Review Article Open Access
Current Trends in Ubiquitous Biosensing
Dimitra N Stratis-Cullum* and Amethist S Finch
US Army Research Laboratory, RDRL-SEE-B, Adelphi, USA
- *Corresponding Author:
- Dr. Stratis-Cullum DN
US Army Research Laboratory, RDRL-SEE-B
2800 Powder Mill Road, Adelphi, MD 20783, USA
E-mail: dimitra.stratiscullum1@us.army.mil
Received date: March 19, 2013; Accepted date: May 15, 2013; Published date: May 17, 2013
Citation: Stratis-Cullum DN, Finch AS (2013) Current Trends in Ubiquitous Biosensing. J Anal Bioanal Tech S7:009. doi:10.4172/2155-9872.S7-009
Copyright: © 2013 Stratis-Cullum DN, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
Biosensing technology is not currently capable of widespread use outside of a laboratory environment due to significantly limitation in bioreceptor function and production, as well as in the overall size, weight, and cost of the sensing platform. However, as technology continues to advance, biosensors could truly become ubiquitous, employing social media and personal electronic devices for mundane yet powerful capabilities. In the near future, point-of-care diagnostics in third-world countries could save millions of lives and revolutionize the healthcare industry worldwide. Recent trends show many of the traditional barriers to realizing this vision will soon be overcome. In this paper we review exciting trends in the development of synthetic reagents, fluidic integration, and mobile platforms that are necessary for ubiquitous biosensing capabilities.
Keywords
Biosensing; Ubiquitous sensing; Synthetic molecular recognition; Synthetic reagents; Peptides; smart phone; Personal electronic devices; Bacterial display; Biomolecular recognition; Biosensor
Abbreviations
SWaP-C: Size, Weight, Power and Cost; PEDs: Personal Electronic Devices; POC: Point of Care; SERS: Surface Enhanced Raman Spectroscopy
Introduction
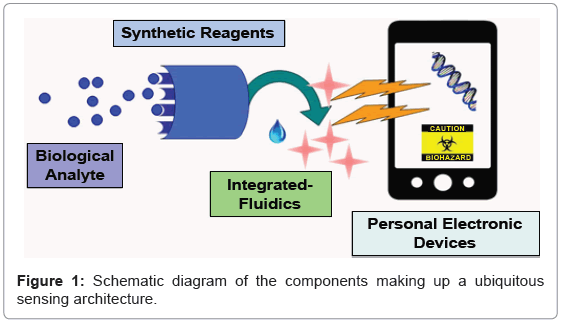
Biosensors of the future promise to be as ubiquitous as mobile phones, and if current sensing trends continue, the mobile phone itself could become an integrated platform for everyday use. For example, biosensors could bring about the next revolution in public health monitoring in third-world countries, providing low-cost point-of-care solutions with advanced diagnostic capabilities. Homeland security and the spread of disease could be monitored in real time, and the impact could be minimized through embedded sensors throughout the public transportation system. As technology continues to advance, biosensors could truly become a part of everyday life, employing social media and personal electronic devices to fuse biosensed data from the real world into virtual realities. There are several technological barriers that must be overcome before this scope and intensity is fully realized [1]. In this paper we review exciting trends in the development of synthetic reagents, fluidic integration, and personal electronic devices as platforms that are necessary for ubiquitous biosensing capabilities (Figure 1).
