Detection and Epidemiology of Tick-Borne Pathogens in Free-Ranging Livestock in Mongolia
Received: 20-Nov-2012 / Accepted Date: 08-Jan-2013 / Published Date: 10-Jan-2013 DOI: 10.4172/2161-0681.S3-006
Abstract
Abstract
A cross-sectional epidemiologic investigation was undertaken to identify tick-borne pathogens in Mongolian
livestock across two provinces (aimags) from 2007 to 2008. Serology and PCR were used to identify exposure to and
infection with Borrelia burgdorferi sensu lato, Anaplasma phagocytophilum, A. ovis, and spotted fever group rickettsiae
species in the animals sampled. Factors evaluated for association with pathogen prevalence included region, body
condition score, gender, and species. Khuvsgul livestock had high seroprevalence to
A. phagocytophilum (64%), and A. ovis (77%); Khenti livestock had a high exposure to spotted fever group
rickettsiae species (48%). Females and intact males had higher prevalence to A. phagocytophilumthan castrated
males, as did small ruminants compared to cattle and horses. Animals with lower BCS, or animals living at higher
elevations, had a greater prevalence odds of exposure to pathogens for spotted fever group rickettsiae and A. ovis,
respectively. Reports of the newly identified Rickettsia and Borrelia species in the neighbouring provinces of northern
China combined with the data from this study warrant further investigation of tick-borne pathogens to identify reservoir
hosts and infection in Mongolian herders.
Keywords: Anaplasma phagocytophilum, Anaplasma ovis, Borrelia burgdorferi, SFG rickettsiae, Mongolia
316860Introduction
Tick-borne organisms in North America and Eurasia are important health threats to agricultural animals causing acute and chronic debilitating illness, and leading to decreased milk, meat, or fiber production, diminished foraging, and reduced utility as pack animals. Borrelia burgdorferi, Anaplasma phagocytophilum, A. ovis, and Spotted Fever Group (SFG) rickettsiae are among the most studied ticktransmitted organisms, pathogenic to livestock species, with disease sequelae that lead to economic losses through premature death, loss of production, and culling of affected individuals [1,2].
Borrelia burgdorferi, a gram negative, microaerophilic spirochete, the agent of Lyme disease, causes multisystemic disorders in certain animals, with abnormalities in the skin, musculoskeletal system, heart, and nervous system [3,4].The ecology of Lyme disease is well-studied but complex, and B. burgdorferi persists within an ecosystem due to the non-specific feeding behavior of the ticks and abundance of reservoir and amplifying hosts [4].
In animals clinical signs may include rash, fever, joint pain, lameness, weight loss, and overall decreased productivity [1].
Anaplasma phagocytophilum, formerly, Ehrlichia phagocytophila, causes anaplasmosis, Human Granulocytic Anaplasmosis (HGA), and a similar disease in horses [5]. The first human case of HGA and equine case of E. equi (reclassified as A. phagocytophilum) were described in 1994 and 1969, respectively, in the United States (U.S.), with cases reported in European horses during the 1990’s [5]. During the 1930’s E. phagocytophila was recognized in livestock species throughout Europe [5-7]. Anaplasma phagocytophilum, a gram negative, intracellular, rickettsial pathogen affects neutrophils and occasionally endothelial cells and macrophages, causing a febrile illness with a sudden onset of fever, headache, myalgia, nausea, gastrointestinal signs, and arthralgia in humans [4].This pathogen is responsible for tick-borne fever in cattle and granulocytic anaplasmosis in horses [6,7]. In some livestock, the disease may be self-limiting, but reports of sheep mortality up to 24% have been published from the United Kingdom (UK).
Anaplasma ovisis an intraerythrocytic rickettsial pathogen responsible for sudden death in small ruminants and other species [8,9]. In the U.S., A. ovis has been identified in sheep as a subclinical infection and may cause decreased productivity in infected animals [10]. In Mongolia, A. ovis has been implicated in the sudden death of Mongolian reindeer [8].
Spotted Fever Group (SFG) rickettsial organisms are gram-negative obligate intracellular coccoid shaped bacteria [4]. SFG rickettsiae invade monocytes/macrophages, thrombocytes, neutrophils, and erythrocytes causing anaemia, thrombocytopenia, and leukopenia in a variety of mammalian species, including livestock [11,12].
Humans and larger mammal species are incidental hosts for B. burgdorferi, A. phagocytophilum, and SFG rickettsiae, but can become severely ill or fatally affected by these pathogens [4]. One study conducted in people across four Mongolian provinces in 2003 showed seroprevalenceto B. burgdorferi, and A. phagocytophilum ranging from 2%–15% indicating tick-borne pathogens may be an important public health concern in this nation [13].
Investigations into tick-borne diseases in Asia revealed a number of Borrelia and Spotted Fever Group (SFG) rickettsiae species transmitted by different tick genera [14-22]. In Inner Mongolia, a new rickettsial organism was reported in 2000, R. heilongjiangensis [21]. North Asian tick typhus, caused by R. sibirica, is widespread in Siberia and central Asia, has been isolated from ticks as well as humans, and was recently identified as an emerging pathogen in Eurasia [19,23].
The Mongolian grassland steppe supports an estimated 40 million head of livestock, accounting for approximately 24% of the country’s entire gross national product. Three landscape types predominate in Mongolia, desert, grassland, and forest steppe, with pockets of taiga situated in the north-central region of the country along the Siberian-Mongolian border. Over a third of Mongolians are pastoralists where millennia-old traditional livestock management persists. Transhumance, migration, and translocation may contribute to tick and tick-borne pathogens becoming established and surviving in environments and hosts where they have not existed previously.
There is a dearth of information about the role domesticated animals, landscape, or pastoral agricultural practices play in the epidemiology and disease ecology of tick-borne pathogens in Mongolia. Investigations identifying exposure or infection to B. burgdorferi sensu lato (s.l.), A. phagocytophilum, A. ovis, and SFG rickettsiae have not been pursued in Mongolian animals for over 45 years [24]. North Asian tick typhus, (R. sibirica), widespread in Siberia, Far Eastern Russia, central Asia, and Eurasia, has been isolated from a region adjacent to the eastern part of Mongolia suggesting that investigations to identify spotted fever in Mongolia is warranted. In north central Mongolia, reindeer (Rangifer tarandus) tested for tick-borne pathogens had high seroprevalence and infection of A. ovis, A.phagocytophilum, and B. burgdorferi s.l. (Papageorgiou, unpublished data).
The objective of this research is to identify tick-borne pathogens in Mongolian livestock, across three different Mongolian landscapes (ecosystems), and to focus on tick-borne pathogens that have been isolated in neighbouring regions of Russia and China [15,20,21,25-29]. The hypothesis is that livestock in different ecosystems vary in their exposure to and prevalence of tick-borne pathogens. The aims of this research are to: 1) measure seroprevalence and infection of tick-borne pathogens in livestock, 2) identify and evaluate potential prevalence factors that may contribute to tick-borne pathogen exposure or infection, and 3) propose possible mechanisms of transmission for tick-borne pathogens in four species of Mongolian livestock (cattle, horse, sheep, and goat) across two provinces (aimags).
Materials and Methods
Site description
Sampling was performed in two different aimags in northern Mongolia: 1) three sites in Khenti aimag between June 26 to July 5, 2007; 2) one site in Khuvsgul aimag between June 10 to June 18, 2007; and 3) three sites in Khuvsgul aimag from June 14 to June 27, 2008, one previously sampled in 2007 (Table 1 and Figure 1). Cattle, sheep and goats were sampled at all sites, but at site three in Khuvsgul only goats were sampled (Table 1 and Figure 1). Horses were sampled at one site in Khenti and one in Khuvsgul.
| Region | Site | Year | Mean Altitude (ma) | Latitudeb | Longitudeb | Landscape Type |
|---|---|---|---|---|---|---|
| Khenti | 1 | 2007 | 1325 | 48° 3' 59" | 109° 30' 13" | Grassland-Forest Steppe |
| 2 | 2007 | 1128 | 48° 57' 23" | 110° 37' 32" | Grassland-Forest Steppe | |
| 3 | 2007 | 1099 | 47° 19' 44" | 110° 36' 11" | Grassland | |
| Khuvsgul | 4 | 2007 | 1582 | 51° 18' 45" | 99° 16' 0” | Grassland-Forest Steppe |
| 4 | 2008 | 1571 | 51° 18' 45" | 99° 16' 0” | Grassland-Forest Steppe | |
| 5 | 2008 | 1682 | 51° 33' 46" | 99° 25' 50" | Grassland-Forest Steppe | |
| 6 | 2008 | 2218 | 51° 11' 27" | 98° 54' 59" | Taiga |
am=meter; bdegrees minute
Table 1: Summary of altitude (m), latitude, longitude, and site descriptions within two Mongolian regions (Khenti and Khuvsgul aimags), during 2007 and 2008 field seasons.
