : | Eduardo Guimarães Hourneaux de Moura Av. Dr. Enéas de Carvalho Aguiar 255, Ambulatory Building - 6th floor Endoscopy Unit, 05403-900, São Paulo, Brazil Tel: 55-11-30696460 E-mail: eduardoghdemoura@gmail.com |
| Received October 17, 2011; Accepted April 17, 2012; Published April 19, 2012 | |
| Citation: De Moura EGH, Martins BDC, Lopes GS, Orso IRB, De Oliveira SL, et al. (2012) Six Months Results of the Duodenal-Jejunal Bypass Liner for the Treatment of Obesity and Type 2 Diabetes. J Gastrointest Dig Syst S2:003. doi: 10.4172/2161-069X.S2-003 | |
| Copyright: © 2012 De Moura EGH, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Gastrointestinal & Digestive System
Abstract
Background: The duodenal-jejunal bypass liner (DJBL) is an endoscopic device that excludes the duodenum and initial portion of the jejunum from the contact with the chyme. It has been shown to be effective for weight loss and may improve the control of type 2 diabetes mellitus (T2DM). The objective of this study is to evaluate the efficacy of the DJBL in weight loss and in T2DM control over a 24-week period.
Patients and Methods: Obese (BMI ≥ 35 kg/m²) and T2DM patient candidates for bariatric surgery were prospectively assigned to the implant of the DJBL at an academic endoscopy referral center (São Paulo - Brazil) Results: Twenty-two patients were submitted to the implant procedure, with technical success of 100%. At week 24, mean weight loss was 14 kg (p<0.001). BMI dropped on average 5.4 points and excess weight loss was 22.2%. Fasting glucose (baseline = 171.8 mg/dl; W24 = 141.5 mg/dl) and glycosylated hemoglobin levels (8.8% to 7.3%) were significantly reduced. There was also a reduction in the usage of anti-diabetic medications (except metformin).
Conclusions: The DJBL is an effective weight loss method and also promotes glucose control over a 24-week period. Long-term studies with post-removal follow-up are required to determine its role in the management of type 2 diabetes.
| Keywords |
| Obesity; Diabetes mellitus type 2; Endoscopy; Weight regulation and obesity; Humans |
| Abbreviations |
| T2DM: Type 2 Diabetes Mellitus; BMI: Body Mass Index; DJBL: Duodeno Jejunal Bypass Liner; RYGB: Roux-en-Y Gastric Bypass; HbA1c: Glycosylated Hemoglobin; EWL: Excess Weight Loss; SD: Standard deviation |
| Introduction |
| The World Health Organization (WHO) considers obesity and overweight as one of the greatest pandemics of the century, and estimates that over 2.3 billion adults will be overweight and more than 700 million will be obese by 2015. Obesity is closely related to the development of other metabolic diseases, mainly type 2 diabetes mellitus (T2DM), whose risk of development is 38-fold higher in people with a body mass index (BMI) over 35 kg/m2 [1]. |
| Bariatric surgery has been established as the treatment of choice for morbidly obese patients because it is an effective, lasting treatment that reduces late mortality compared to the untreated population [2,3]. However, bariatric surgery is not free from complications and often requires endoscopic procedures to correct problems such as fistulas and band and ring removal [4]. |
| In addition to the obvious benefit of weight loss, bariatric surgery has shown to be an effective method for the resolution of T2DM, which improves early after the surgical procedure, even prior to significant weight loss [5,6]. Particularly, procedures that exclude the duodenum from the alimentary tract result in higher rates of improvement of T2DM, suggesting that gastrointestinal tract rearrangement leads to improvements in glucose homeostasis [7-9] . |
| Endoscopic procedures offer an alternative therapeutic option for patients who refuse surgery or those patients who are poor surgical candidates. In order to mimic bariatric procedures with malabsorptive components, an endoscopic device capable of providing the functional exclusion of the duodenum and proximal jejunum was developed (Duodeno Jejunal Bypass Liner - EndoBarrier, GI Dynamics, Inc., Lexington, MA). |
| Prior studies demonstrated safety and efficacy of the Duodenal- Jejunal Bypass Liner (DJBL) for short-term weight loss (3 months) in morbidly obese patients [10-12]. An additional unintended effect observed in these studies was the control of T2DM in non-insulin dependent patients. This fact motivated the development of a specific protocol in our Institution to assess the efficacy of the device maintained for 12 months in patients with T2DM who are candidates for bariatric surgery. This article will discuss the 6 months results after the implant procedure. |