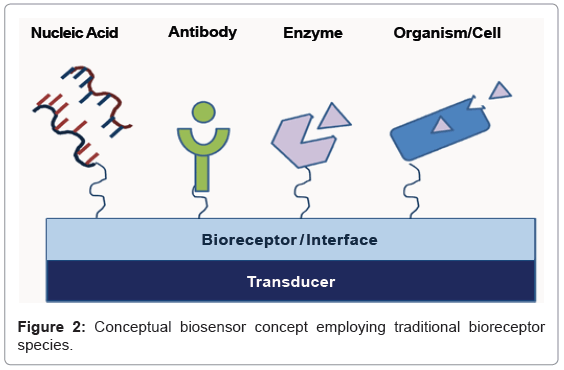
Biosensors comprise two primary components: the bioreceptor, or recognition reagent responsible for specific binding to the biological analyte of interest, and the transducer, which converts this binding event to a measureable signal (Figure 2) [1,2]. A variety of bioreceptors (nucleic acid, antibody, etc.) can be used to bind to an analyte or target of interest, and similarly, a variety of transduction mechanisms have been used to translate this recognition event into an electronic signal (e.g., mass, electrochemical, optical, magnetic, label-free, etc.) [3,4]. Significant limitations in bioreceptor function and production, as well as in the overall size, weight, and cost (SWaP-C) of the transducer, have been the primary technological barriers to the vision of ubiquitous biosensing. Ideally, a bioreceptor reagent should have the characteristics outlined in table 1 to enable ubiquitous implementation across a variety of material systems and operational environments.
| Desired Reagent Feature | Necessary for Ubiquitous Biosensing |
|---|---|
| Robust | Temperature, pH, enzyme degradation, and a long shelf life |
| Thermoplastic | Maintains full function under extreme temperature conditions |
| Universal | Encompasses all biosensing analytes irrespective of charge, size, etc. |
| Rapid Discovery | Rapid development without extensive knowledge of the target analyte, critical to adapt technology to new and emerging threats |
| Manufacturing Scale Production | Cost-effective production necessary for ubiquitous scale |
| On-Demand Production | Circumvent any shelf life or supply/demand issues |
| Low Cost | Critical for universal implementation |
| Adaptable | Readily incorporate into variety of platforms; drop-in replacement technology |
| High Affinity | Equivalent (or better) than antibody gold standard to meet sensing requirements |
| High Specificity | Critical for practical application in complex environments |
Table 1: Table of affinity reagent features important to ubiquitous sensing.
The bioreceptor (i.e., reagent) must be robust and resistant to degradation stressors encountered in real-world applications, including temperature extremes (cold and heat), pH variations, enzymes, etc. [5]. Furthermore, the ideal bioreceptor should be not only robust, but also thermoplastic in nature (i.e., operational at elevated temperatures). The current state-of-the-art in reagent technology (i.e., antibody bioreceptors) capable of recognizing protein biomarkers and toxins (i.e., nongenetic materials) is fraught with difficulties including poor mass production, stability, and large overall production costs.
Many research groups have investigated and developed synthetic chemical and biological affinity reagent alternatives in an attempt to overcome limitations in natural protein antibody-based recognition. Most commonly these include nucleic acid aptamer, phage display, yeast display bacterial display, mRNA display, and one-bead-onecompound solid phase chemical libraries technologies [6-17].
Synthetic Reagents
Recent advances in synthetic affinity reagent technology and discovery have shown the potential to meet all of the desired features of ubiquitous biosensor bioreceptors outlined in table 1. In addition to the stability, a key advantage of many synthetic reagent technologies is the speed at which they can be discovered, a critical capability when considering rapidly changing detection needs, including newly engineered threat agents to which no current bioreceptor technologies exist. Antibodies usually require several weeks to months to isolate from living hosts, whereas recent advances have demonstrated that synthetic routes can be employed using various synthetic peptide recognition element technologies (e.g., bacterial display and phage display) to produce bioreceptors in as little as a few days to a couple of weeks [5,12,18,19].
Coupled with the speed at which a new synthetic bioreceptor can be manufactured is the scale and cost of the manufactured product. Currently, major issues in antibody technology include time to supply and shelf-life stability. To avoid these standard pitfalls, ideal bioreceptor materials should be stable and manufacturable on demand [17]. In the case of ubiquitous biosensing, a plethora of platforms and materials integration issues will be utilized, making adaptability a key feature of the synthetic bioreceptor technology [1,3]. Of course, any synthetic reagent needs to meet affinity and specificity requirements to a standard of equivalency to antibody technology for practical use in any biosensor system and real-world operational environments full of interfering background species [20].
