Research Article Open Access
Biosensor-based Binding Assay for Platelet-Derived Growth Factor Receptor-�?± Autoantibodies in Human Serum
Massimiliano Cuccioloni1†*, Gianluca Moroncini2,3†, Matteo Mozzicafreddo1, Katarzyna N Pozniak2, Giulia Nacci4, Antonella Grieco2, Chiara Paolini2, Cecilia Tonnini2, Ada Funaro4, Mauro Angeletti1, and Armando Gabrielli2,3
1School of Biosciences and Biotechnology, University of Camerino, Camerino, Italy
2Dipartimento di Scienze Cliniche e Molecolari, Università Politecnica delle Marche, Ancona, Italy
3Clinica Medica, Ospedali Riuniti Ancona, Italy
4Dipartimento di Scienze Mediche, Università di Torino, Italy
†These authors equally contributed to the manuscrip
- *Corresponding Author:
- Massimiliano Cuccioloni
School of Biosciences and Biotechnology
University of Camerino
62032 Camerino, Italy
Tel: +39 0737 403247
E-mail: massimiliano.cuccioloni@unicam.it
Received date: March 27, 2013; Accepted date: May 15, 2013; Published date: May 17, 2013
Citation: Cuccioloni M, Moroncini G, Mozzicafreddo M, Pozniak KN, Nacci G, et al. (2013) Biosensor-based Binding Assay for Platelet-Derived Growth Factor Receptor-α Autoantibodies in Human Serum. J Anal Bioanal Tech S7:010. doi:10.4172/2155-9872.S7-010
Copyright: © 2013 Cuccioloni M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
Biosensors are versatile tools for monitoring molecular interactions. Herein, we report a novel assay designed to detect anti-platelet-derived growth factor receptor α (PDGFRα) autoantibodies in the serum of patients affected by systemic sclerosis (SSc), an autoimmune disease of the connective tissue. The assay was based on resonant mirror sensing, and used recombinant PDGFRα as molecular “bait” for anti-PDGFRα autoantibodies (IgG class). The selection and optimization of the analytical procedure required a preliminary investigation on the preservation of the native-like conformation of the receptor following the immobilization procedure. The PDGFRα-based biosensor was used to test IgG purified from sera of SSc patients and healthy controls (HC). The specificity and the reversibility of the interaction permitted a rapid analysis, a single detection test requiring only a few minutes. Remarkably, this assay could discriminate between SSc patients and HC. This receptor-based biosensor, based on the reversible interaction between a blocked macromolecule and soluble ligands, and with major advantages such as the rapidity, the reusability of the capturing surface and the low cost per single test, could represent a promising approach for the diagnosis of SSc and other diseases characterized by anti-receptor autoantibodies or other bioactive ligands to cellular receptors.
Keywords
Systemic sclerosis; Scleroderma; PDGFR; Autoantibodies; Biosensor
Introduction
Systemic sclerosis, or scleroderma, (SSc) is a chronic autoimmune disease of the connective tissue characterized by clinical heterogeneity, unpredictable course, high morbidity/mortality and lack of adequate disease-modifying drugs [1]. The variety and the clinical relevance of autoantibodies in SSc patients have been extensively studied [2-5]. Agonistic autoantibodies (abs) targeting the PDGF receptor (PDGFR) have been detected by a cell-based bioassay based on the production of reactive oxygen species (ROS) with immunoglobulins (IgG) purified from serum of SSc patients [6] and proposed as novel biomarkers of SSc. However, subsequent reports questioned the presence of agonistic anti-PDGFR abs in SSc [7,8]. To address this issue, we sought to circumvent the pitfalls associated with cell-based assays and obtain a specific binding assay for detection of anti-PDGFRα IgG. Thus, we developed a solid-phase assay based on a recombinant human PDGFRα conformer spanning the extracellular, transmembrane and part of the intracellular regions fused to a poly-Histidine tail (His tag, PDGFRα-His) to be used as a capturing molecule for autoantibodies. Besides providing adequate sensibility/specificity, the resonant mirror sensing platform was chosen since it allows the conformational control of the immobilized recombinant PDGFRα, which we hypothesized to be critical for the specific detection of agonistic IgG likely binding to conformational epitopes of the receptor. On such background, herein we propose a rapid method for the detection of anti-PDGFRα IgG purified from human serum, based on the increase in the response signal upon their addition to a PDGFRα-derivatized biosensor, eventually enabling discrimination between SSc and healthy controls.