Figure 1: Map of Mongolia with detail of NW Khuvsguland Khenti aimags in Mongolia outlining the five livestock study sites. WS=west steppe, ES=east steppe, WT=west taiga; star=township of Tsaaganuur, block arrow=Lake Tsaaganuur, outline arrow=Lake Khuvsgul; filled cross=sites in Khenti; square outline=capitol of Mongolia, UlaanBaatar.
Sites one and two in Khenti aimag were a mosaic of forest and grassland steppe amid hills and site three was a uniformly flat grassland steppe ecosystem (Figure 1). Two of the three sites, in northern Khuvsgul aimag, were separated into west and east regions by a segment of Lake Tsaaganuur (Figure 1). The third Khuvsgul site is situated within the taiga ecosystem adjacent to the west steppe site with hills, larch and pine forests, bounded by grassland and forest steppe where livestock graze (Figure 1).
Latitude, longitude, and elevation (m) were recorded where each herd was sampled using a Global Positioning System (GPS) handheld device (eTrex Legend® HCx, Garmin, Olathe, KS).
Sample collection
Each herder in this study maintained cattle, horses, sheep and goats, although not all herders’ horses were sampled. Animals from 15 herds were sampled using manual restraint. Animal selection for sampling was opportunistic. Livestock were herded into makeshift corrals and captured for blood collection until approximately 30 each of sheep and goats, and 10 each of cattle and horses were sampled. Blood samples were collected from the jugular vein using a Vacutainer® needle system (18 or 20 gauge) and transferred to EDTA-containing and sterile additive-free tubes. Skin biopsies, sampled from the base of the ear or cervical aspect of the neck from sheep and goats in western Khuvsgul aimag were collected using a sterile, disposable, 2mm biopsy punch (Miltex, Inc., York, PA). Skin samples were stored in 70% alcohol at ambient temperature and received no further treatment until laboratory analysis.
Body Condition Score (BCS), gender, age, and location were recorded for each animal sampled.
Sample handling/storage/processing
Blood collected into additive-free tubes were allowed to form a clot, and the serum transferred into sterile additive-free tubes. To maintain a cold chain while in the field, samples were stored in plastic locking bags within nylon containers, placed in large plastic bags that were immersed in river water, and secured with rocks.
Samples were transported to the Institute of Veterinary Medicine in Ulaanbaatar, Mongolia and stored at 20°C. Serum aliquots were heat-treated at 70°C for one hour. Aliquots of EDTA-preserved whole blood samples were treated with 0.2% glutaraldehyde solution. Treatments complied with the United States Department of Agriculture regulations to ensure only foot-and-mouth disease virus-free samples entered the United States. All samples were transported on ice to the University of California, Davis and stored at -20°C.
Serology
Serum samples were analysed for antibodies to B. burgdorferi, A. phagocytophilum, and SFG rickettsiae using indirect Immunofluorescent Antibody (IFA) testing. Samples were prepared as follows: serum was diluted in phosphate buffered saline (PBS) at 1:25, from which 25 μl were applied to commercially prepared slides with the following antigens: B.burgdorferi (VMRD, Pullman, WA), A. phagocytophilum (ProtaTek International, Inc., St. Paul, MN), and R. rickettsii (ProtaTek Intl.). A low dilution was used in order to maximize analytical sensitivity during this exploratory phase in the absence of cutoff values for these species. Slides were incubated at the 37°C in moist air for 40 min. and washed three times with PBS. Thirty μl of secondary antibody (fluorescein isothiocyanate-labelled goat anti-bovine (cattle samples), rabbit anti-sheep (goat and sheep samples), and goat anti-equine (horse samples) IgG heavy and light chain antibodies) (Kirkgaard & Perry Laboratories (KPL), Gaithersburg, MD) at 1:30 dilution in PBS were added to each slide well and incubated with moisture at 37°C for 40 min [6,30]. Slides were washed with PBS twice. Two to three drops of eriochrome T-black were added to the PBS and allowed to stain the slide for 3 min. Positive controls included naturally-infected black bear (Ursus americanus) and woodrat (Neotoma spp.) for B. burgdorferi, horse and cow for A. phagocytophilum, and dog and human for R. rickettsii with species-appropriate secondary antibodies. Distilled water was used as a negative control. Tests were considered positive when shiny green fluorescent spirochetes, cytoplasmic inclusion bodies, or a ‘starry night’ array for Borrelia, Anaplasma, and SFG rickettsiae, respectively, were observed under epi-fluorescent microscopy.
PCR
DNA was extracted from whole blood and skin samples using the Qiagen DNeasy® kit and DNeasy Blood & Tissue protocols (Qiagen, Valencia, CA).
Real-time quantitative PCR was performed for B. burgdorferi, A. phagocytophilum, and Rickettsia species. The B. burgdorferi PCR targeted thep66 gene with primers and probes as previously described (forward and reverse primers, 5’-GCTGTAAACGATGCACACTTGGT-3’ and 5’-GGCGGCACACTTAACACGTTAG-3’, respectively; probes used were 6FAM-TTCGGTACTAACTTTTAGTTAA and VIC-CGGTACTAACCTTTCGATTA, for the forward and reverse primers, respectively) [31,32]. The PCR for A. phagocytophilum targeted the msp2 gene using forward and reverse primers, 903F, 5’-AGTTTGACTGGAACACACCTGATC-3’ and 1024R, 5’-CTCGTAACCAATCTCAAGCTCAAC-3’ and probe 939p-TTAAGGACAACATGCTTGTAGCTATGGAAG-GCA[33]. For SFG rickettsiae PCR, the citrate synthase gene (gltA) was amplified with forward primer 692F, 5’-AATGCTTCTACTTCAACAGTCCGAAT-3’ and reverse 773R, 5’-GTGAGGCAATACCCGTGCTAA-3’, and probe 724T-CTCATCCGGAGCTAACCCTTTTGCTTGT [33].
Twelve μl PCR reactions for all three pathogens contained 5 μl DNA, 1X TaqMan Universal Master Mix (Applied Biosystems, Menlo Park, CA), 2nmoll each primer, and 400pmoll of the probe. For B. burgdorferi, PCR conditions were: 94°C for 3 min followed by 40 cycles of 94°C for 1 min; 50°C for 2 min; and 74°C for 2 min. For the other two pathogens conditions were: 50°C for 2 min, 95°C for 10 min, and 50 cycles at 95°C for 15 sec, followed by 60°C for 1 min. PCR amplification was performed with an ABI 7700 Prism Sequence Detector (Applied Biosystems, Menlo Park, CA), and the products were analyzed with the accompanying software. Negative controls were used for each PCR assay. For all TaqMan PCR reactions, samples were considered positive if they had a Cycle Threshold (CT) value <40 and characteristic amplification plots.
Amplification of the groEL gene for A. ovis was performed using traditional PCR as described, with modification, using forward and reverse primers 5’-TAAAAGCCAAGGAGGCTGTG-3’ (H0011) and 5’-TTGCTCTCCTCGACCGTTAT-3’ (H0012), respectively [8]. PCR reactions for A. ovis consisted of 42 μl containing 5 μl of buffer, 3 μl MgCl2, 1 μl dNTPs, 2 μl of each primer, and 0.25 μl Taq. PCR reaction conditions were 94°C for 2 min, then 40 cycles for 30 sec each alternating at 94°C, 60°C, and 72°C, and a final extension for 10 min at 72°C performed in an EppendorfMastercycler EP Gradient instrument [8]. PCR products (181 bp) were visualized on 1.5% agarose gels stained with GelStar (Lonza, Rockland, ME) using UV transillumination.
Sequencing
Of the 123 PCR positive A. ovis samples, one positive sample from a goat was extracted for sequencing using a Qiagen® tissue extraction kit. DNA sequencing was performed at a commercial sequencing facility (Davis Sequencing, University of California, Davis, CA). The resulting sequence was compared with sequences available on GenBank (National Center for Biotechnology Information (NCBI), using the BLAST algorithm searching nr/nt nucleotide databases [34].
DNA extractions and PCR reaction preparations and analysis were performed in separate laboratory rooms.
