Candidaemia in Immune-Compromised Hosts: Incidence and Drugs Susceptibility
Received: 21-Sep-2012 / Accepted Date: 17-Oct-2012 / Published Date: 20-Oct-2012 DOI: 10.4172/2161-0681.1000131
Abstract
Abstract
We analyzed the different Candidae species isolated from bloodstream infections and the related antifungal
susceptibility pattern over a five-year period (2007-2011) at Policlinico Umberto I of Rome (Italy).
The overall incidence of candidaemia during this range of time accounted for 6.4% with a marked increase from
2007 to 2011 (from 3.83% to 9%). The species isolated were the following: C. albicans (42%) and non-albicans (58%).
In the period 2007-2009, C.krusei was the most frequent species detected among non-albicans species (29.4%),
followed by C.glabrata (20%), whereas during 2010-2011 we found a shift versus C. parapsilosis increasing from 4.6%
to 33%.
C.albicans showed a good susceptibility to the most antifungal agents. C. krusei exhibited a raising resistance to
flucytosin and fluconazole. As for fluconazole, it shows an intrinsic resistance. In C. glabrata we observed increased
resistance especially to fluconazole for which this species shows a Susceptibility Dose-Dependent (SDD). C.tropicalis
had only a resistance to fluconazole (MIC 90%=16 mcg/ml) In vitro activity of the echinocandins resulted to be very
strong for all Candidae species. These drugs have a fungicidal activity and their use is recommended in the most
severe diseases.
Our study confirms the high incidence of candidaemia in the setting of critically ill patients. A strong association of
Candidae infection with the presence of Central Venous Catheter (CVC) has been markedly noticed especially in the
years 2009 and 2011 due to a greater use of CVC in our patients. The overall rate of resistance increased over the
study period in all Candidae strains.
Keywords: C. albicans and non-albicans; Candidaemie; Antimycotic agents susceptibility; Azoles; Echinocandins; Central Venous Catheter (CVC)
308532Introduction
Candidaemia is increasing all over the world in recent years mainly in critically ill patients. Invasive Candidiasis (IC) is becoming an important nosocomial infection. Bloodstream Infections (BSIs) caused by Candidae spp are a major reason of morbidity and mortality in immune-compromised hosts. The prevalence of fungal infections has progressively increased, because the population of immune-deficient subjects has expanded due to advances in supportive therapy as well as the number of elderly people in our society. In these patients, the most common infections caused by Candidae spp are invasive candidiasis and Candidaemia [1,2].
The incidence of IC was remarkably consistent in US increasing from 1990 to 2009 of about 300% in parallel with the increase of bacterial infections. In US, as well as wordlwide, C. albicans was the most frequently isolated fungal species with 43.6% of occurrence followed by C. glabrata ( 26.7%) , C.parapsilosis (16%) , C. tropicalis (10%) ,C. krusei (2%) etc. The distribution of Candidae spp is quite different in US and in Europe. In Europe and in Canada, blood-stream infections by Candidaeare lower than that reported from United States where C. glabrata is the most frequent species among non-albicans Candidae.. In any case, a shift to non-albicans species and a growing resistance to the common antifungal drugs have been noticed over the last years [3,4].
Candidae spp are part of the normal flora of human skin and mucosa gastrointestinal tract: the Candidae species are differently distributed in the various districts of the organism. Based on experimental studies and using molecular biology techniques, it has been confirmed that the most frequent origins of Candidaemia are the gastrointestinal tract (via bacterial translocation) and the skin (in critically ill patients with venous catheters). [5]
A model for IC can be explained through an insult on the epithelial cells of gastro-intestinal tract and the use of antibiotics affecting the normal intestinal flora. This may select Candidae species consequently promoting the infection , the following translocation from gastrointestinal tract to bloodstream finally leading to the disseminated disease and eventual colonization of Central Venous Catheter (CVC).
In non-albicans Candidae the presence of risk factors must be deeply evaluated especially as far as prophylaxis with fluconazole is concerned [6]. The prophylaxis with fluconazole is considered an important risk factor for these Candidae. In fact its use in the prophylactic strategy can lead to the selection of resistant strains especially in C. glabrata and C. krusei. C. glabrata resistance is dependent on the dose of fluconazole used in therapy whereas C. krusei is intrinsically resistant to azoles [7].