Materials and Methods
Reagents, chemicals, and devices
The carboxylate-functionalized cuvette and the immobilization chemicals (N-hydroxysuccinimide; 1-ethyl-3- (3-dimethylaminopropyl)-carbodiimide; ethanolamine) were obtained from Neosensors (Crewe, UK). NaOH, NaCl, NaH2PO4 were obtained from J.T. Baker (Netherlands). Tween-20 and SDS were obtained from Sigma-Aldrich (Milan, Italy). All chemicals were of analytical grade. Biosensor analyses were performed on an IAsys plus device, Affinity Sensors (Cambridge, UK). Conformational control ligands were recombinant human PDGF-BB (R&D Systems) and mouse antihuman PDGFRα monoclonal antibody mab 322 (R&D Systems).
Generation and purification of human PDGFRα-His
Two variants of recombinant human His-tagged PDGFRα (rhPDGFRα-His) were generated by inserting cDNA encoding amino acids 1-834 or 1-560 in the pcDNA V5 HIS A vector (Invitrogen), in order to fuse the COOH-terminal of the protein with a 6x Histidine tag. Upon sequencing, the constructs were stably transfected into HeLa cells (ATCC) using Lipofectamine 2000 (Invitrogen). Transfected cells were cloned in selection medium containing G418 (1 mg/mL, Sigma- Aldrich) and cells with the highest membrane expression of PDGFRα were selected by FACS analysis using anti-PDGFRα mab 322 followed by FITC-labeled secondary antibody (Jackson Immunoresearch) staining. rhPDGFRα-His was purified from total protein extracts by metal-ion affinity chromatography (HiTrap chelating columns, Amersham) and dialyzed against PBS to remove imidazole traces. The quality of purified rhPDGFRα-His was assessed by immunoblotting with a rabbit anti-human PDGFRα antibody (D01P, Abnova) followed by HRP-conjugated goat anti-rabbit IgG (Santa Cruz).
Patients
Patients (n=8) with a definite diagnosis of SSc [9] and sex- and age-matched healthy controls HC (n=8) were chosen upon informed consent as serum donors. The clinical features of SSc patients included in the study are described in table 1.
| Characteristics of SSc patients (n=8) | |
|---|---|
| Sex; female/male ratio | 2/1 |
| Age; median age [age range] (years) | 60.7[38-72] |
| Limited/diffuse ratio | 1.75/1 |
| Average duration of disease (years) | 6.5 |
| Average Rodnan Skin Score | 12.5 |
| ANA (% positive) | 92% |
| Anti-Scl 70 (% positive) | 55.6% |
| Anticentromere antibody (% positive) | 29.5% |
| Internal organ involvement; % of lung and/or GI tract and/or heart involvement | 65.7% |
Table 1: Characteristics of SSc patients.
Purification of IgG from serum
IgG were purified by individual protein A/G resin columns (Pierce) from sera of 8 SSc patients and 8 healthy controls (HC). After elution with glycine at pH 2.2 and neutralization with Tris buffer, the fractions containing IgG were subjected to size exclusion chromatography (5000 MWCO, Thermo Scientific) to remove trace amounts of contaminating cytokines. The absence of PDGF contaminations in IgG preparations was verified by immunoblotting with a primary polyclonal rabbit anti-human PDGF-BB antibody (Abcam) with detection limit of 0.1 ng cytokine/200 μg IgG.
Immobilization of rhPDGFRα-His
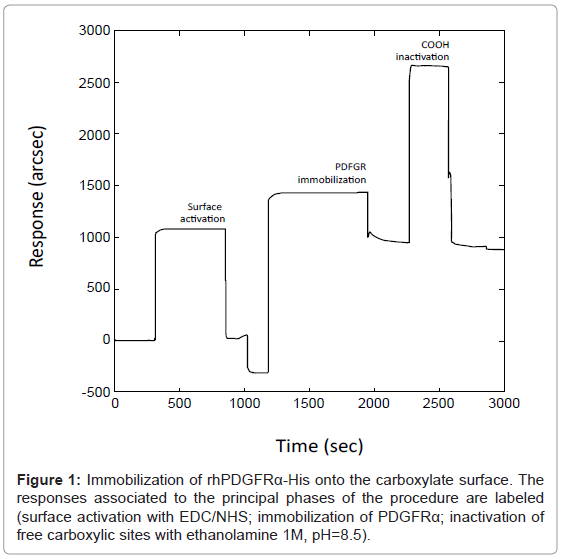
The sensing chamber was thermostatted at 25°C. The carboxylate surface was washed and equilibrated with PBS buffer (10 mM Na2HPO4, 2.7 mM KCl, 138 mM NaCl, pH=7.4), then activated by addition of an equimolar mixture of N-hydroxysuccinimide and N-ethyl-N-(dimethylaminopropyl) carbodiimide hydrochloride [10]. rhPDGFRα-His was dialyzed against 10 mM CH3COONa buffer pH 5, then anchored to the carboxylic surface via the N-terminus of histidine tail. Free carboxylic sites on the sensor surface were deactivated by injection of 1 M ethanolamine, pH 8.5. The surface was finally re-equilibrated with PBS. Conformational control ligands (namely, PDGF-BB and mab322) were added, and binding kinetics were monitored up to equilibrium. Dissociation steps were performed by a single 1 min wash (80 μL) with fresh PBS buffer, whereas surface regeneration was achieved by serial PBS washes (the number of washing cycles depending on the levels of captured ligand) to rhPDGFRα baseline recovery prior to any further addition of ligands. Raw data were globally fitted both to monophasic and biphasic models. The validity of each model to fit a particular time course was assessed by a standard F-test procedure [11].