Statistical analyses
Differences in animal Body Condition Score (BCS) and gender between aimags were evaluated using Mann-Whitney U and chi-square homogeneity tests, respectively. Ninety-five percent Confidence Intervals (CIs) for prevalences were calculated using OpenEpi®
Logistic regression analyses were performed to identify associations between outcomes (presence/absence for each pathogen) and prevalence factors (variables: species, gender, BCS, and elevation). Categorical variables were defined as: 1) species: cattle (referent category), small ruminants (sheep/goat), and horse; and 2) gender: female (referent category), male, and male castrate. Body condition score was evaluated by the principal investigator assessing the prominence of the ilia, spine, ribs, and epaxial muscling using an ordinal scale of 1-5, (1=emaciated, 2=thin, 3=normal, 4=overweight, 5=obese).BCS (ordinal) and elevation (continuous) were categorized to evaluate the assumption of linearity in the log odds of the dependent variable for each regression model by plotting the log odds against the midpoint of the categories for BCS and elevation. All prevalence factors were included in all models. Two-way interactions between prevalence factors were assessed for model inclusion using likelihood ratio tests. Equids were excluded from the A. ovis model because horses are not infected with this pathogen, and the seven goats sampled from the taiga were excluded from the regression analyses because of the small number sampled. Results are presented as prevalence odds ratios (ORprev) and 95% CIs.
Analyses were performed using SPSS® (v. 11.0, Chicago, IL), and Egret® (v. 2.0.1, Cytel Corporation, Cambridge, MA). P-values ≤ 0.05 were considered significant.
Results
Study sites and samples
Whole blood and serum samples from 460 animals and skin samples from 57 animals were collected (Table 2). Khenti livestock had higher BCS than livestock sampled in Khuvsgul (p<0.001, data not shown).The sex distribution differed by aimag (p=0.03, data not shown). In Khuvsgul, 85% of the livestock sampled were female and 14% were male, compared to Khenti where 75% were female and 24% were male. Pathogen prevalence by species, gender, and site is shown in Table 3.
| Year | Aimag | Site | Species and Gender | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mongolian Cattle | Mongolian Fuzzy Goat | Fat-Tailed Sheep | Mongolian Horse | |||||||||||
| M | F | MC | M | F | MC | M | F | MC | M | F | MC | |||
| 2007 | Khenti | 1 | 0 | 28 | 0 | 12 | 19 | 0 | 18 | 30 | 0 | 1 | 2 | 0 |
| 2 | 0 | 23 | 2 | 12 | 15 | 1 | 22 | 28 | 0 | 0 | 0 | 0 | ||
| 3 | 4 | 14 | 0 | 13 | 26 | 0 | 28 | 20 | 0 | 0 | 0 | 0 | ||
| 2008 | Khuvsgul | 4 | 2 | 12 | 1 | 8 | 14 | 3 | 1 | 17 | 6 | 3 | 5 | 12 |
| 5 | 0 | 4 | 1 | 1 | 2 | 22 | 1 | 6 | 13 | 0 | 0 | 0 | ||
| 6 | 0 | 0 | 0 | 2 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Species Total | 91 | 155 | 190 | 23 | ||||||||||
agender missing for one horse sampled
Table 2: Summary of livestock species sampled (n=460) in Mongolia from 2007-2008 by gender, site, province, and year.
A
| Seroprevalence (n) | |||||||
|---|---|---|---|---|---|---|---|
| Pathogen | Overall | Khenti | Khuvsgol | ||||
| Site 1 | Site 2 | Site 3 | Site 4 | Site 5 | Site 6 | ||
| Borreliaburgdorferi s.l. | 11.3 (48) | 1.1 (1) | 2.9 (3) | 10.7 (11) | 24.1 (19) | 29.8 (14) | 0 (0) |
| Anaplasmaphagocytophilum | 35.8 (149) | 17.2 (15) | 29.1 (30) | 20.4 (19) | 62.0 (49) | 76.5 (36) | 0 (0) |
| SFG rickettsia | 21.6 (89) | 3.4 (3) | 47.9 (45) | 30.0 (30) | 10.3 ( 8) | 6.5 (3) | 0 (0) |
B
| A. ovis Infection % (n) | ||||||
|---|---|---|---|---|---|---|
| Khenti | Khuvsgol | |||||
| Species | Site 1 | Site 2 | Site 3 | Site 4 | Site 5 | Site 6 |
| Mongolian Cattle | 5 (1) | 16.7 (2) | 0 (0) | 80 (12) | 20 (1) | n/a |
| Fat-Tailed Sheep | 33.3 (5) | 60 (6) | 42.1 (8) | 88 (22) | 40 (8) | n/a |
| Mongolian Fuzzy Goat | 64.7 (11) | 50 (4) | 71.4 (10) | 64 (16) | 44 (10) | 0 (7) |
A=Anaplasma
Table 3: Seroprevalence (A) for three tick-borne pathogens detected in four Mongolian livestock species, (n=436), and (B) A. ovis infection (excludes horses), sampled from 2007-2008 showing overall and regional prevalence.
Univariate analyses and serostatus
Univariate analyses contrasting species, gender, BCS, and elevation with presence/absence of the pathogen were performed. Body condition score was linear in the log odds for all four pathogens. Elevation was linear in the log odds in the A. phagocytophilum and A. ovis models, but not in the B. burgdorferi s.l. and SFG rickettsiae models. For the B. burgdorferi s.l., model elevation was dichotomized into low (1050-1350 m) and high (>1500 m) categories for model inclusion. A square root transformation was performed to fit elevation in the SFG rickettsiae model. Comparisons of species, gender, BCS and elevation among sites using prevalence odds ratios are summarized in Table 4 for the pathogen outcomes that were significant and final models for B. burgdorferi s.l, A. phagocytophilum, SFG rickettsiae, and A. ovis (Table 5).
A
| Khenti | Gender | Pathogena | Khuvsgul | Gender | Pathogena | ||||
|---|---|---|---|---|---|---|---|---|---|
| Species | Bb | Ap | Rr | Species | Bb | Ap | Rr | ||
| Mongolian | F | 0 (0) | 100 (2) | 0 (0) | Mongolian | F | 60.0 (3) | 100 (5) | 80.0 (4) |
| Horse | M | 0 (0) | 100.0 (1) | 0 (0) | Horse | M | 33.3 (1) | 100 (3) | 0 (0) |
| MC | - | - | - | MC | 36.4 (4) | 63.6 (7) | 18.2 (2) | ||
| Total | 0 (0) | 100 (3) | 0 (0) | Total | 42.1 (8) | 78.9 (15) | 31.6 (6) | ||
| Mongolian | F | 12.5 (7) | 47.8 (22) | 2.0 (1) | Mongolian | F | 12.5 (2) | 6.3 (1) | 18.8 (3) |
| Cattle | M | 0 (0) | 42.3 (2) | 0 (0) | Cattle | M | 0 (0) | 0 (0) | 50 (1) |
| MC | - | 100 (1) | - | MC | 0 (0) | 50 (1) | 0 (0) | ||
| Total | 11.3 (7) | 43.9 (25) | 1.9 (1) | Total | 10.0 (2) | 10.0 (2) | 20.0 (4) | ||
| Mongolian | F | 3.9 (3) | 17.3 (13) | 37.3 (28) | Mongolian | F | 26.3 (5) | 73.7 (14) | 0 (0) |
| Fat-Tailed Sheep | M | 3.1 (2) | 12.3 (8) | 37.5 (24) | Fat-Tailed Sheep | M | 50.0 (1) | 100 (2) | 0 (0) |
| MC | - | - | - | MC | 44.4 (8) | 66.7 (12) | 5.6 (1) | ||
| Total | 3.5 (5) | 15.0 (21) | 37.4 (52) | Total | 35.0 (14) | 72.5 (28) | 2.8 (1) | ||
| Mongolian | F | 5.9 (3) | 19.1 (9) | 22.0 (11) | Mongolian | F | 0 (0) | 57.1 (12) | 0 (0) |