Not surprisingly, synthetic peptide receptors show some of the greatest potential as synthetic antibody alternatives, as the mechanisms for specific binding from peptides (protein building blocks) are similar to antibody-antigen interactions [21]. However, rapid development of stable synthetic antibody replacements can be accomplished through bioengineered combinatorial libraries and advanced screening methods followed by mass-production through standard synthesis techniques. Several variations of combinatorial peptide technologies, including yeast, phage, and bacterial display and other chemically synthetic techniques, are currently used to isolate synthetic reagents. In peptide display, a small section of protein from the surface of a biological system (bacterium, virus, etc.) is engineered to present a randomized segment of amino acids (the building blocks of proteins) [11,16,22,23].
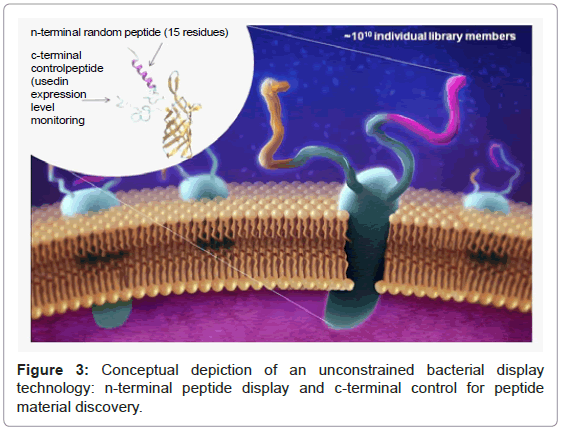
However, with unconstrained bacterial peptide display technology, the extremely fast replication rate of bacteria is exploited, and biological components (e.g., modified proteins) on an E. coli cell surface are harnessed to produce the peptide library of binders (Figure 3) [11,12,19]. This creates billions of individual peptide display clones, together creating a combinatorial library of peptide binding elements (i.e., candidates of synthetic affinity reagents). The large diversity of sequences presented by this library is then exposed to a target of interest, and stringency controls, in an alternating fashion. A built-in expression tag allows normalization of the expression library and affinity screening-a key enabling feature for reproducible reagent discovery. Although discovery is rapid, the characterization, optimization, and integration into assays and devices can still be a bottleneck to successful biosensor development using synthetic alternatives.
More recently, advances in peptide in-situ click chemistry have allowed scientists to develop a new class of highly manufacturable synthetic antibody alternatives: protein-catalyzed capture (PCC) Agents [7,17]. In this technology, the target protein is used as a highly selective catalytic scaffold for assembling its own capture agent. PCC Agents are assembled stepwise from comprehensive, chemically synthesized peptide libraries that incorporate non-natural amino acids into the starting library to produce a final product that is highly stable, both on the shelf and in vivo. Additional functionality can be built into the initial design to produce a synthetic “drop-in replacement” that is highly adaptable and easily integrated into assays and sensor systems. The process is repeated to build biligand and triligand structures, each time refining the binding affinity and specificity through avidity with the target. The final synthetic peptide can be scaled up on-demand by automated chemical synthesis, avoiding the problem of batch-to-batch reproducibility.
Integrated Fluidics
The combined advances in microfabrication and micro-optic devices in optofluidics are key to enabling sensing of biological analytes, which are typically performed in aqueous systems. In the last decade, exciting and fundamental advances have been made in the synergistic combination of research in the fields of microfluidics and optics, coined “optofluidics” [24-26]. Optical techniques have long been used to analyze and characterize biological samples. Optical systems have traditionally served as a laboratory workhorse and include such instruments as flow cytometers, spectrophotometers, and microplate readers. More recently, these systems have been implemented in microdevices, such as on-chip waveguides and resonators. In parallel, we have seen the miniaturization of device architectures using microfabrication and clean-room techniques for the development of microfluidic devices [27]. Advances in the rapid fabrication of nano- and microfluidic devices bridge the SWAP-C requirement gap and enable low-cost, small-volume sample handling and processing. Specifically for ubiquitous and mobile biosensing applications, optofluidics incorporates sample preparation and delivery with integrated transduction. The integrated optical transduction can take a variety of forms, including refractive index, fluorescence, Raman scattering, absorption, and polarization used individually or in concert to generate a robust signal output [24,26,28].