Aim of the study was to analyze the different Candidae species isolated from blooadstream infections and the related antifungal susceptibility pattern over a five-year period (2007-2011) at Policlinico Umberto I° in Rome (Italy).
Materials and Methods
Ninety-five critically ill patients coming from Surgery, Neonatal and Paediatric Intensive Care Unit, Paediatric Onco-haematology, Internal Medicine, Infectious Disease wards etc. were examined for blood-stream infections by Candidae spp through the period 2007- 2011 at Policlinico Umberto I° of Rome (Italy). The patients age ranged from less than 1 year to 78 years. All subjects were evaluated for the presence of risk factors such as age, Candidae colonization, use of broad-spectrum antibiotics, parenteral nutrition, severity of underlying disease, CVC, length of hospital stay etc. Each one presented one or more risk factors for this infection (see below).
We totally examined in the period under study 16704 blood samples. One hundred sixty-seven episodes of candidaemia occurred in the 95 patients during 2007 through 2011. Only a single strain of Candidae was found in each patient with the exception of two subjects. The mean number of positive blood cultures for patient was two (range 1-10).
Candidaemia is defined as an infection where at least one positive blood culture yields Candidae spp in patients with fever or other clinical signs of infection. Nosocomial candidaemia is a candidaemia occurring in patients after 48-72h from the hospitalization. Candidaemia related to indwelling catheters is considered when a semi-quantitative culture of the catheter tip yields more than 15 colony-forming units (CFU/ ml) of Candidae. Candidaemia attributable mortality is defined as a candidaemia regarded as the primary cause of death in patients died with microbiological, histological and/or clinical events of fungal infection without any other cause of death [8].
The blood samples were inoculated in Bactec Mycosis IC/F or Bactec Plus Aerobic Broth and inserted in an automated culture system Bactec 9420 ( Becton-Dickinson). Catheter tip specimens (CVC) were inoculated into thioglyconate medium. All cultures were monitored using an automated culture system. In the positive samples, passages to Blood agar and Sabouraud dextrose agar were performed.
If Candidae strains were detected, they were plated out in chromogenic media (Oxoid) in order to have a presumptive species identification. C. albicans was also identified by morphological criteria ( germ tube and chlamydospore formation). A definitive identification was made by API ID 32C System (Biomerieux Diagnostic System). Finally the susceptibility to antimycotic agents (Amphotericin B, Posaconazole, Fluconazole, Voriconazole, Itraconazole, 5-Flucytosine, Micafungin, Anidulafungin, Caspofungin) were tested through the Sensititre Yeast One 10. For the susceptibility test, the breakpoints were followed according to EUCAST document and the results were expressed as Susceptible (S), Intermediate (I) and Resistant (R) and correspondent with the minimum inhibitory concentrations [9].
Statistical Analysis
Categorical variables were analyzed by the Chi-square test with Epi Info and Fisher’s exact test. Chi-square for linear trend analysis was performed for evaluating the association of candidaemia with the presence of CVC through the years under consideration. A p-value of <0.05 was considered statistically significant [10].
Results and Discussions
In our study, most of candidaemiae were nosocomial infections (70-80%) occurring in patients after 48-72h from the hospitalization whereas community infections accounted approximately for 20-30%.
Table 1 shows the overall blood cultures examined during study period (16704), the blood cultures positive (2604) and among them, those positive for Candidae (167). The percentage of candidaemiae was 3.83%, 3.08% and 8.7% respectively in 2007, 2008 and 2009 with a mean value during the 3 years period of study of 5.4%. In the years 2010 and 2011 candidaemiae accounted for 6% and 9% respectively. This marked increase in 2009 and 2011 could be the result of the higher presence, in our patients, of CVC. In all the period under study (2007- 2011), the mean presence of Candidae sepsis was 6.4%.
| Years | Blood cultures total | Blood cultures positive (%) | Candidaemia positive (%) |
|---|---|---|---|
| 2007 | 2868 | 365 (12,7%) | 14 (3,8%) |
| 2008 | 2499 | 389 (15,5%) | 12 (3,1%) |
| 2009 | 2207 | 450 (20,3%) | 39 (8,7%) |
| 2010 | 5333 | 800 (15,0%) | 48 (6,0%) |
| 2011 | 3797 | 600 (15,8%) | 54 (9,0%) |
| Total | 16704 | 2604 (15,5%) | 167 (6,4%) |
Table 1: Incidence of candidaemie during study period (2007-2011).