| Fuzzy Goat | M | 0 (0) | 17.1 (6) | 37.1 (13) | Fuzzy Goat | M | 0 (0) | 54.5 (6) | 0 (0) |
| MC | - | 0 (0) | 100 (1) | MC | 40.9 (9) | 95.5 (21) | 0 (0) | ||
| Total | 3.4 (3) | 18.1 (18) | 29.1 (25) | Total | 16.7 (9) | 72.2 (39) | 0 (0) | ||
| Overallb | 5.1 (15) | 22.6 (64) | 27.7 (64) | Overallb | 24.8 (33) | 63.9 (84) | 8.4 (11) | ||
awithin species positives bwithin site positives cwithin species totals
Bb: Borrelia burgdorferi; Ap: Anaplasma phagocytophilum; Rr: SFG rickettsiae; “-“: no samples available in this category
B
| Seroprevalence (n)* | |||||
|---|---|---|---|---|---|
| Pathogen | Horses | Cattle | Goats | Sheep | p-value |
| B.burgdorferi s.l. | 16.7 (8) | 18.8 (9) | 25 (12) | 39.6 (19) | 0.002 |
| A.phagocytophilum | 12.1 (18) | 18.1 (27) | 36.2 (54) | 33.6 (50) | <0.001 |
| SFG rickettsiae | 6.7 (6) | 5.6 (5) | 28.1 (25) | 59.6 (53) | <0.001 |
*Values presented are % within pathogen; B=Borrelia; A= Anaplasma
C
| Seroprevalence (n)* | |||||
|---|---|---|---|---|---|
| Site | Pathogen | Horses | Cattle | Goats | Sheep |
| Khenti | B.burgdorferi s.l. | 0 (0) | 2.4 (7) | 1.0 (3) | 1.7 (5) |
| A.phagocytophilum | 1.1 (3) | 8.8 (25) | 5.3 (15) | 7.4 (21) | |
| SFG rickettsiae | 0 (0) | 0.4 (1) | 8.9 (25) | 18.4 (52) | |
| Khuvsgol | B.burgdorferi s.l. | 6.0 (8) | 1.5 (2) | 6.8 (9) | 24.8 (33) |
| A.phagocytophilum | 11.3 (15) | 1.5 (2) | 29.3 (39) | 21.8 (29) | |
| SFG rickettsiae | 4.6 (6) | 3.1 (4) | 0 (0) | 0.8 (1) | |
% = % within site; n = positive count within species in each site
D
| A. ovis Prevalencea (n)* | |||||||
|---|---|---|---|---|---|---|---|
| Khenti | Khuvsgul | ||||||
| Site | Site | ||||||
| Species | 1 | 2 | 3 | 4 | 5 | ||
| Mongolian Cattle | F | 5 (1) | 20.0 (2) | 0 (0) | 83.3 (10) | 25.0 (1) | |
| M | - | - | 0 (0) | 50.0 (1) | - | ||
| MC | - | 0 (0) | - | 100 (1) | 0 (0) | ||
| Totalc | 5.0 (1) | 16.7 (2) | 0 (0) | 80.0 (12) | 20 (1) | ||
| Mongolian Fuzzy Goat | F | 72.7 (8) | 25.0 (1) | 77.8 (7) | 64.3 (9) | 100 (2) | |
| M | 50.0 (3) | 75.0 (3) | 60.0 (3) | 87.5 (7) | 0 (0) | ||
| MC | - | - | - | 0 (0) | 40.9 (9) | ||
| Total | 64.7 (11) | 50.0 (4) | 71.4 (10) | 64.0 (16) | 44.0 (11) | ||
| Mongolian Fat-Tailed | F | 12.5 (1) | 55.6 (5) | 66.7 (6) | 94.1 (16) | 50.0 (3) | |
| Sheep | M | 57.1 (4) | 100 (1) | 20.0 (2) | 0 (0) | 100 (1) | |
| MC | - | - | - | 83.3 (5) | 30.8 (4) | ||
| Total | 33.3 (5) | 60.0 (6) | 42.1 (8) | 88.0 (22) | 40.0 (8) | ||
| Overallb | 32.7 (17) | 40.0 (12) | 50.0 (18) | 76.9 (50) | 40.0 (20) | ||
awithin species positives; bwithin site positives; cwithin species totals; donly goats sampled; emissing gender classification; ‘-‘ no samples available in this category
Table 4: Tick-borne pathogen prevalence in four species of Mongolian livestock (n=436) sampled from 2007-2008: (A) shown by region, species, and gender; (B) showing significant differences across species; (C) categorized by species and site; and (D) A. ovisinfection identified by aimag and site in three species of Mongolian livestock (n=223, horses not included).
| Pathogen | Variables | Prevalence | 95% CI | p-value | |
|---|---|---|---|---|---|
| Odds Ratio | Lower | Upper | |||
| B. burgdorferi s.l. | BCS | 0.90 | 0.39 | 2.08 | 0.80 |
| Goat/Sheep | 0.56 | 0.20 | 1.55 | 0.27 | |
| Horse | 1.99 | 0.47 | 8.38 | 0.35 | |
| Male | 0.48 | 0.14 | 1.63 | 0.24 | |
| Castrated male | 1.92 | 0.73 | 5.05 | 0.18 | |
| Elevation: 1110-1340 meters | 0.02 | 0.01 | 0.08 | <0.001 | |
| Anaplasma | BCS | 241.20 | 8.25 | 7049.00 | 0.00 |
| phagocytophilum | Male | 0.61 | 0.34 | 1.11 | 0.11 |
| Castrated male | 2.38 | 1.01 | 5.60 | 0.05 | |
| Goat/Sheep | 0.94 | 0.50 | 1.80 | 0.86 | |
| Horse | 3.45 | 0.77 | 15.39 | 0.10 | |
| Elevation | 1.01 | 1.01 | 1.02 | < 0.001 | |
| SFG rickettsiae | Goat/Sheep | 4.22 | 1.56 | 11.40 | <0.005 |
| Horse | 42.34 | 7.31 | 245.00 | <0.001 | |
| Male | 1.19 | 0.68 | 2.10 | 0.55 | |
| Castrated male | 0.48 | 0.10 | 2.32 | 0.36 | |
| BCS | 1.17 | 0.68 | 2.00 | 0.58 | |
| Elevation: SquareRoot | 0.69 | 0.59 | 0.81 | <0.001 | |
| Anaplasma ovis | BCS | 0.13 | 0.01 | 2.55 | 0.18 |
| Elevation | 1.00 | 0.99 | 1.01 | 0.60 | |
| Goat/Sheep | 1883.00 | 4.01 | 884200.00 | 0.02 | |
| BCS × Elevation | 1.00 | 1.00 | 1.00 | 0.28 | |
| Goat/Sheep * Elevation | 1.00 | 0.99 | 1.00 | 0.05 | |
| Male | 0.87 | 0.41 | 1.83 | 0.71 | |
| Castrated male | 0.24 | 0.11 | 0.56 | <0.001 | |
BCS: body condition score; Goat/Sheep: indicates goat and sheep combined into one category
Table 5: Summary of logistic regression final models for each of four tick-borne pathogens, B.burgdorferi s.l., A. phagocyophilum and Rickettsia spp., with seroprevalence as outcome, and A.ovis infection as outcome, from four livestock species (n=436) sampled in five sites within two aimags in Mongolia in 2007 and 2008. Outcomes were positive or negative based on laboratory analyses. Reference categories are as follows: BCS: <2; Species: cattle; Gender: female.
Overall seroprevalence
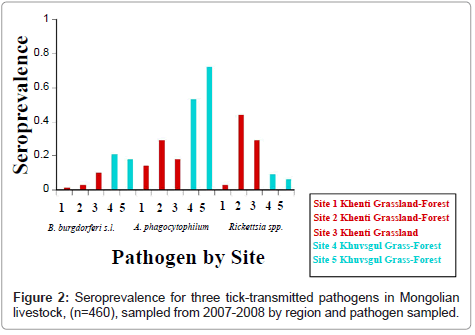
Overall seroprevalence of tick-borne pathogens in the animals sampled was 11.3% (CI=8.5%-14.5%), 35.8% (CI=31.3%-40.5%), and 21.6% (CI=17.8%-25.7%) for B. burgdorferi s.l., A. phagocytophilum, and SFG rickettsiae, respectively (Table 3).
Differences in seroprevalence between the two aimags and among species for exposure to all three pathogens were measured (Table 4A). In Khenti there was similar exposure to A. phagocytophilum and SFG rickettsiae, 22.6% (CI=18.0%-27.8%) and 27.7% (CI=22.7%-33.1%), respectively; in Khuvsgul
A. Phagocytophilum seroprevalence was higher by 63.9% (CI=55.5%-71.7%) livestock exposed, and only 8.4% (CI=4.5%-14.1%) SFG rickettsiae seropositive (Table 4A and Figure 2). Similarly, Khuvsgul had higher B. burgdorferi s.l. seroprevalence (24.8%, CI=18.0%-32.7%) compared to Khenti (5.1%, CI=3.0%-8.1%) (Table 4A and Figure 2). In site six at Khuvsgul the goats (n=7) sampled were negative for all three pathogens.
Significant differences among all livestock species were detected for exposure to B. burgdorferi s.l., A. phagocytophilum, and SFG rickettsiae (Table 4B). Sheep had a higher seroprevalence of B. burgdorferi s.l. and SFG rickettsiae, but slightly lower seroprevalence of A. phagocytophilum than goats (Table 4A). Cattle and horses had substantially lower seroprevalence to B. burgdorferi s.l. and A. phagocytophilum than measured in the small ruminants (Table 4A).