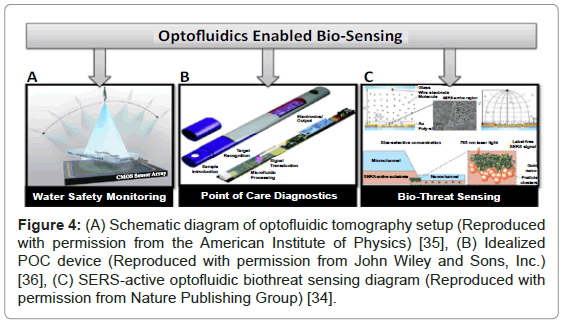
The versatility and broad application space of optofluidics is evidenced by a number of recent reviews and publications [24,26,28,29]. Figure 4 illustrates several examples of optofluidic technologies that enable biosensing applications. Technologies such as lens-free imaging and on-chip optofluidic tomography (Figure 4A) may prove to be invaluable where high-throughput, 3D lab-on-a-chip imaging of the specimen is necessary, such as water safety monitoring [30]. Optofluidics is also well-poised to revolutionize the field in point of care (POC) diagnostics. An idealized and ruggedized POC device is illustrated in Figure 4B, where the optofluidics is integrated into an all-encompassing device, which includes everything from sample introduction to electronic output [31]. Finally, the area of biothreat sensing has been covered by a number of reviews [20]. Figure 4C shows a sample surface enhanced raman spectroscopy (SERS) active, biothreat sensing modality [28]. This optofluidic sensor could be readily incorporated and modified and multiplexed into a variety of devices for detection of biological threats.
Figure 4: (A) Schematic diagram of optofluidic tomography setup (Reproduced with permission from the American Institute of Physics) [35], (B) Idealized POC device (Reproduced with permission from John Wiley and Sons, Inc.) [36], (C) SERS-active optofluidic biothreat sensing diagram (Reproduced with permission from Nature Publishing Group) [34].
Personal Electronic Devices
Widespread sensing will likely be accomplished across a variety of platforms such as cell phones or other personal devices (PEDs), medical devices, autonomous vehicles, and a host of other data collection systems. Table 2 outlines the primary features needed for ubiquitous sensing including a universal interface, and an open and programmable architecture. Overarching engineering goals of low size, weight, power and cost (SWAP-C) have been the focus of point-of-care and persistent surveillance in particular, but are key to widespread use. Integration and fusion of onboard sensor suites and reporting through an agile and archival network will also be necessary. Recent trends in smartphone and other personal electronic device platforms show tremendous advances over the last several years, rapidly positioning smart-phone technology as a ubiquitous sensing platform [32]. Smartphones are open and programmable personal electronic devices that are equipped with a growing number of powerful embedded sensors, such as an accelerometer, digital compass, gyroscope, GPS, microphone, and camera. Combined these enable new sensing applications across a wide variety of domains such as mobile health, transportation, social networking, gaming, entertainment, and education.
| Desired Platform Feature | Necessary for Ubiquitous Biosensing |
|---|---|
| Standardized | Universal interface capable of operation across platforms |
| Open and Programmable | Necessary for graphical user interface and software application to be rapidly modifiable |
| SWAP-C | For disruptive technologies, platforms must meet economic demands, including size, weight, power, and cost |
| Other Standard Capabilities to Leverage | Integrated archival data storage (GPS, time, date, readout, images, etc.) available for after-action processing |
| Networked | Data transmitted and recorded for further analysis and processing |
| Easy to Use | Operation and readout on platform must be simple for broad acceptance |
| Multiplexed Analysis | Extendable to include biothreat sensing, POC diagnostics, small molecule (cocaine, TNT, etc.), and nuclear materials |
Table 2: Mobile platform reagent features important to ubiquitous sensing.