Predisposing factors associated with C. albicans. and non- albicans. species are presented in table 2.
| C. albicans | C. non-albicans | |
|---|---|---|
| Candidae colonisation (≥2 sites) | X | X |
| Age | X | X |
| Days of ICU permanence (LOS) | X | |
| Severity of illness (Apache II score) on admission to ICU | X | |
| Total parenteral nutrition (TPN) | X | |
| Broad-spectrum antibiotics | X | X |
| Neutropenia, chemotherapy | X | |
| Presence of indwelling catheter (CVC) | X | |
| Major abdominal surgery/pancreatitis | X | X |
| Invasive procedures | X | |
| Urinary catheter | X | |
| Prior hospitalisation | X | |
| Haematological malignancies | X | |
| Previous fluconazole prophylaxis or therapy | X |
LOS=Length of Stay (Hospitalization)
CVC=Central Venous Catheter
TPN=Total Parenteral Nutrition
Table 2: Risk factors for invasive candidiasis.
Patients with C. albicans were more likely to have had urinary catheter, prolonged hospitalization (days of Intensive Care Unit [ICU] permanence) and presence of indwelling catheters. Patients with non- albicans species were more likely to have had neutropenia, prior hospitalization, Total Parenteral Nutrition (TPN) , severity of illness (Apache II score) on admission to ICU, invasive procedures, haematological malignancies and mainly previous fluconazole prophylaxis or therapy . Candidae colonization in more than 2 nonsterile sites is a crucial risk factor for both Candida albicans and non- albicans. In fact Candidae colonization, defined as growth of yeasts from non-sterile sites, is usually the first stage for most cases of invasive candidiasis in critically ill patients and it has been considered as an early marker of deep infection. [11] In fact, we demonstrated that 60-80% of patients with candidaemia were previously colonized by the same species.
The use of broad spectrum antibiotics is a risk factor for all Candidae owing to yeasts selection in the human commensal flora.The patient’s age is important in determining the C. albicans or nonalbicans infection [12].
In our cases, we noticed a correlation between invasive candidiasis and patient age. First of all, invasive candidiasis is mostly present at the extreme age of the patient’s life (less than 1 year or less than 19 or more than 65). The older patients have an increased risk of fungemia and also appeared to have an increased risk of dying from this event. C. krusei during years 2007-2009 and C. parapsilosis during years 2010-2011 were detected more in younger people (less than 19 years) probably due to the correlation of these species with haematological malignancies well represented in our records. C. albicans resulted to be the most frequent species in all age groups (42%) with the exception of the group 1-19. The range 19-49 is the less affected population with about the same percentage of the different Candidae. species.(3%). In the range 50-64 only C. albicans occurred (7%) and in the range more than 65, both C. albicans(13%) and C. glabrata (7%) were present confirming the greater presence of C.glabrata in the oldest group (data not shown).
In (Table 3), MIC 50% and MIC 90% for all Candidae against the tested antimycotic agents (Amphotericin B, Flucytosine, Fluconazole, Voriconazole and the Echinocandins) are reported.