Species and Landscape Differences (by pathogen)
Borrelia burgdorferi sensu lato
In Khenti livestock, B. burgdorferi s.l. seroprevalence was substantially higher (10.7%, CI=5.7%-17.8%) in site 3, a dry grassland landscape devoid of forest, than livestock residing in the grassland and mixed grassland-forest steppe in sites one and two (Table 4C ). However, Khuvsgul livestock had a 4.9 times higher prevalence odds of B. burgdorferi s.l. exposure than livestock in Khenti. Additionally, Khuvsgullivestock in site 5 had higher overall exposure (29.8%; CI=18.1%-43.9%) to B. burgdorferi s.l. than animals in site 4 (24.1%; CI=15.6%-34.4%) (p<0.001).
Animals (regardless of species) at elevations greater than 1500 m had a higher prevalence odds of exposure to B. burgdorferi s.l. than animals at elevations less than 1500 m (ORprev=6.9, CI=0.7-66.7). The association between B. burgdorferi s.l. seroprevalence and gender revealed that castrated males had an increased prevalence odds of exposure to B. burgdorferi s.l. when compared with females and males, respectively (ORprev=1.9, CI=0.7-5.0 and ORprev=4.0, CI=0.7-23.2).
SFG rickettsiae species
For SFG rickettsiae exposure, site 2 (47.9%, CI=37.9%-58.0%),a denser forested area, in Khenti had a higher seroprevalence than sites one and three (3.4%, CI= 0.9%-9.0%; 30.0%, CI= 21.6%-39.5%, respectively). There was a significantly higher prevalence of SFG rickettsiae (p<0.001) in Khenti than in comparable mixed grassland-forest landscapes at sites 4 and 5 in Khuvsgul aimag (10.3%, CI=4.9%-18.5%; 6.5% CI=1.7%-16.7%, respectively) (Table 3A).
Small ruminants and horses had 4.2 and 42.3 (CI=1.5-11.4 and CI=7.3-245.0, respectively) greater odds of being seropositive for SFG rickettsiae compared to cattle (Table 4D). When compared to castrated males (ORprev=0.48, CI=0.1–2.3), females and intact males had an approximate twofold increase in prevalence odds for the outcome of SFG rickettsiae (ORprev=2.1, CI=0.4-10.2; ORprev=2.5, CI=0.4-14.3, respectively) in contrast to what was observed in the B. burgdorferi s.l. analysis (Table 5).
The multivariate model included BCS, gender, and species as predictors for SFG rickettsiae exposure and a square root transformation to fit elevation in the model (Tables 4 and 5). The coefficients for species and elevation in this model were significant, but coefficients for BCS and gender were not significant (Table 5). The effects of changes in elevation on SFG rickettsiae seroprevalence were not consistent. For example, in castrated males with a BCS ≤2, SFG rickettsiae seroprevalence was approximately 12% at low elevations (<1100 m), then decreased linearly as elevation increased from 1100 m until 1350 m, and leveled off to approximately 1% between 1350 m to 1650 m.
Anaplasma phagocytophilum
There were differences in exposure to A. phagocytophilum and SFG rickettsiae among species and sites. Specifically, sheep and goats had higher exposure to A. phagocytophilum in Khuvsgul and to SFG rickettsiae in Khenti than cattle and horses in these regions (Table 4C).
The highest exposure to A. Phagocytophilumin Khenti was detected on site 2 (29.1%, CI=21.0%-38.4%), but Khuvsgul sites 4 and 5 had overall higher A. phagocytophilum seroprevalence (62.0%, CI=51.0%-72.2% and 76.5% CI=63.0%-87.0%, respectively). Both sites in Khuvsgul were situated adjacent to the taiga region where the grassland-forest steppe was more densely forested than similar landscape regions sampled in Khenti (Table 3B).
Predictor variables for A. phagocytophilum seroprevalence included BCS, gender, species, elevation, and an interaction term (BCS × elevation) (Tables 5 and 6). There was a greater prevalence odds of animals seropositive to A. phagocytophilum at the higher (1500 m or 1700 m) elevations (ORprev (1500 m)=2.6, CI=1.1-6.1 and ORprev(1700 m)=7.0, CI=1.3-37.7) than the lower regions (1300 m) (Table 6).
| Pathogen | Variables | Prevalence | 95% CI | |
|---|---|---|---|---|
| Odds Ratio | Lower | Upper | ||
| Anaplasma | ||||
| phagocytophilum | Female & MC | 4.52 | 2.57 | 7.94 |
| Male & MC | 6.41 | 2.28 | 18.01 | |
| 1500 m & 1300 m | 2.65 | 1.14 | 6.14 | |
| 1700 m & 1300 m | 7.00 | 1.30 | 37.71 | |
| Cattle & Goat/Sheep | 3.28 | 1.78 | 6.03 | |
| BCS 2 & 3 compared at Elevation 1300 m | 1.74 | 1.26 | 2.40 | |
| BCS 2 & 3 compared at Elevation 1700 m | 3.62 | 1.57 | 8.36 | |
| BCS 2 & 4 compared at Elevation 1300 m | 3.03 | 1.59 | 5.78 | |
| BCS 2 & 4 compared at Elevation 1700 m | 13.11 | 2.46 | 69.85 | |
| SFG rickettsiae | ||||
| 1300 m & 1500 m | 2.71 | 1.75 | 4.19 | |
| 1300 m & 1700 m | 6.86 | 2.95 | 15.97 | |
| 1500 m & 1700 m | 2.54 | 1.69 | 3.81 | |
| Cattle & Goat/Sheep | 4.20 | 1.56 | 11.11 | |
| Cattle & Horse | 42.35 | 7.32 | 245.14 | |
| BCS 3 & 2 compared at Elevation 1300 m | 1.74 | 1.26 | 2.40 | |
| BCS 3 & 2 compared at Elevation 1700 m | 1.17 | 0.68 | 2.00 | |
| BCS 4 & 2 compared at Elevation 1700 m | 1.36 | 0.46 | 4.00 | |
| Anaplasma ovis | ||||
| Cattle & Goat/Sheep | 1881.83 | 3.06 | 1155806.62 | |
| BCS of 3 compared at Elevations of 1300 and 1500 m | 2.88 | 1.23 | 6.76 | |
| BCS of 3 compared at Elevations of 1300 and 1700 m | 8.30 | 1.51 | 45.73 | |
| BCS of 4 compared at Elevations of 1300 and 1700 m | 3.61 | 1.24 | 10.52 | |
| BCS of 4 compared at Elevations of 1300 and 1700 m | 13.03 | 1.53 | 110.78 |
m: meter; MC: castrated male; BCS: body condition score; Goat/Sheep: indicates goat and sheep combined into one category
Table 6: Summary of significant prevalence odds ratio comparisons of BCS, gender, species, elevation, and any appropriate interactions, for all tick-borne pathogen logistic regression analyses of Mongolian livestock in 2007-2008. Reference categories are as follows: BCS: <2; Species: cattle; Gender: female; Elevation=1300 m.
There was a significant interaction between BCS and elevation for seroprevalence of A. phagocytophilum. For example, at 1300 m livestock with a one-unit increase in BCS had a 1.7 times greater prevalence odds (CI=1.3-2.4) of exposure to this pathogen which was lower than animals with the same unit increase in BCS at 1700 m (ORprev=3.6; CI=1.6-8.4) (Table 6).
PCR
All DNA extracts tested for SFG rickettsiae and B. burgdorferi were PCR negative. Two of the 223 whole blood samples, extracted from adult sheep in Khuvsgul during 2008, were PCR positive for A. phagocytophilum, but sequencing was not pursued because of the weak signal detected with TaqMan PCR in these samples.
Anaplasma ovis
Of the 240 samples tested for A. ovis, 51.3% (n=123, CI=45.0%-57.5%) were PCR positive. Comparison of A. ovis infection showed that Khuvsgul aimag livestock had higher (p<0.001) infection prevalence (61.9%, CI= 53.1%-70.3%) than Khenti animals (39.8%, CI=31.3%-48.9%). Contrasting species, goats had higher prevalence of A.ovis infection (47.2%, CI=38.5-56.0%) than sheep and cattle (39.8%, CI=31.5-48.7% and 13.0%, CI=7.9%-19.6%, respectively. Furthermore, when the association between species and A. ovis was controlled for site (using the variable elevation) there was a higher prevalence odds of infection in goats (ORprev=7.4, CI=3.1–18.0) than when site was not in the model (ORprev=2.1, CI=1.2–3.7), and the prevalence odds was still greater than cattle or sheep for this pathogen. The association between A. Ovid infection and gender when controlled for the site showed that females had a 3.9 times the greater prevalence odds of A. ovis infection (CI=1.1–14.4) than males in this same region.
For A. ovis infection the predictor variables in the model included species, gender, elevation, BCS, and two interaction terms (BCS × elevation and species × elevation). The interaction of BCS and elevation on outcome was significant and showed that animals with a BCS of 3 had an increased prevalence odds of A. ovis infection when elevation increased from 1300 m to 1500 m (ORprev=2.9, CI=1.2-6.8).