| Methods |
| Patients |
| Twenty-two patients underwent endoscopy to implant the DJBL, intended to be maintained for 1 year. Inclusion criteria were patients between 18 to 65 years old with BMI ≥ 35 kg/m2, type 2 diabetes, candidates for bariatric surgery and a history of unsuccessful weight reduction with nonsurgical methods. Main exclusion criteria were anticoagulation therapy, severe coagulopathy, inflammatory bowel disease, biliopancreatic lithiasis, pregnancy, alcohol or drug dependence, previous gastrointestinal surgery that could affect device placement, and patients taking weight loss medication. This study was approved by the Institutional Review Board of our institution (protocol number 0052/07) and written informed consent was obtained from all patients. |
| After the implant, patients were followed through regular weekly outpatient visits during the first month and monthly visits after then. Oral intake was resumed in the first day after the procedure, starting with liquids and increasing the consistency of the meal progressively until achieving the regular diet without restrictions in the third week. All patients received double dose of proton pump inhibitor (omeprazole 80mg) during the study. At the routine visits, laboratory tests and abdominal radiography were performed, weight and waist circumference were measured, and a specific questionnaire for registering symptoms and adverse events was filled out. An upper GI endoscopy was repeated in all patients at week 20, in order to ensure the proper position of the device. |
| Diabetes control was defined as a decrease below 7.0% in glycosylated hemoglobin (HbA1c) levels and partial control was defined as a reduction of more than 1.0% in HbA1c levels, although sustained over 7.0%. |
| Device description |
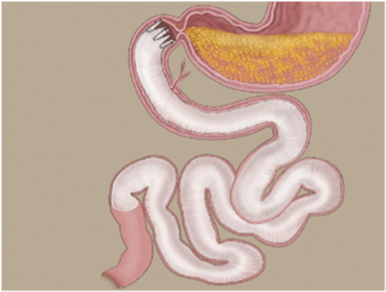
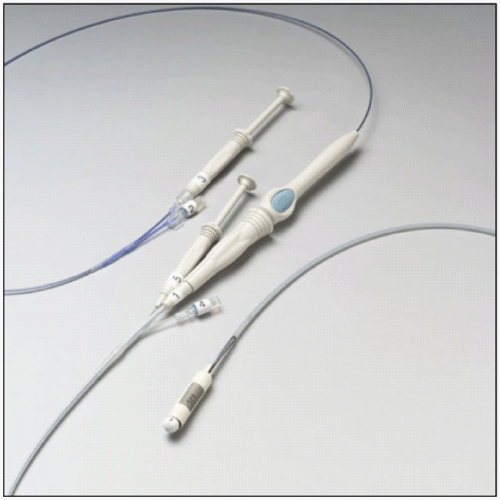
| The DJBL is an endoscopic device consisting of a nitinol anchoring system with lateral fixation barbs and a 62-cm long, ultrathin, impermeable fluoropolymer liner that prevents the contact of the chyme with the duodenal mucosa (Figure 1 and 2) [13]. Implant and explants procedures have been described in previous studies [10,14] and no changes on the delivery technique was done. The procedures were performed under general anesthesia with endotracheal intubation. Initial access to the stomach and duodenum is achieved by a standard gastro scope through which a guide wire is advanced into the duodenum. The encapsulated device on the delivery system catheter (Figure 3) is tracked over the guide wire into the duodenum under fluoroscopic control. The catheter has an atraumatic ball end which is advanced through the intestine extending the sleeve behind itself. After full extension of the sleeve, the anchor is deployed in the duodenal bulb 0.5 cm distally from the pylorus. |
| Removal of the DJBL is achieved with a custom grasper that grasps the polypropylene catheter on the anchor. A foreign body retrieval hood at the tip of the endoscope is used to incorporate the device to avoid any damage of the stomach or esophagus on the way out of the patient’s body. |
| Statistical study |
| Statistical analysis was performed with the use of the PASW™ 18 software (IBM - SPSS renamed in its most recent version). |
| The analysis was carried out through ANOVA (analysis of variance) with repeated measures. The evaluation of data distribution normality in each period was carried out through Kolmogorov-Smirnov test. Whenever there was no adjustment, nonparametric ANOVA with repeated measures test was used. |
| By performing ANOVA with repeated measures, the premise of equality of variances at each time point (Mauchly sphericity test) was examined and the Greenhouse-Geisser correction was used whenever necessary. In the evidence of inequalities between time points, multiple comparisons were carried out between baseline, week 4, week 12, and week 24. A level of significance at 0.05 (α = 5%) was adopted. Descriptive levels (p) lower than these values were considered to be significant. |
| Results |
| Twenty two patients met the inclusion criteria and the DJBL was successfully implanted in all of them (100%). The majority of the patients were female (86.3%), with mean age of 50.8 years (Table 1). The most prevalent comorbidity was arterial hypertension (72.7%) and hepatic steatosis (33.3 %). |
| Safety |