| Amph B (mcg/ml) | Flucytosine (mcg/ml) | Fluconazole (mcg/ml) | Voriconazole (mcg/ml) | Caspofungin (mcg/ml) | Anidulafungin (mcg/ml) | Micafungin (mcg/ml) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC 50% | MIC 90% | MIC 50% | MIC 90% | MIC 50% | MIC 90% | MIC 50% | MIC 90% | MIC 50% | MIC 90% | MIC 50% | MIC 90% | MIC 50% | MIC 90% | |
| C. albicans(70) | 0.12 | 0.25 | 0.12 | 1 | 0.25 | 1 | 0.03 | 0.06 | 0.5 | 0.5 | 0.03 | 0.03 | 0.08 | 0.15 |
| C. glabrata(28) | 0.25 | 0.5 | 0.12 | 0.12 | 8 | 32 | 0.25 | 1 | 0.5 | 1 | 0.03 | 0.12 | 0.25 | 0.5 |
| C. parapsilosis(34) | 0.25 | 0.5 | 0.12 | 0.12 | 1 | 2 | 0.03 | 0.12 | 2 | 2 | 0.06 | 0.06 | 0.25 | 1 |
| C. tropicalis(11) | 0.25 | 0.5 | 0.12 | 0.5 | 0.5 | 16 | 0.06 | 2 | 0.5 | 0.5 | 0.03 | 0.12 | 0.03 | 0.06 |
| C. krusei(19) | 0.25 | 0.5 | 4 | 32 | 32 | >64 | 0.5 | 1 | 1 | 2 | 0.06 | 0.12 | 0.5 | 1 |
| Others (5) | 0.12 | 0.5 | 0.12 | 0.12 | 0.5 | 4 | 0.03 | 0.25 | 1 | 2 | 0.06 | 0.25 | 0.03 | 0.03 |
Table 3: Minimum inhibitory concentration (MIC50%/MIC90%) of antifungal agents against the 167 Candidae strains isolated.
It is clear that most Candidae spp are usually susceptible in vitro to the agents used in therapy, but resistance can be detected in some species such as C. glabrata and C. krusei and at a less extent C. parapsilosis.
As shown, it is possible to underline that C. krusei is completely resistant to Fluconazole (MIC 50% equal to 32 mcg/ml and MIC 90% > 64 mcg/ml) .This can be expected because C. krusei is intrinsically resistant to Fluconazole (Fc) [13].
C. glabrata also results resistant to Fc (MIC 50% and 90% 8 and 32mcg/ml respectively) but this Candida may show a susceptibility to this drug depending on the dose used in therapy. In C. parapsilosis we found a higher MIC than C. albicans against Fluconazole and a MIC of 2 mcg/ml for Caspofungin (for the latter there are no defined breakpoints values).
C. albicans results to be the most susceptible species to anti-mycotic agents (MICs for all agents are very low ranging from 0.12 to 1 mcg/ml)) whereas C. tropicalis shows a slight resistance to Fluconazole due to the fact that, even if the MIC 50% corresponds to 0.5 mcg/ml, the MIC 90% is 16 mcg/ml. The Echinocandins show a good activity against the tested Candidae . However so far, for micafungin whose breakpoints are still in preparation and for caspofungin due to significant interlaboratory variation in MIC ranges in vitro, breakpoints have not been yet established [9]. For Fluconazole, there are no corresponding values for C. krusei (intrinsic resistance) and C. glabrata (susceptible dosedependent) owing to the fact that these yeasts don’t represent a good target for therapy with this drug.
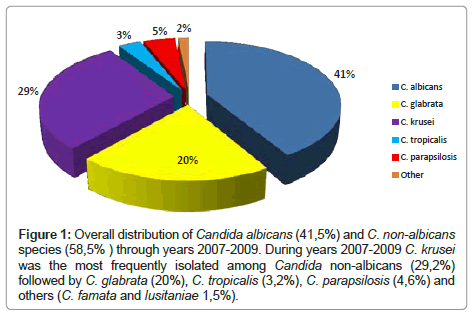
In (Figure 1), we report the distribution of the different Candidae species during the first period of study (2007-2009). We found that C. albicans resulted to be the most frequently isolated fungal species with 41.5% of detection. The overall presence of Candidae nonalbicans accounted for 58.5% and included C. krusei (29.2%) the most represented species in that period followed by C. glabrata (20%), C. parapsilosis (4.6%) and C. tropicalis (3.2%).
Figure 1: Overall distribution of Candida albicans (41,5%) and C. non-albicans species (58,5% ) through years 2007-2009. During years 2007-2009 C. krusei was the most frequently isolated among Candida non-albicans (29,2%) followed by C. glabrata (20%), C. tropicalis (3,2%), C. parapsilosis (4,6%) and others (C. famata and lusitaniae 1,5%).