Most recently, simple, cost-effective, compact, and lightweight imaging has been achieved on personal electronic platforms through the use of a smart-phone in conjunction with lens-free imaging. When lens-free imaging is used, high-resolution images are possible on a field-portable platform, which is ideal for affordable POC devices and is broadly extendable to ubiquitous sensing applications (e.g., food safety, environmental monitoring, homeland security). Researchers are exploring the use of personal electronic devices for biosensing applications ranging from food and water defense to explosive checkpoint analysis to point-of-care diagnostics [32-34]. Bridging the dimensional gap between single-channel analysis to microscopy provides a powerful approach to portable systems.
Conclusion
To conclude, biosensing is a powerful tool used in research and clinical laboratories that if implemented on a global scale could transform our ability to monitor biological species in any location and environment. Specific advances in lab-on-a-chip technology to optofluidics as well as low-cost, highly networked mobile platforms open up the possibility, for the first time, to truly have a ubiquitous and archived biosensing network. Advances in the ability to produce highly manufacturable and robust synthetic bioreceptor alternatives now make biosensing in austere environments a reality. For example, smart skins used to be considered science fiction; several reports of patch electronic devices that stretch and conform to skin have been produced for physiological monitoring and drug delivery [35,36]. If trends continue, the fusion of these technological advances could revolutionize biosensing, with far-reaching impact, and make way for more commercial applications.
Acknowledgements
The authors would like to thank Mr. Eric Proctor and Mr. William Parks for their conceptual rendition of the bacterial display technology.
References
- Bohlen M, Ilya M, Paul Lloyd S, Silvia Z, Nicole L, et al. (2012) Prototyping Ubiquitous Biosensing Applications through Speculative Design. Intelligent Environments (IE), 2012 8th International Conference on, 198-205.
- Sooter LJ, Stratis-Cullum DN, Zhang Y, Rice JJ, Ballew JT, et al. (2009) Hand Held Biowarfare Assays: Rapid Biowarfare Detection Using the Combined Attributes of Microfluidic in vitro Selections and Immunochromatographic Assays. ACS Symposium Series 1016: 73-83.
- Gubala V, Harris LF, Ricco AJ, Tan MX, Williams DE (2012) Point of care diagnostics: status and future. Anal Chem 84: 487-515.
- Iqbal SS, Mayo MW, Bruno JG, Bronk BV, Batt CA, et al. (2000) A review of molecular recognition technologies for detection of biological threat agents. Biosens Bioelectron 15: 549-578.
- Stratis-Cullum D, Kogot JM, Sarkes DA, Val-Addo I, Pellegrino PM (2011) Bacterial display peptides for use in biosensing applications. In On Biomimetics, edited by Pramatarova DL, InTech.
- Agnew HD, Rohde RD, Millward SW, Nag A, Yeo WS, et al. (2009) Iterative in situ click chemistry creates antibody-like protein-capture agents. Angew Chem Int Ed Engl 48: 4944-4948.
- Millward SW, Agnew HD, Lai B, Lee SS, Lim J, et al. (2013) In situ click chemistry: from small molecule discovery to synthetic antibodies. Integr Biol (Camb) 5: 87-95.
- Nguyen KV (2006) Selection of peptide ligands specific for baculovirus DNA-binding protein from the flitrx random peptide display library. Anal Lett 39: 99-112.
- Bessette PH, Hu X, Soh HT, Daugherty PS (2007) Microfluidic library screening for mapping antibody epitopes. Anal Chem 79: 2174-2178.
- Bessette PH, Rice JJ, Daugherty PS (2004) Rapid isolation of high-affinity protein binding peptides using bacterial display. Protein Eng Des Sel 17: 731-739.
- Daugherty PS (2007) Protein engineering with bacterial display. Curr Opin Struct Biol 17: 474-480.
- Kogot JM, Zhang Y, Moore SJ, Pagano P, Stratis-Cullum DN, et al. (2011) Screening of peptide libraries against protective antigen of Bacillus anthracis in a disposable microfluidic cartridge. PLoS One 6: e26925.