Binding assays
IgG purified from serum of patients and HC were identified with a letter code and tested individually in blind fashion. IgG solutions were added to the PDGFRα-functionalized surface, and each response kinetic was monitored up to equilibrium. The dissociation of the complexes and the regeneration of the PDGFRα monolayer were carried out by serial PBS washes. Each IgG sample was analyzed in triplicate. Values falling outside the 95% confidence interval were considered significantly different from controls. Detection procedures were replicated on different days both on the same and on different PDGFRα-functionalized surfaces. Additionally, assessment of the number of regeneration cycles that a sensor surface can withstand without significant loss of sensitivity and accuracy of the assay and evaluation of the stability of the sensing surface throughout multiple measurements were performed.
Results
Optimization of the binding assay
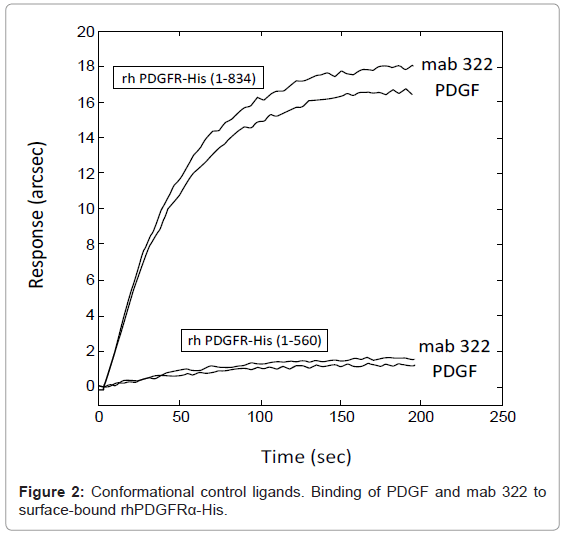
To develop an efficient biosensor-based assay for the detection of ligand binding to PDGFRα, the best experimental conditions were achieved taking into account all the parameters such as PDGFRα and ligand concentrations, immobilization pH, temperature, buffering. The binding surface containing immobilized receptor was obtained as described in the Experimental Section (Figure 1). A shift in sensor response (ΔR ≈ 1000 arcsec) upon rh PDGFRα-His immobilization was reported: these conditions resulted in the coupling of a partial ‘Langmuir’ monolayer (70% surface occupied) corresponding to a final surface concentration of 1.7 ng/mm2 (approximately equivalent to 7 mg/mL). These data were adequate, since immobilization levels should be moderately high for screening applications, in order to maximize the number of available binding sites, and to limit steric hindrance/shielding effects due to overcrowding, which could affect the analysis. The immobilization protocol was optimized after several experiments based on the variation of receptor concentration and immobilization pH value. The immobilization pH value of 5 (chosen upon the PDGFRα isoelectric point=5.5) allowed an optimal immobilization of rh PDGFRα-His with preservation of its native-like conformation as assessed by conformational control ligands. A receptor concentration of 300 μg/mL was chosen to obtain the optimal surface density of PDGFRα molecules: in fact, the assay sensitivity may be impaired not only by an insufficient surface density, but also by an excessive surface density potentially hindering the accessibility of soluble ligands to the binding domains of the receptor or favoring dimerization between blocked PDGFRα macromolecules in absence of ligands, consequently reducing the number of available binding sites on the sensing surface. Preservation of the native-like conformation of immobilized rhPDGFRα-His was tested each time using PDGF-BB and mab 322, ligands binding only to conformational epitopes of the extracellular PDGFRα domain. As highlighted in figure 2, disruption of the native folding caused loss of rhPDGFRα-His binding to these ligands. Local and global fit analysis of the interaction data generally revealed monophasic kinetics. Specifically, monoexponential analysis of association curves residuals was not affected by measurable systematic errors (a biexponential model did not considerably improve the quality of the fit as judged by an F-test, 95% confidence).