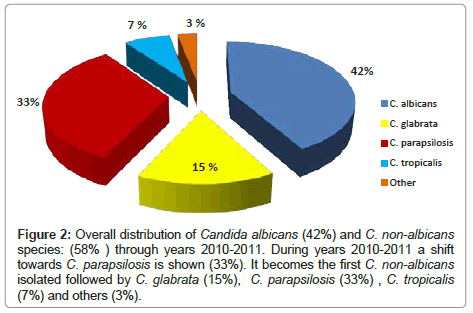
In the second period of study (2010-2011), we found a completely different situation (Figure 2) because we did not find C. krusei whereas C. parapsilosis became the second most representative species, following the US trend [4] where this species results to be the third detected Candida after C. albicans and C. glabrata.
Figure 2: Overall distribution of Candida albicans (42%) and C. non-albicans species: (58% ) through years 2010-2011. During years 2010-2011 a shift towards C. parapsilosis is shown (33%). It becomes the first C. non-albicans isolated followed by C. glabrata (15%), C. parapsilosis (33%) , C. tropicalis (7%) and others (3%).
In our case, we mainly found C. albicans (42%) followed by C. parapsilosis (33%), C. glabrata (15%) and C. tropicalis (7%). The nonalbicans Candidae accounted for 58% as well as the previous period (2007-2009).
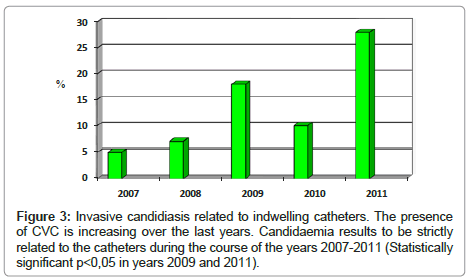
Invasive candidiasis are strongly correlated to the presence of indwelling catheters as it is shown in (Figure 3) in which during 2009 and 2011 owing to a higher presence of patients with catheters, the increase of Candidaemie is statistically significant (p<0.05).
Invasive Candidiasis (IC) is increasing over the last 20 years due to the fact of a greater presence of immune-compromised subjects in our hospital. They represent an increasing challenge for the relevance to intensive care units and for the fact that the mortality is higher than in bacterial infections. In our patients the mortality ranged from 40 to 55% depending on many factors. These factors can be referred to omitted, delayed or inadequate antifungal therapy, to treatment with an agent to which the organism is resistant, to Apache II score at the admission (severity of illness) and to infection with biofilm forming Candidae species [5]. For example, C. parapsilosis that is mainly involved in nosocomial candidaemie, is an exogenous pathogen that may be found on skin rather than mucosal surfaces. C. parapsilosis is notorious for the ability to form biofilms on catheters and other implanted devices for nosocomial spread by hand carriage and for persistence in the hospital environment [8]. Antifungal agents show reduced activity against Candidae biofilm and poor penetration into vegetations in infectious endocarditisn [7].
The diagnosis of candidaemie can be difficult for the following reasons: clinical manifestations are non specific, the blood cultures are usually positives late in the course of infection and the usefulness of serological test (β-D-glucan, mannans) is controversial [5]. In fact these serological tests can be considered as surrogate markers of illness in a high-risk host before clinical signs and symptoms of overt disease develop and they can be only useful for the establishment of the preemptive therapy defined as an early treatment of this kind of infection. Culture evidence is the mainstay for the diagnosis and the culture of sterile sites is definitive whereas the culture of non- sterile sites only defines colonization.
Risk factors for Invasive Candidiasis (IC) such as the presence of CVC, neutropenia, Candidae colonization, broad-spectrum antibiotic use, severity of illness (Apache II score) have been identified and must be accurately taken into account [1,5,14,15]. Models were developed for identifying factors that are predictive of IC and these factors were used to build clinically relevant scores that may help clinicians to identify an optimal therapeutic approach.