- Hyman P (2012) Bacteriophages and Nanostructured Materials. Adv Appl Microbiol 78: 55-73.
- Lee SY, Choi JH, Xu Z (2003) Microbial cell-surface display. Trends Biotechnol 21: 45-52.
- Mäkelä AR, Oker-Blom C (2008) The baculovirus display technology--an evolving instrument for molecular screening and drug delivery. Comb Chem High Throughput Screen 11: 86-98.
- Moore SJ, Olsen MJ, Cochran JR, Cochran FV (2009) Cell Surface Display Systems for Protein Engineering. In Protein engineering and Design, edited by Park SJ, Cochran JR, 23-50.
- Millward SW, Henning RK, Kwong GA, Pitram S, Agnew HD, et al. (2011) Iterative in situ click chemistry assembles a branched capture agent and allosteric inhibitor for Akt1. J Am Chem Soc 133: 18280-18288.
- Stratis-Cullum DN, Joshua MK, Paul MP (2010) Rapid Peptide Reagent Isolation in a Disposable Microfluidic Cartridge. ARL-TR-5357.
- Stratis-Cullum DN, Joshua MK, Michael SS, Margaret MH, Deborah AS, et al. (2012) Development of bacterial display peptides for use in biosensing applications. SPIE Proceedings 8358.
- Gooding JJ (2006) Biosensor technology for detecting biological warfare agents: Recent progress and future trends. Analytica Chimica Acta 559: 137-151.
- Pavan S, Berti F (2012) Short peptides as biosensor transducers. Anal Bioanal Chem 402: 3055-3070.
- Kumara MT, Srividya N, Muralidharan S, Tripp BC (2006) Bioengineered flagella protein nanotubes with cysteine loops: self-assembly and manipulation in an optical trap. Nano Lett 6: 2121-2129.
- Arap MA (2005) Phage display technology - Applications and innovations. Genet Mol Biol 28: 1-9.
- Monat C, Domachuk P, Eggleton BJ (2007) Integrated optofluidics: A new river of light. Nature Photonics 1: 106-114.
- Boas G (2011) Optofluidics and the Real World: Technologies Evolve to Meet 21st Century Challenges. Photonics Spectra 45: 52.
- Schmidt H, Hawkins AR (2011) The photonic integration of non-solid media using optofluidics. Nature photonics 5: 598-604.
- Pan T, Wang W (2011) From cleanroom to desktop: emerging micro-nanofabrication technology for biomedical applications. Ann Biomed Eng 39: 600-620.
- Fan X, White IM (2011) Optofluidic Microsystems for Chemical and Biological Analysis. Nat Photonics 5: 591-597.
- Liu AQ, Yang C (2012) Themed issue: optofluidics. Lab Chip 12: 3539.
- Isikman SO, Bishara W, Zhu H, Ozcan A (2011) Optofluidic Tomography on a Chip. Appl Phys Lett 98: 161109.
- Gervais L, de Rooij N, Delamarche E (2011) Microfluidic chips for point-of-care immunodiagnostics. Adv Mater 23: H151-176.
- Zhu H, Isikman SO, Mudanyali O, Greenbaum A, Ozcan A (2013) Optical imaging techniques for point-of-care diagnostics. Lab Chip 13: 51-67.
- Ilhan HA, Dogar M, Özcan M (2013) Fast autofocusing in digital holography using scaled holograms. Optics Communications 287: 81-84.
- Coskun AF, Wong J, Khodadadi D, Nagi R, Tey A, et al. (2013) A personalized food allergen testing platform on a cellphone. Lab Chip 13: 636-640.
- Celik-Butler Z, Butler DP (2006) Flexible sensors - A review. J Nanoelectron Optoe 1: 194-202.
- Wang SD, Li M, Wu J, Kim DH, Lu N, et al. (2012) Mechanics of Epidermal Electronics. J Appl Mech-T Asme 79.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 16029
- [From(publication date):
specialissue-2013 - Nov 25, 2025] - Breakdown by view type
- HTML page views : 11312
- PDF downloads : 4717