Application of the binding assay
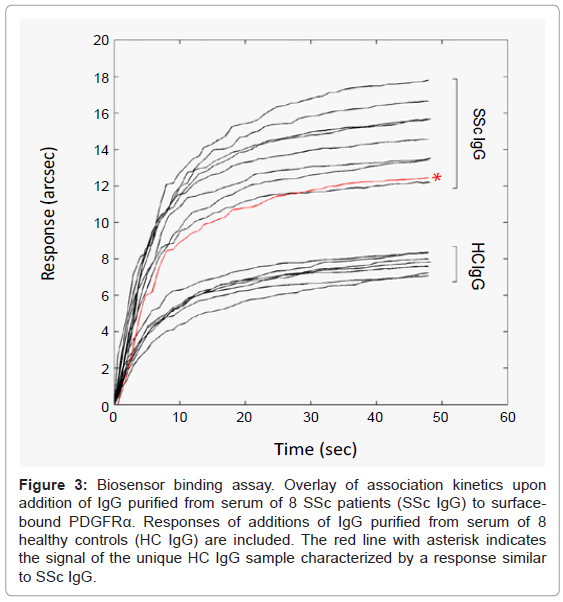
Biosensor-based assay was applied to human IgG purified from serum. The test allowed the identification of anti-PDGFRα IgG fractions in total IgG pools. The final in-assay concentration of total IgG granting an unambiguous response within 1 min was 120 μg/mL (0.8 μM). Most importantly, based on the extent of the responses, the analysis of the binding of IgG to rhPDGFRα-His generally discriminated between SSc and HC group (Figure 3). SSc signals were always higher than 2 SD above the mean value of the HC population (ΔR= 8.35 ± 1.77 arcsec): specifically, all SSc IgG samples generated responses in the range of 12-18 arcsec, whereas responses obtained with HC samples were lower than 8.2 arcsec, except for one HC sample (12 arcsec).
Figure 3: Biosensor binding assay. Overlay of association kinetics upon addition of IgG purified from serum of 8 SSc patients (SSc IgG) to surfacebound PDGFRα. Responses of additions of IgG purified from serum of 8 healthy controls (HC IgG) are included. The red line with asterisk indicates the signal of the unique HC IgG sample characterized by a response similar to SSc IgG.
Reusability and efficiency of the biosensor
The effect of different regeneration agents on the biosensor reusability was studied. The complete dissociation of the IgG-receptor complexes was performed by exposure to both acidic and neutral buffer solutions (PBS binding buffer). Performing two rapid 15 sec pulses of 10 mM HCl (80 μL each), the biosensor surface could be used without loss of activity for 10 measurement cycles before any significant loss of binding capacity was observed. On the other hand, the sensing surface resisted to a higher number of experimental cycles if it was washed repeatedly with 80 μL of PBS buffer (biosensor response did not change more than 5% after 50 regeneration cycles). In this case, the number of washes varied with the levels of the captured ligand and with the affinity of the ligand-receptor complex. Consequently, the use of PBS provided an efficient (although slower) desorption of the ligand without degrading the immobilized receptor.
Conclusion
Biosensors are versatile tools with a wide range of applications [12-14]. From the immunological perspective, biosensors allow the real-time and label-free monitoring of the recognition events associated with the formation of antigen-antibody complexes. To our knowledge, the use of biosensors for autoantibody detection in serum of patients affected by autoimmune diseases has never been reported.
Herein we described the development of a human PDGFRα biosensor for the detection of anti-PDGFRα autoantibodies in the serum of patients affected by systemic sclerosis, whose biological implications have been discussed in the introduction. The PDGFRα biosensor assay is based on the ability of anti-PDGFRα IgG purified from serum to specifically recognize a natively folded, surface-bound human PDGFRα, and makes use of a biosensor-based method to measure the response upon binding of the analytes, i.e. IgG purified from serum of different SSc patients and healthy controls. The PDGFRα biosensor assay displayed some general advantages such as the rapidity of the test and the minimal consumption of reagents. In fact, it was generally possible to obtain unequivocal results suitable for rapid testing of the biological samples within 1 minute, using only few microliters of IgG preparations, and the biosensor could be stored at 4°C for weeks after the first experimental session and reused for subsequent analytical sessions afterwards. Specific advantage of this assay was the excellent sensitivity/specificity ratio, enabling detection of the very small anti-PDGFRα autoantibody fractions within the total IgG pools in all cases analyzed, but with a clear cutoff value discriminating between the IgG samples obtained from SSc patients and those obtained from HC. Critical, from this perspective, was the preservation of the PDGFRα native-like conformation in the biosensor, that can certainly account both for the confirmation of the presence of anti-PDGFRα autoantibodies in the serum of SSc patients and for the identification of a difference between SSc and HC IgG. The only HC subject characterized by a biosensor response over the cutoff value will undergo clinical follow-up in order to monitor the possible onset of SSc.