In our research we found that C. albicans is the most frequently isolated fungal species (~ 42% of all Candidae isolated) whereas nonalbicans Candidae account for 58 %. C. krusei (29.2%) through years 2007-2009 and C. parapsilosis (33%) through years 2010-2011, result to be the second most frequent species respectively. This shift from C.krusei versus C. parapsilosis in the latest period can be of relevant importance for the epidemiology of our candidaemie.
The reason for the variation in the frequency of non-albicans Candidae as a cause of bloodstream infections are unclear but they may include the following: exposure to azoles that is different in the various wards of our Hospital and during the course of the years, prophylactic strategy, patient age, severity or underlying disease and indiscriminate use of broad spectrum antibacterial agents. The different geographic areas are a further factor affecting the detection of different Candidae species in other countries [16].
The prophylaxis with fluconazole has been identified as a risk factor especially for C. glabrata and C. krusei. For azoles, an extra class of Susceptible Dose-Dependent (SDD) is considered. This value is important for C. glabrata that is strongly dependent on the concentration of drugs used in therapy. That means that this species can be cleared from the BSIs only with high doses of fluconazole whereas C. krusei is intrinsically resistant to fluconazole. In this way, this prophylactic strategy can select the presence of resistant Candidae [6].
Available drugs to treat invasive candidiasis and candidaemia include Amphotericin B, Fluconazole, Voriconazole, Posaconazole, Itraconazole and the Echinocandins (Caspofungin, Anidulafungin and Micafungin) . Echinocandins are the most recently introduced class of antifungal drugs. They have fungicidal activity and are strongly active against the most part of Candidae. Moreover, they are better tolerated than conventional drugs and have less side-effects and drugrelated toxicity [17]. This can be of crucial importance for the patients admitted in an ICU especially in those with renal failure. Echinocandins and especially anidulafungin display a potent activity in vitro against sessile Candidae cells within biofilms as C. parapsilosis (in our study this species shows a very low MIC against anidulafungin) and a good penetration into vegetations and blood clots in experimental models of infectious endocarditis [7,18]. Micafungin is the newest echinocandin introduced in the market: so far in fact the antimycotic breakpoints are still in preparation [9].
In any case the Echinocandins can be considered a valid alternative to the use of azoles and Amphotericin B in therapy. In fact they represent a valid choice for Candidae treatment especially for the patients with more severe illness and with a higher Apache II score. They are in fact generally used for the haemodinamically unstable patients whereas the less severe diseases and the stable patients can be treated with azoles [5,19]. Most Candidae spp are usually susceptible to these antifungal drugs but slight resistance has been described [20,21]. In fact no class of antifungal agents is immune to the development of resistance. In our study, C. albicans results to be the most susceptible yeast (MIC50% and 90% ranging from 0.25 to 1mcg/ml), whereas C. krusei results to be the most resistant especially to azoles such as Fluconazole. Voriconazole, even if it is recommended as first- line therapy in invasive aspergillosis, shows a potential role in candidiasis.
Conclusions
In conclusion we can say that fungal infection constitutes an increasing challenge for the relevance in ICU, for the high rate of mortality, for the difficulty in making an early diagnosis, for the question of when to start the treatment, for the balance of risk and benefit in anti-fungal prophylaxis and for the establishment of a correct therapy in consequence of the growing detection of drug resistance.
References
- Concia E, Azzini AM, Conti M (2009) Epidemiology, incidence and risk factors for invasive candidiasis in high-risk patients. Drugs 1: 5-14.
- Pfaller MA, Diekema DJ (2007) Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev 20: 133-163.
- Hajjeh R.A, Sofair A.N, Harrison I.H, Lyon G.M, Arthington-Shaggs B.A, et al. (2004) Incidence of bloodstream infections due to Candida species and in vitro susceptibilities of isolates collected from 1998 to 2000 in a population-based active surveillance program. J. Clin. Microbiol 42: 1519-1527.
- Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, et al. (2004) Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis 39: 309-317.
- Guery B, Arendrup M, Auzinger G, Azoulay E, Borges SÃ M, et al. (2009) Management of invasive candidiasis and candidemia in adult non -neutropenic intensive care unit patients: Part I. Epidemiology and diagnosis. Intensive Care Med 35: 55-62.