| The most common adverse events observed were abdominal pain (81.8%), nausea (68.2%) and vomiting (54.5%). All events were mild to moderate in intensity and were controlled with oral medications, except for one patient who was diagnosed with an ovarian cancer at the 20 week. Eighteen patients completed 24 weeks of the study. Beside the patient that was explanted early due to an unrelated ovarian cancer, another patient was removed because of noncompliance with the study protocol. Therefore, two patients were actually explanted due to complications related to the device: 1 due to bleeding at 4 weeks, and one due to a persistent abdominal pain at 20 week (Table 3). These four patients were excluded from the final analysis. |
| Weight loss |
| The mean absolute weight decreased from 120.6 kg at the baseline to 106.6 kg at 24 weeks. The mean BMI was 45 kg/m2 at the baseline, and decreased to 39.6 kg/m2 at 24 weeks, a mean decrease of 5.4 kg/m2. The average percentage of excess weight loss (%EWL) at 4, 12 and 24 weeks was 14.8%, 20% and 22.8% respectively. The waist circumference decreased from 133.7 to 122.5 cm. By comparing the baseline values with each time point of the study, a significant difference (p < 0.001) was found in the percentage of excess weight loss, mean absolute weight, body mass indices and waist circumference (Table 4). |
| Glycemic control |
| The changes in plasma glucose, HbA1c, insulin and c-peptide are shown in the Table 4. Plasma glucose decreased as early as 1 week after implantation and remained lower until the 24 week. HbA1c decreased from 8.8% at the baseline to 7.3% at 6 months (p<0,001). Diabetes control occurred in 11 patients (61,1%), partial control was observed in six patients (33.3%) and one (5.5%) did not show any improvement. Considering both control and partial control of diabetes, 94.5% of patients presented an improvement with DJBL. |
| The insulin and c-peptides values decreased until the 12 week (p=0.006 and p=0.04 respectively) but then there was a raise in the 24 week that was not statistically different compared to the baseline. We also find a reduction in the use of oral hypoglycemic agents, so that at the end of the study, there was a prevalence of the use of metformin alone (Table 5). |
| Discussion |
| Bariatric surgery is a well-established method for the treatment of morbid obesity [15] and this type of procedure has progressively been recognized as an effective, long-lasting treatment for T2DM [5,16,17]. The rapid improvement in diabetes after these procedures and the observation that those involving a duodenal bypass present better diabetes improvement indices, suggests a possible hormonal role of the gut in glucose homeostasis [18]. |
| Rubino et al. [19] have shown that the duodenal exclusion plays a fundamental role in the regulation of glucose levels in diabetic rats, and recently, these authors suggested that T2DM might be an operable intestinal disease and proposed a possible regulatory mechanism that places special importance on the proximal small bowel in the process [7]. |
| Therefore, the DJBL device emerges as a nonsurgical option for duodenal exclusion and diabetes control [13,14,20]. Its liner acts as a barrier and probably alters the normal release of hormones originated by the contact of the chyme with the duodenal mucosa and the proximal jejunum. It is extremely thin and flexible, allowing peristaltic movements to normally occur [10,20]. |
| Rodriguez-Grunert et al. [10] published the first experience with a duodenal exclusion endoscopic device in human subjects and the mean excess weight loss was 23.6% after 12 weeks in the 10 patients enrolled in the study. An unintended result was the observation that the four T2DM patients normalized their glycemic levels and discontinued diabetes medications. This fact motivated our study that included only patients with T2DM. Tarnoff et al. [11] published the first randomized controlled study for weight loss involving 25 patients implanted with DJBL versus 14 patients as a diet control group. The device was maintained for 12 weeks and both groups received the same counseling to follow a low-calorie diet. Excess weight loss was greater in the device group (22% versus 5% - p < 0.001) demonstrating its efficacy in shortterm weight loss. In another multicenter study conducted by Schouten et al. [12], 26 morbidly obese patients were implanted with DJBL and 11 patients were in the diet control group. Both groups received the same dietary instructions. Mean excess weight loss after 3 months was 19.0% for device patients versus 6.9% for control patients (p < 0.002), and absolute BMI change at 3 months was 5.5 and 1.9 kg/m2, respectively. An additional effect was the improvement in 7 of 8 patients with T2DM. |