Sequencing was performed on one of the positive PCR goat samples from Khuvsgul aimag for A. ovis. Analysis for this A. ovis sequence showed that the sequence was identical to sequences obtained from reindeer (Rangifer tarandus) for A. ovis from the taiga region directly adjacent to this steppe region (GenBankAccession numbers JF260865 to JF260870).
Discussion
Regional differences to tick-borne pathogen exposure
High infection prevalence of tick-borne pathogens was identified among livestock across two aimags in Mongolia. These pathogens could have pernicious or subtle effects on the animals and could be threats to human health as well.
There were clear differences in exposure to infectious disease agents between the two aimags and among sites within the regions. In general, seroprevalences greater than 20% for B. burgdorferi s.l., A. phagocytophilum, and A. ovis was identified in Khuvsgul. SFG rickettsiae were more prevalent in Khenti than in Khuvsgul, particularly at site two, which was located within a mixed grassland-forest ecosystem. The only site in Khenti where B. burgdorferi s.l was prominent was site three, a strictly grassland ecosystem. It is probable that the varied ecosystems and elevations across both aimags may support diverse species of ticks and reservoir hosts capable of maintaining different tick-host-pathogen cycles. A few possible tick-borne pathogen host species observed during field sampling in both aimags included marmots (Marmota spp.), pikas (Ochotona pallasi), and squirrels (Spermophilus spp) in Khenti, while long-tailed ground squirrels (Urocitellus undulatus) predominated in Khuvsgul with voles (Microtus spp) and field mice (Apodemus spp) spottedat the taiga boundary near the transition zone to the grassland steppe. In Khenti marmots and pikas may be more competent hosts of Borrelia and SFG rickettsiae species than the long-tailed ground squirrels observed in Khuvsgul.
Climate may influence the disease ecology of these pathogens. Conditions in Khenti were cooler in the northern, mixed grassland-forest steppe than in site three. The Khuvsgul sites were on average 400 m higher in elevation, and located at higher latitudes, than the Khenti sites. The higher elevations and latitudes, combined with the effects generated by the snow-capped mountains in Khuvsgul, have colder,more severe weather, in Khuvsgulthan in Khenti and may support different small mammal, tick, and vegetation species contributing to the variable tick-borne pathogen prevalence between the two aimags. Marmots and pikas were not observed at the Khuvsgul study sites. The absence of these potential tick-borne pathogen reservoir hosts may explain the lower prevalence of SFG rickettisae and B. burgdorferi in this area as compared to Khenti where these rodents were frequently observed and may be part of the tick-pathogen cycles for these organisms.
Little is known about the spatial extent of tick-borne pathogens in Mongolia or Central Asia. Researchers in China are reporting novel tick-borne organisms in northwestern China are bordering Mongolia [19,21,22,35]. Most of the samples collected in these investigations were from ticks and rodents, with the discovery of a novel species of SFG rickettsiae (R. heilongjiangensis) [22,36,37]. Borrelia sinica sp. B. afzelii, and B. garinii are also newly identified tick-borne pathogen in northeastern China [15,22]. One recent study reported that B. burgdorferi s.l,
A. phagocytophilum, and Tick-Borne Encephalitis (TBE) virus were endemic in Mongolia based on a serosurvey of 545 human blood samples, and in 1997 R. sibirica was identified in a traveller from Mongolia, although none of these data originated in the two aimags in this study [13,37]. Other studies of tick-transmitted pathogens in Mongolia primarily focused on piroplasms such as Babesia and Theileria spp. in equids and ticks but no current work has pursued investigating B. burgdorferi s.l, A. phagocytophilum, A. ovis, and SFG rickettsiae in livestock in this country [38-40]. During this investigation high seroprevalence to B. burgdorferi s.l, A. phagocytophilum, A. Ovid, and SFG rickettsiae in livestock was evidence which indicates that people may also be at risk for exposure to these tick-borne pathogens. Mongolia’s large pastoral population relies on livestock for subsistence (milk, meat, fiber, transportation), lives in the grassland and forest steppe, and may be at greater risk for tick-borne pathogen exposure from constant animal contact and daily interaction with the environment.
Factors affecting tick-borne disease in Mongolian livestock
Animal-specific factors evaluated in this study to assess the relationship with tick-borne pathogen exposure included species, age, gender, and BCS. Body condition score was relevant because animals in already poor condition could be more susceptible to one or more tick-borne infections; conversely, chronic infection from tick-borne pathogens could negatively affect BCS. The animals in Khenti had higher average BCS and overall lower prevalence of tick-transmitted pathogens than those in Khuvsgul; however, the potential causal relationships for this association are obscured, because of the cross-sectional study undertaken in this investigation.
The sites sampled in Khuvsgul are at higher elevations and latitudes than those in Khenti. Climatic conditions in Khuvsgul contribute to shorter growing seasons, so forage may be less nutritious due to longer, colder winters and shorter summer and fall seasons in this area as compared to Khenti. Decreased nutritional intake can lead to lower BCS, and possibly, a higher prevalence of tick-borne pathogens in livestock for Borrelia and Anaplasma phagocytophilum, and A. ovis [7,41,42]. An animal with a higher BCS is more likely to be better nourished and have a healthier immune system that helps clear tick-borne pathogens before they cause any illness, than an animal with a lower BCS and a weaker immune system.
Gender differences in exposure to tick-borne pathogens were evident. Several factors might increase risk in fertile animals, including reproductive hormone levels, hormone-related behavioral territoriality and breeding, and energy expended for reproduction, birth, and lactation. Research exploring the effects of testosterone on Babesia microti infection indicated that male voles had higher levels of parasitemia than their castrated counterparts, and showed significant increases in peak percent parasitemia, earlier and longer onset of infectivity, and higher mortality [43,44]. The females in this investigation had higher seroprevalence in three of the four pathogens tested,
B. burgdorferi, SFG rickettsiae, and A. ovis. This finding may be the result of reproductive stresses the females undergo in pastoral management systems that may weaken their immune system during spring and summer birth and lactation periods when ticks are also questing. The condition of females during this time may subject them to an increase in tick-borne pathogen exposure and infection.
Small ruminants had the highest levels of exposure to tick-borne pathogens. This finding may be because: 1) they are inherently more susceptible to these infections than cattle and horses; 2) they range in landscape types with higher tick density; or 3) they harbor more ticks in their thicker wool and hair coats than cattle and horses. During milking of animals, the herders visualize and remove ticks from cattle and horses because of the short hair coat, but cannot do so easily in woolly sheep or long-haired goats where ticks can be hidden and remain attached longer, thus transmitting tick-borne pathogens before they are detected by herders. Anaplasmosis and borreliosis have previously been described in Eurasian sheep [7,42,45-47]. Ogden [45] showed that pastured sheep maintained enzootic B. burgdorferi in a region devoid of the usual rodent reservoir hosts, and that
A. phagocytophilum infection in sheep was modulated by tick density on sheep [45,46]. Small ruminants in Mongolia may also function as reservoirs for some of these pathogens [48-50].
A. ovis has been identified in free-ranging livestock and wildlife species. Specifically, this pathogen has been well-described in small ruminants in Africa and the U.S., but pathogenicity of A.ovis differs in small ruminants in these two continents [41,51-54]. In Mongolian reindeer (Rangifer tarandus) A. ovis was implicated in the sudden death of animals during 2004 [8]. Goff et al. [53] showed that bighorn sheep (Ovis canadensis canadensis) were susceptible to infection by this pathogen, and elk (Cervus elaphus) were susceptible under experimental conditions [53,55]. Other hoofed ruminants studied for Anaplasma spp. Exposure included white and black-tailed deer (Odocoileus virginianus, O. hemionus columbianus), mule deer (O. hemionus), and pronghorn antelope (Antilocapra americana americana) in the U.S., with infections of A. marginale and other Anaplasma spp reported in a variety of Asian and African species [56-58].
In dairy cattle B. burgdorferi and A. phagocytophilum cause clinical signs including lameness, joint swelling, anemia, fever, and decreased milk production [45]. Most cattle operations in Europe and the U.S. cull low producing or sick animals, so the course of tick-borne pathogen infections in cattle and outcomes of treatment are not well known. Investigations of tick-borne pathogens are warranted in Mongolia because the insidious nature of tick diseases does not readily cause herders to cull individual animals from their herds. Since herders maintain lower producing animals longer in Mongolia, pathogen persistence in incidental hosts can be measured over time and the information implemented in preventive medicine programs.