- Bassetti M, Ansaldi F, Nicolini L, Malfatto E, Molinari MP, et al. (2009) Incidence of candidaemia and relationship with fluconazole use in an intensive care unit. J Antimicrob Chemother 64: 625-629.
- Venditti M (2009) Clinical aspects of invasive candidiasis: endocarditis and other localized infections. Drugs 1: 39-43.
- Celebi S, Hacimustafaoglu M, Ozdemir O, Ozkaya G (2008) Nosocomial candidaemia in children: results of a 9-year study. Mycoses 51: 248-257.
- European Committee on Antimicrobial Susceptibility Testing – (EUCAST) Breakpoint tables for interpretation of MICs and zone diameters, version 2.0, 2012.
- Dean A.D, Dean J.A, Burton H, Dicker R.C (1990) Epi Info, vers 5: a words processing, database, and statistics program for epidemiology on micro-computers. Centers for Disease Control, Atlanta.
- Diekema D, Arbefeville S, Boyken L, Kroeger J, Pfaller M (2012) The changing epidemiology of healthcare-associated candidemia over three decades. Diagn Microbiol Infect Dis 73: 45-48.
- Garnacho-Montero J, DÃaz-MartÃn A, Cayuela-Dominguez A (2008) Management of invasive Candida infections in non-neutropenic critically ill patients: from prophylaxis to early therapy. Int J Antimicrob Agents 2: S137-141.
- Nakamura T, Takahashi H (2006) Epidemiological study of Candida infections in blood: susceptibilities of Candida spp. to antifungal agents, and clinical features associated with the candidemia. J Infect Chemother 12: 132-138.
- Kotwal A, Biswas D, Sharma JP, Gupta A, Jindal P (2011) An observational study on the epidemiological and mycological profile of Candidemia in ICU patients. Med Sci Monit 17: CR663-668.
- Bassetti M, Taramasso L, Nicco E, Molinari MP, Mussap M et al. (2011) Epidemiology, species distribution, antifungal susceptibility and outcome of nosocomial candidmia in a tertiary care hospital in Italy. Plos One 6: e24198
- Canton E, Peman J, Quindos G, Eraso E, Miranda-Zapico I, et al. (2011) Prospective multicenter study of the epidemiology, molecular identification, and antifungal susceptibility of Candida parapsilosis, Candida orthopsilosis, and Candida metapsilosis isolated from patients with candidemia. Antimicrob Agents Chemoter 55: 5590-5596.
- Beyda ND, Lewis RE, Garey KW (2012) Echinocandin resistance in Candida species: mechanisms of reduced susceptibility and therapeutic approaches. Ann Pharmacother 46: 1086-1096.
- Bonfietti LX, Martins Mdos A, Szeszs MW, Pukiskas SB, Purisco SU, et al. (2012) Prevalence, distribution and antifungal susceptibility profiles of Candida parapsilosis, Candida orthopsilosis and Candida metapsilosis bloodstream isolates. J Med Microbiol 61: 1003-1008.
- Pfaller MA (2012) Antifungal drug resistance: mechanisms, epidemiology, and consequences for treatment. Am J Med 125: S3-13.
- Reboli AC, Shorr AF, Rotstein C, Pappas PG, Kett DH, et al. (2011) Anidulafungin compared with fluconazole for treatment of candidemia and other forms of invasive candidiasis caused by Candida albicans: a multivariate analysis of factors associated with improved outcome. BMC Infect Dis. 11: 261
- Iatta R, Caggiano G, Cuna T, Montagna MT (2011) Antifungal susceptibility testing of a 10-year collection of Candida spp. isolated from patients with candidemia. J Chemother 23: 92-96.
Citation: Mascellino MT, Raponi G, Oliva A, Mastroianni CM, Vullo V (2012) Candidaemia in Immune-Compromised Hosts: Incidence and Drugs Susceptibility. J Clin Exp Pathol 2:131. DOI: 10.4172/2161-0681.1000131
Copyright: © 2012 Mascellino MT, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 16287
- [From(publication date): 11-2012 - Oct 21, 2025]
- Breakdown by view type
- HTML page views: 11436
- PDF downloads: 4851