| In this study, we found a mean weight loss of 14 kg after 24 weeks of device implant. The average percentage of EWL was 22.2% (p < 0,001) and there was a significant reduction in waist circumference (11.2 cm) and body mass index (5.4 kg/m2). These results are similar to the results described in a large review of 3.608 patients submitted to intragastric balloon therapy: weight loss of 14.7 kg, BMI reduction of 5.7 kg/m2, and 32.1% reduction of the excess weight [21]. Similarly to the intragastric balloon, our patients also reported an increased sense of fullness, resulting in reduction of oral intake. |
| Glycemic control could be observed as soon as 4 weeks after the procedure with a continued decrease up to 24 weeks, which corroborates the thesis, that duodenal exclusion - and not weight loss - is the major factor responsible for this control. Conversely, the reduction in glycosylated hemoglobin levels was slower and more constant because it is a long-term marker. Type 2 diabetes improvement with the DJBL was 94.5%, and one patient (5.5%) did not present any change in glycemic control (characterized by HbA1c drop less than 1%). |
| A substantial reduction in the use of hypoglicemic drugs was observed. The number of patients using sulphonylurea decreased 62.5% and 5 patients was out of hypoglycemic at 6 months. The majority of subjects (13/18) remained under treatment with metformin. Since this is the first line of treatment, it was established that it would be the last to be suspended. It is important to emphasize that during the study, 4 hypoglycemia episodes were reported by 4 patients. All episodes were considered to be mild and were resolved with no squeal. |
| The DJBL mimics some of the features of the Roux-en-Y gastric bypass (RYGB), such as exclusion of the proximal intestine from the flow of nutrients, arrival of ingested nutrients directly to the jejunum, segregation of digestive secretions from the flow of nutrients, and arrival of nutrients partially digested to the distal intestine [22,23], which in part could explain the similarities between the two procedures in controlling the metabolic syndrome. |
| Limitations to this protocol are the lack of a control group to assess the efficacy of diabetes treatment and non-dosage of gastrointestinal hormones that might collaborate to understand diabetes resolution mechanisms. |
| This temporary endoscopic device for duodenal-jejunal exclusion envisages a non-operative therapeutic possibility, positioned between pharmacologic drugs and bariatric surgery. It promotes weight loss, reduction of body mass index and waist circumference and also improves the control of glucose, glycosylated hemoglobin, insulin, and C-peptide levels throughout 24 weeks. The effects triggered by the presence of this device allowed the reduction and even the suspension of medications to control type 2 diabetes. Long-term studies with postremoval follow up are required to determine its role in the management of type 2 diabetes. |
References
- Colditz GA, Willett WC, Rotnitzky A, Manson JE (1995) Weight gain as a risk factor for clinical diabetes mellitus in women. Ann Intern Med 122: 481-486.
- Blackburn GL, Hutter MM, Harvey AM, Apovian CM, Boulton HR, et al. (2009) Expert panel on weight loss surgery: executive report update. Obesity (Silver Spring) 17: 842-862.
- Williamson DF, Thompson TJ, Thun M, Flanders D, Pamuk E, et al. (2000) Intentional weight loss and mortality among overweight individuals with diabetes. Diabetes Care 23: 1499-1504.
- Neto MP, Ramos AC, Campos JM, Murakami AH, Falcao M, et al. (2009) Endoscopic removal of eroded adjustable gastric band: lessons learned after 5 years and 78 cases. Surg Obes Relat Dis 6: 423-427.
- Lima J, Helena L, Oliveira S, Viana L, Godoy E (2005) Rapid resolution of diabetes after gastric bypass. Obes Surg 15: 448-449.
- Torquati A, Lutfi R, Abumrad N, Richards WO (2005) Is Roux-en-Y gastric bypass surgery the most effective treatment for type 2 diabetes mellitus in morbidly obese patients? J Gastrointest Surg 9: 1112-1116.
- Rubino F (2008) Is type 2 diabetes an operable intestinal disease? A provocative yet reasonable hypothesis. Diabetes Care 31 Suppl 2: S290-296.
- Naslund E, Kral, J.G (2006) Impact of Gastric Bypass Surgery on Gut Hormones and Glucose Homeostasis in Type 2 Diabetes. Diabetes 55: S92-S97.