Although only a small number of equids were sampled, there was serologic evidence of exposure to A. phagocytophilum in both aimags and all three pathogens in Khuvsgul (except A. ovis), where most of the horses were tested. While some studies suggest that horses exposed to Borrelia spp. do not exhibit clinical signs, Parker [1] reported that horses suffer with musculo-skeletal problems from borreliosis such as joint swelling and laminitis, [1,59,60]. Equine infection with A. phagocytophilum causes overt clinical signs of fever, limb edema, inappetance, and depression, although the infection can be treated and eliminated with appropriate antibiotics [61,62]. Horses sampled in Khuvsgul are used as riding and pack animals for research, tourism, and transporting goods to the neighboring steppe and taiga areas. These horses are in direct contact with Mongolian reindeer that have high sero- and infection prevalence of tick-borne pathogens and may act as mechanical vectors transporting ticks to and from the taiga and steppe areas where reindeer and livestock respectively reside.
Based on the high seroprevalence (>30% at two of three sites) of SFG rickettisae in Khenti and
B. burgdorferi (>20% at two of three sites) in Khuvsgul, it is surprising that PCR analysis was negative for both pathogens. The Borrelia burgdorferi probe used may not detect the spectrum of B. burgdorferi s.l. species that represent B. burgdorferi genospecies found in Eurasia [15-17]. The R. rickettsii PCR probes used to detect SFG rickettisae in the samples should have cross-reacted with the SFG rickettisae found regionally in eastern Asia, such as R. sibirica, R. heilongjiangensis, or R. raoulti. The PCR negative SFG rickettsiae results indicate a possible absence of active infection of rickettsial pathogens in the animals sampled, that there were too few organisms for the PCR probe to detect, or the appropriate gene and probe were not used to target this pathogen.
Overview of investigation and conclusion
Opportunistic sampling was used in this study, which may have introduced selection bias and falsely elevated prevalence values. Most cattle and horses in the herd were corralled and all small ruminants were available for sampling. The herders did not omit any animals from the sampling pool; however, the animals caught may have been less thrifty or inherently tamer and less likely to elude capture once in the enclosure. A cross-sectional investigation limits the ability to assess causation. However, this study design serves to identify the prevalence of disease within a region and can constitute an initial step in supporting further research in disease ecology or epidemiological investigations.
This is the first phase of an investigation since Pavlovsky’s field research, during the 1960s, to initiate a comprehensive investigation of tick-borne pathogen presence in northern Mongolia [24]. Results from this research can guide surveillance and efforts to curb tick-borne diseases in these regions. Assessment of human exposure to tick-borne pathogens are also important, although societal constraints among some herding communities limit blood sampling from herders. The unique Mongolian culture, livestock management practices, and landscape create an interesting and challenging opportunity to further research of important tick-borne infections of animals and humans in that country.
Acknowledgements
The authors gratefully acknowledge financial support received from The Gifford Grant, University of California, Davis, the Lewis & Clark Grant from the Philosophical Society, and the Morris Animal Foundation for funding this research endeavour. The investigators are grateful to the herding communities in Mongolia for their hospitality, allowing the field team to camp, facilitating fieldwork at their homesteads, and access to their animals; to the Mongolian ministry directors and staff for permit support and allowing us to conduct field research in their country; and to Ms. Marta Zoess Purtzer, Dr. Bruce Smith, and Dr. Alice E. Bacon for their invaluable assistance in the field.
References
- Parker JL, White KK (1992) Lyme borreliosis in cattle and horses: a review of the literature. Cornell Vet 82: 253-274.
- Gritsun TS, Lashkevich VA, Gould EA (2003) Tick-borne encephalitis. Antiviral Res 57: 129-146.
- Estrada-Peña A (2002) Increasing habitat suitability in the United States for the tick that transmits Lyme disease: a remote sensing approach. Environ Health Perspect 110: 635-640.
- Sonenshine DE (1993) Ecology, behavior, and host-parasite interactions, Oxford University Press, Norfolk, VA.
- Parola P, Davoust B, Raoult D (2005) Tick- and flea-borne rickettsial emerging zoonoses. Vet Res 36: 469-492.
- Dumler JS, Choi KS, Garcia-Garcia JC, Barat NS, Scorpio DG, et al. (2005) Human granulocytic anaplasmosis and Anaplasma phagocytophilum. Emerg Infect Dis 11: 1828-1834.
- Stuen S (2007) Anaplasma phagocytophilum - the most widespread tick-borne infection in animals in Europe. Vet Res Commun 1: 79-84.
- Haigh JC, Gerwing V, Erdenebaatar J, Hill JE (2008) A novel clinical syndrome and detection of Anaplasma ovis in Mongolian reindeer (Rangifer tarandus). J Wildl Dis 44: 569-577.
- Ndung'u LW, Aguirre C, Rurangirwa FR, McElwain TF, McGuire TC, et al. (1995) Detection of Anaplasma ovis infection in goats by major surface protein 5 competitive inhibition enzyme-linked immunosorbent assay. J Clin Microbiol 33: 675-679.
- Tibbitts T, Goff W, Foreyt W, Stiller D (1992) Susceptibility of two Rocky Mountain bighorn sheep to experimental infection with Anaplasma ovis. J Wildl Dis 28: 125-129.
- Pusterla N, Huder JB, Feige K, Lutz H (1998) Identification of a granulocytic Ehrlichia strain isolated from a horse in Switzerland and comparison with other rickettsiae of the Ehrlichia phagocytophila genogroup. J Clin Microbiol 36: 2035-2037.
- Dumler JS (1997) Is human granulocytic ehrlichiosis a new Lyme disease? Review and comparison of clinical, laboratory, epidemiological, and some biological features. Clin Infect Dis 1: S43-47.
- Walder G, Lkhamsuren E, Shagdar A, Bataa J, Batmunkh T (2006) Serological evidence for tick-borne encephalitis, borreliosis, and human granulocytic anaplasmosis in Mongolia. Int J Med Microbiol 296 Suppl 40: 69-75.
- Ai CX, Hu RJ, Hyland KE, Wen YX, Zhang YG, et al. (1990) Epidemiological and aetiological evidence for transmission of Lyme disease by adult Ixodes persulcatus in an endemic area in China. Int J Epidemiol 19: 1061-1065.
- Masuzawa T, Takada N, Kudeken M, Fukui T, Yano Y, et al. (2001) Borrelia sinica sp. nov., a lyme disease-related Borrelia species isolated in China. Int J Syst Evol Microbiol 51: 1817-1824.
- Masuzawa T, Iwaki A, Sato Y, Miyamoto K, Korenberg EI, et al. (1997) Genetic diversity of Borrelia burgdorferi sensu lato isolated in far eastern Russia. Microbiol Immunol 41: 595-600.
- Mediannikov OY, Ivanov L, Zdanovskaya N, Vorobyova R, Sidelnikov Y, et al. (2005) Diversity of Borrelia burgdorferi sensu lato in Russian Far East. Microbiol Immunol 49: 191-197.
- Lewin MR, Bouyer DH, Walker DH, Musher DM (2003) Rickettsia sibirica infection in members of scientific expeditions to northern Asia. Lancet 362: 1201-1202.
- Cao WC, Zhan L, De Vlas SJ, Wen BH, Yang H, et al. (2008) Molecular detection of spotted fever group Rickettsia in Dermacentor silvarum from a forest area of northeastern China. J Med Entomol 45: 741-4.
- Mediannikov OY, Sidelnikov Y, Ivanov L, Mokretsova E, Fournier PE, et al. (2004) Acute tick-borne rickettsiosis caused by Rickettsia heilongjiangensis in Russian Far East. Emerg Infect Dis 10: 810-817.
- Zhang JZ, Fan MY, Wu YM, Fournier PE, Roux V, et al. (2000) Genetic classification of "Rickettsia heilongjiangii" and "Rickettsia hulinii," two Chinese spotted fever group rickettsiae. J Clin Microbiol 38: 3498-3501.
- Yu X, Jin Y, Fan M, Xu G, Liu Q, et al. (1993) Genotypic and antigenic identification of two new strains of spotted fever group rickettsiae isolated from China. J Clin Microbiol 31: 83-88.
- de Sousa R, Barata C, Vitorino L, Santos-Silva M, Carrapato C, et al. (2006) Rickettsia sibirica isolation from a patient and detection in ticks, Portugal. Emerg Infect Dis 12: 1103-1108.
- Pavlovsky EN (1966) Natural nidality of transmissible diseases: With special reference to the landscape epidemiology of zooanthroponoses, University of Illinois Press.
- Cao WC, Zhan L, He J, Foley JE, DE Vlas SJ, et al. (2006) Natural Anaplasma phagocytophilum infection of ticks and rodents from a forest area of Jilin Province, China. Am J Trop Med Hyg 75: 664-668.
- Postic D, Korenberg E, Gorelova N, Kovalevski YV, Bellenger E, et al. (1997) Borrelia burgdorferi sensu lato in Russia and neighbouring countries: high incidence of mixed isolates. Res Microbiol 148: 691-702.