This receptor-based biosensor could represent a promising tool for the diagnosis and clinical monitoring of SSc. The same methodological approach may be rewarding in other diseases characterized by antireceptor autoantibodies or by bioactive pathological ligands to cellular receptors.
Acknowledgements
This work was supported by a Young Investigator Award from Gruppo Italiano Lotta alla Sclerodermia (GILS) to G.M., grants from Ministero Italiano per l’Università e la Ricerca Scientifica to A.G., and A.F., grants from Fondazione Cariverona and Fondazione Italiana Ricerca Artrite (FIRA) to A.G., and from Fondazione di Medicina Molecolare e Terapia Cellulare, Università Politecnica delle Marche, Ancona, Italy.
References
- Gabrielli A, Avvedimento EV, Krieg T (2009) Scleroderma. N Engl J Med 360: 1989-2003.
- Chizzolini C, Raschi E, Rezzonico R, Testoni C, Mallone R, et al. (2002) Autoantibodies to fibroblasts induce a proadhesive and proinflammatory fibroblast phenotype in patients with systemic sclerosis. Arthritis Rheum 46: 1602-1613.
- Gabrielli A, Svegliati S, Moroncini G, Avvedimento EV (2007) Pathogenic autoantibodies in systemic sclerosis. Curr Opin Immunol 19: 640-645.
- Hamaguchi Y (2010) Autoantibody profiles in systemic sclerosis: predictive value for clinical evaluation and prognosis. J Dermatol 37: 42-53.
- Riemekasten G, Philippe A, Näther M, Slowinski T, Müller DN, et al. (2011) Involvement of functional autoantibodies against vascular receptors in systemic sclerosis. Ann Rheum Dis 70: 530-536.
- Baroni SS, Santillo M, Bevilacqua F, Luchetti M, Spadoni T, et al. (2006) Stimulatory autoantibodies to the PDGF receptor in systemic sclerosis. N Engl J Med 354: 2667-2676.
- Loizos N, Lariccia L, Weiner J, Griffith H, Boin F, et al. (2009) Lack of detection of agonist activity by antibodies to platelet-derived growth factor receptor alpha in a subset of normal and systemic sclerosis patient sera. Arthritis Rheum 60: 1145-1151.
- Classen JF, Henrohn D, Rorsman F, Lennartsson J, Lauwerys BR, et al. (2009) Lack of evidence of stimulatory autoantibodies to platelet-derived growth factor receptor in patients with systemic sclerosis. Arthritis Rheum 60: 1137-1144.
- Johnson SR, Feldman BM, Hawker GA (2007) Classification criteria for systemic sclerosis subsets. J Rheumatol 34: 1855-1863.
- Davies RJ, Edwards PR, Watts HJ, Lowe CR, Buckle PE, et al. (1994) The resonant mirror: a versatile tool for the study of biomolecular interactions. Protein Chemistry.
- Mozzicafreddo M, Cuccioloni M, Bonfili L, Eleuteri AM, Fioretti E, et al. (2008) Antiplasmin activity of natural occurring polyphenols. Biochim Biophys Acta 1784: 995-1001.
- Cuccioloni M, Amici M, Eleuteri AM, Biagetti M, Barocci S, et al. (2005) Binding of recombinant PrPc to human plasminogen: kinetic and thermodynamic study using a resonant mirror biosensor. Proteins 58: 728-734.
- Cuccioloni M, Mozzicafreddo M, Barocci S, Ciuti F, Pecorelli I, et al. (2008) Biosensor-based screening method for the detection of aflatoxins B1-G1. Anal Chem 80: 9250-9256.
- Cuccioloni M, Mozzicafreddo M, Spina M, Tran CN, Falconi M, et al. (2011) Epigallocatechin-3-gallate potently inhibits the in vitro activity of hydroxy-3-methyl-glutaryl-CoA reductase. J Lipid Res 52: 897-907.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 14369
- [From(publication date):
specialissue-2013 - Nov 17, 2025] - Breakdown by view type
- HTML page views : 9665
- PDF downloads : 4704