- Martins MCDC, Souza, A.A.P (2007) Mechanisms of surgical control for type 2 diabetes mellitus after bariatric surgery Rev Col Bras Cir 34: 343-346.
- Rodriguez-Grunert L, Galvao Neto MP, Alamo M, Ramos AC, Baez PB, et al. (2008) First human experience with endoscopically delivered and retrieved duodenal-jejunal bypass sleeve. Surg Obes Relat Dis 4: 55-59.
- Tarnoff M, Rodriguez L, Escalona A, Ramos A, Neto M, et al. (2009) Open label, prospective, randomized controlled trial of an endoscopic duodenal-jejunal bypass sleeve versus low calorie diet for pre-operative weight loss in bariatric surgery. Surg Endosc 23: 650-656.
- Schouten R, Rijs CS, Bouvy ND, Hameeteman W, Koek GH, et al. (2010) A multicenter, randomized efficacy study of the EndoBarrier Gastrointestinal Liner for presurgical weight loss prior to bariatric surgery. Ann Surg 251: 236-243.
- Fishman E, Melanson D, Lamport R, Levine A (2008) A novel endoscopic delivery system for placement of a duodenal-jejunal implant for the treatment of obesity and type 2 diabetes. Conf Proc IEEE Eng Med Biol Soc 2008: 2501-2503.
- Gersin KS, Keller JE, Stefanidis D, Simms CS, Abraham DD, et al. (2007) Duodenal- jejunal bypass sleeve: a totally endoscopic device for the treatment of morbid obesity. Surg Innov 14: 275-278.
- Arteaga JR, Huerta S, Basa NR, Livingston EH (2003) Interval jejunoileal bypass reduces the morbidity and mortality of Roux-en-Y gastric bypass in the super-obese. Am Surg 69: 873-878.
- Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, et al. (2004) Bariatric surgery: a systematic review and meta-analysis. JAMA 292: 1724-1737.
- Scopinaro N, Marinari GM, Camerini GB, Papadia FS, Adami GF (2005) Specific effects of biliopancreatic diversion on the major components of metabolic syndrome: a long-term follow-up study. Diabetes Care 28: 2406-2411.
- Bose M, Oliván B, Teixeira J, Pi-Sunyer FX, Laferrère B (2009) Do Incretins play a role in the remission of type 2 diabetes after gastric bypass surgery: What are the evidence? Obes Surg 19: 217-229.
- Rubino F, Marescaux J (2004) Effect of duodenal-jejunal exclusion in a non-obese animal model of type 2 diabetes: a new perspective for an old disease. Ann Surg 239:1-11.
- Milone L, Gagner M, Ueda K, Bardaro SJ, Ki-Young Y (2006) Effect of a polyethylene endoluminal duodeno-jejunal tube (EDJT) on weight gain: a feasibility study in a porcine model. Obes Surg 16: 620-626.
- Imaz I, Martínez-Cervell C, García-Alvarez EE, Sendra-Gutiérrez JM, González-Enríquez J (2008) Safety and effectiveness of the intragastric balloon for obesity. A meta-analysis. Obes Surg 18: 841-846.
- Goldfine AB, Shoelson SE, Aguirre V (2009) Expansion and contraction: treating diabetes with bariatric surgery. Nat Med 15: 616-617.
- Mun EC, Blackburn GL, Matthews JB (2001) Current status of medical and surgical therapy for obesity. Gastroenterology 120: 669-681.
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 | Table 4 | Table 5 |
Figures at a glance
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 |
Relevant Topics
- Constipation
- Digestive Enzymes
- Endoscopy
- Epigastric Pain
- Gall Bladder
- Gastric Cancer
- Gastrointestinal Bleeding
- Gastrointestinal Hormones
- Gastrointestinal Infections
- Gastrointestinal Inflammation
- Gastrointestinal Pathology
- Gastrointestinal Pharmacology
- Gastrointestinal Radiology
- Gastrointestinal Surgery
- Gastrointestinal Tuberculosis
- GIST Sarcoma
- Intestinal Blockage
- Pancreas
- Salivary Glands
- Stomach Bloating
- Stomach Cramps
- Stomach Disorders
- Stomach Ulcer
Recommended Journals
Article Tools
Article Usage
- Total views: 13559
- [From(publication date):
specialissue-2011 - Sep 19, 2024] - Breakdown by view type
- HTML page views : 9201
- PDF downloads : 4358