- Sato Y, Miyamoto K, Iwaki A, Masuzawa T, Yanagihara Y, et al. (1996) Prevalence of Lyme disease spirochetes in Ixodes persulcatus and wild rodents in far eastern Russia. Appl Environ Microbiol 62: 3887-3889.
- Shpynov S, Fournier PE, Rudakov N, Tankibaev M, Tarasevich I, et al. (2004) Detection of a Rickettsia closely related to Rickettsia aeschlimannii, "Rickettsia heilongjiangensis," Rickettsia sp. strain rpa4, and Ehrlichia muris in ticks collected in Russia and Kazakhstan. J Clin Microbiol 42: 2221-2223.
- Shpynov S, Fournier PE, Rudakov N, Tarasevich I, Raoult D (2006) Detection of members of the genera Rickettsia, Anaplasma, and Ehrlichia in ticks collected in the Asiatic part of Russia. Ann N Y Acad Sci 1078: 378-383.
- Barlough JE, Madigan JE, DeRock E, Dumler JS, Bakken JS (1995) Protection against Ehrlichia equi is conferred by prior infection with the human granulocytotropic Ehrlichia (HGE agent). J Clin Microbiol 33: 3333-3334.
- Barbour AG (1996) Does Lyme disease occur in the south?: a survey of emerging tick-borne infections in the region. Am J Med Sci 311: 34-40.
- Bunikis J, Tsao J, Luke CJ, Luna MG, Fish D, et al. (2004) Borrelia burgdorferi infection in a natural population of Peromyscus Leucopus mice: a longitudinal study in an area where Lyme Borreliosis is highly endemic. J Infect Dis 189: 1515-1523.
- Drazenovich N, Foley J, Brown RN (2006) Use of real-time quantitative PCR targeting the msp2 protein gene to identify cryptic Anaplasma phagocytophilum infections in wildlife and domestic animals. Vector Borne Zoonotic Dis 6: 83-90.
- Foley JE, Nieto NC, Clueit SB, Foley P, Nicholson WN, et al. (2007) Survey for zoonotic rickettsial pathogens in northern flying squirrels, Glaucomys sabrinus, in California. J Wildl Dis 43: 684-689.
- Chen M, Fan MY, Bi DZ, Zhang JZ, Huang YP (1998) Detection of Rickettsia sibirica in ticks and small mammals collected in three different regions of China. Acta Virol 42: 61-64.
- Zhan L, Cao WC, Chu CY, Jiang BG, Zhang F, et al. (2009) Tick-borne agents in rodents, China, 2004-2006. Emerg Infect Dis 15: 1904-1908.
- Lankester T, Davey G (1997) A lump on the head from Mongolia. Lancet 349: 656.
- Avarzed A, De Waal DT, Igarashi I, Saito A, Oyamada T, et al. (1997) Prevalence of equine piroplasmosis in Central Mongolia. Onderstepoort J Vet Res 64: 141-145.
- Battsetseg B, Lucero S, Xuan X, Claveria F, Byambaa B, et al. (2002) Detection of equine Babesia spp. gene fragments in Dermacentor nuttalli Olenev 1929 infesting mongolian horses, and their amplification in egg and larval progenies. J Vet Med Sci 64: 727-730.
- Boldbaatar D, Xuan X, Battsetseg B, Igarashi I, Battur B, et al. (2005) Epidemiological study of equine piroplasmosis in Mongolia. Vet Parasitol 127: 29-32.
- Splitter EJ, Anthony HD, Twiehaus MJ (1956) Anaplasma ovis in the United States; experimental studies with sheep and goats. Am J Vet Res 17: 487-491.
- Trávnicek M, Stefancikova A, Nadzamová D, Stanko M, Cislákova L, et al. (2002) Seroprevalence of anti-Borrelia burgdorferi antibodies in sheep and goats from mountainous areas of Slovakia. Ann Agric Environ Med 9: 153-155.
- Hughes VL, Randolph SE (2001) Testosterone increases the transmission potential of tick-borne parasites. Parasitology 123: 365-371.
- Hughes VL, Randolph SE (2001) Testosterone depresses innate and acquired resistance to ticks in natural rodent hosts: a force for aggregated distributions of parasites. J Parasitol 87: 49-54.
- Ogden NH, Casey AN, French NP, Woldehiwet Z (2002) A review of studies on the transmission of Anaplasma phagocytophilum from sheep: implications for the force of infection in endemic cycles. Exp Appl Acarol 28: 195-202.
- Ogden NH, Nuttall PA, Randolph SE (1997) Natural Lyme disease cycles maintained via sheep by co-feeding ticks. Parasitology 115: 591-599.
- Zeman P, Januska J, Orolinova M, Stuen S, Struhar V, et al. (2004) High seroprevalence of granulocytic ehrlichiosis distinguishes sheep that were the source of an alimentary epidemic of tick-borne encephalitis. Wien Klin Wochenschr 116: 614-616.
- Stuen S, Casey AN, Woldehiwet Z, French NP, Ogden NH (2006) Detection by the polymerase chain reaction of Anaplasma phagocytophilum in tissues of persistently infected sheep. J Comp Pathol 134: 101-104.
- Stuen S, Djuve R, Bergström K (2001) Persistence of granulocytic Ehrlichia infection during wintertime in two sheep flocks in Norway. Acta Vet Scand 42: 347-353.
- Stuen S, Engvall EO, Artursson K (1998) Persistence of Ehrlichia phagocytophila infection in lambs in relation to clinical parameters and antibody responses. Vet Rec 143: 553-555.
- Shompole S, Waghela SD, Rurangirwa FR, McGuire TC (1989) Cloned DNA probes identify Anaplasma ovis in goats and reveal a high prevalence of infection. J Clin Microbiol 27: 2730-2735.
- de la Fuente J, Atkinson MW, Hogg JT, Miller DS, Naranjo V, et al. (2006) Genetic characterization of Anaplasma ovis strains from bighorn sheep in Montana. J Wildl Dis 42: 381-385.
- Goff W, Stiller D, Jessup D, Msolla P, Boyce W, et al. (1993) Characterization of an Anaplasma ovis isolate from desert bighorn sheep in southern California. J Wildl Dis 29: 540-546.
- Ngeranwa JJ, Venter EH, Penzhorn BL, Soi RK, Mwanzia J, et al. (1998) Characterization of Anaplasma isolates from eland (Taurotragus oryx). Pathogenicity in cattle and sheep and DNA profiles analysis. Vet Parasitol 74: 109-122.
- Zaugg JL, Goff WL, Foreyt W, Hunter DL (1996) Susceptibility of elk (Cervus elaphus) to experimental infection with Anaplasma marginale and A. ovis. J Wildl Dis 32: 62-66.
- Kuttler KL (1984) Anaplasma infections in wild and domestic ruminants: a review. J Wildl Dis 20: 12-20.
- Chomel BB, Carniciu ML, Kasten RW, Castelli PM, Work TM, et al. (1994) Antibody prevalence of eight ruminant infectious diseases in California mule and black-tailed deer (Odocoileus hemionus). J Wildl Dis 30: 51-59.
- Foley JE, Barlough JE, Kimsey RB, Madigan JE, DeRock E, et al. (1998) Ehrlichia spp. in cervids from California. J Wildl Dis 34: 731-737.
- Cohen D, Bosler EM, Bernard W, Meirs D 2nd, Eisner R, et al. (1988) Epidemiologic studies of Lyme disease in horses and their public health significance. Ann N Y Acad Sci 539: 244-257.
- Madigan JE (1993) Lyme disease (Lyme borreliosis) in horses. Vet Clin North Am Equine Pract 9: 429-434.
- Franzén P, Aspan A, Egenvall A, Gunnarsson A, Aberg L, et al. (2005) Acute clinical, hematologic, serologic, and polymerase chain reaction findings in horses experimentally infected with a European strain of Anaplasma phagocytophilum. J Vet Intern Med 19: 232-239.
- Franzén P, Aspan A, Egenvall A, Gunnarsson A, Karlstam E, et al. (2009) Molecular evidence for persistence of Anaplasma phagocytophilum in the absence of clinical abnormalities in horses after recovery from acute experimental infection. J Vet Intern Med 23: 636-642.
Citation: Sophia P, Battsetseg G, Kass Philip H, Foley Janet E (2012) Detection and Epidemiology of Tick-Borne Pathogens in Free-Ranging Livestock in Mongolia. J Clin Exp Pathol S3:006. DOI: 10.4172/2161-0681.S3-006
Copyright: © 2012 Sophia P, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 16250
- [From(publication date): 0-2012 - Dec 21, 2025]
- Breakdown by view type
- HTML page views: 11247
- PDF downloads: 5003