Research Article Open Access
Development of Improved Sandwich Elisa for the In Vitro Detection of Inhibitors of the TNF-TNFR1 Interaction
Jessica M Davis*
Department of Chemistry & Biochemistry, Fairfield University, BNW 315, USA
- *Corresponding Author:
- Jessica M Davis, Ph.D
Assistant Professor
Department of Chemistry & Biochemistry
Fairfield University, BNW 315, USA
Tel: 203-254- 4000 x2123
Fax: 203-254-4034
E-mail: jmdavis@fairfield.edu
Received date: November 04, 2011; Accepted date: December 22, 2011; Published date: December 24, 2011
Citation: Davis JM (2012) Development of Improved Sandwich Elisa for the In Vitro Detection of Inhibitors of the TNF-TNFR1 Interaction. J Anal Bioanal Tech 3:129. doi: 10.4172/2155-9872.1000129
Copyright: © 2012 Davis JM. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
The over-expression of tumor necrosis factor alpha (TNF-alpha) has been associated with various diseases, particularly autoimmune diseases such as rheumatoid arthritis and Crohn’s disease. Some biologic therapies for these diseases specifically target this cytokine, sequestering it, and preventing its interaction with its 55 kD receptor (TNFR 1 ). While these therapies have proven the validity of this approach, they are large proteins that require intravenous administration. Small molecule inhibitors of this interaction are highly desirable and some have been developed. However, such inhibitors have not been reported to have successfully completed clinical trials. One prohibition to this approach is the current state of in vitro testing of such molecules. Assays have been developed that use radio labeled or biotin labeled TNF-alpha. In vivo methods exist, but are cost prohibitive to screening a large selection of molecules. Reported here-in is an inexpensive, highly robust, sensitive assay for inhibitors of the TNF- alpha interaction with TNFR 1 that does not require modification of TNF-alpha. The simplicity of this assay should allow for increased investigation into inhibiting this important interaction.
Abbreviations
TNF: Tumor Necrosis Factor Alpha; TNFR1: Tumor Necrosis Factor 55 kD Receptor; ELISA: Enzyme-Linked Immunosorbant Assay; sTNFR1: Soluble TNFR1; PBS: Phosphate Buffered Saline; BSA: Serum Bovine Albumin; HRP: Horseradish Peroxidase
Introduction
The overexpression of the cytokine tumor necrosis factor alpha (TNFα) has been linked to inflammation in Crohn’s disease and rheumatoid arthritis and has proven to be a viable therapeutic target in the treatment of these diseases [1,2]. Current therapies that target TNFα are the biologics infliximab, etanercept, and adalimumab. These are antibodies or small proteins that work by sequestering TNFα or by blocking the interaction with its 55 kD receptor (TNFR1). Due to the size of these molecules, they require intravenous administration and have poor efficacy [3]. As exemplified in a Current News article in Nature Biotechnology, therapeutics that are orally bioavailable, drug-like molecules with increased specificity and potency over the current treatments are highly desirable [4].
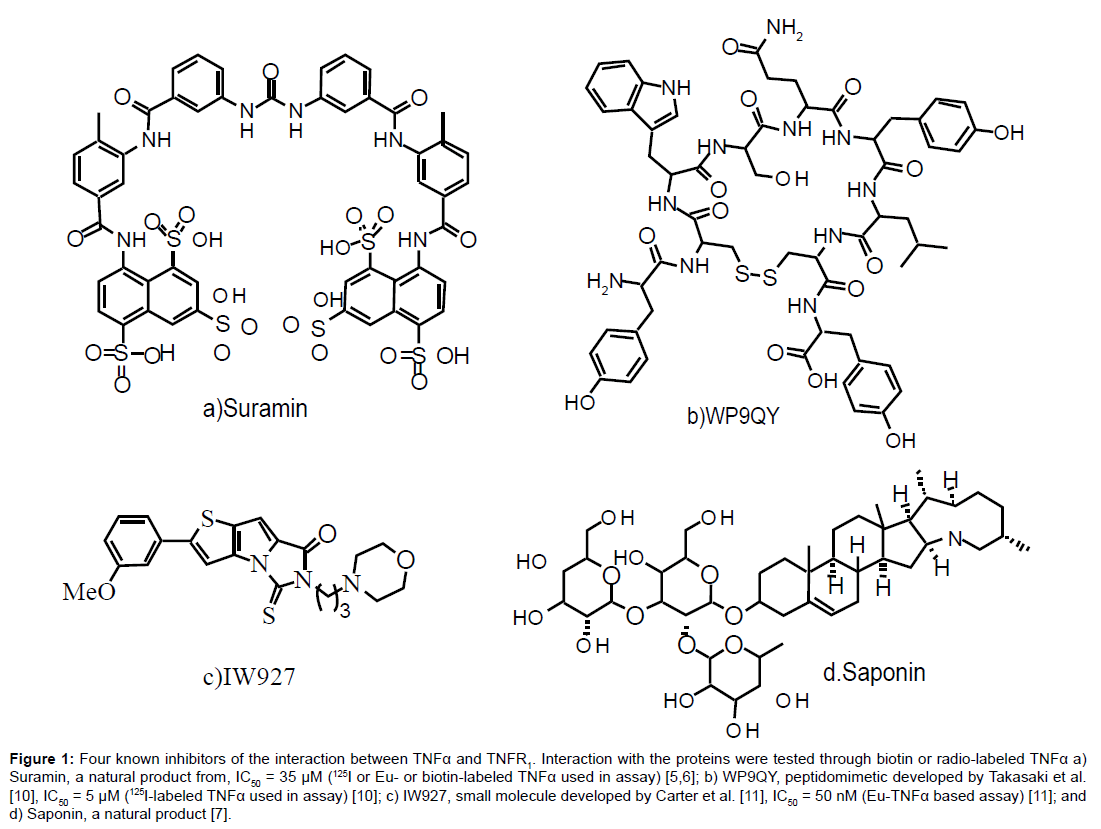
Small molecules that are proposed to function by binding TNFα, such as suramin [5,6] and the saponins [7], have been identified as inhibitors of TNFα function (Figure 1a and d, respectively). Other research groups have developed small peptide antagonists of TNFα, including a library of D-amino acid hexapeptides [8], a derivative of TNFR1 [9], and the peptide WP9QY (Figure 1b) [10]. Targeting this interaction with small molecules that bind TNFR1 has been successful with IW927 (Figure 1c) [11].
Figure 1: Four known inhibitors of the interaction between TNFα and TNFR1. Interaction with the proteins were tested through biotin or radio-labeled TNFα a) Suramin, a natural product from, IC50 = 35 μM (125I or Eu- or biotin-labeled TNFα used in assay) [5,6]; b) WP9QY, peptidomimetic developed by Takasaki et al. [10], IC50 = 5 μM (125I-labeled TNFα used in assay) [10]; c) IW927, small molecule developed by Carter et al. [11], IC50 = 50 nM (Eu-TNFα based assay) [11]; and d) Saponin, a natural product [7].
Considering the validation of this target through current therapeutics, it is disappointing to see a lack of small molecule inhibitors that would be orally available compared to the intravenous treatments currently used. While targeting a large protein-protein interaction with small molecule inhibitors poses significant challenges, it has been successfully achieved for the TNFα interaction with TNFR1 [5-7,11,12]. One prohibitive factor in finding inhibitors of the TNFα interaction with TNFR1 is the lack of easily accessible in vitro assay conditions for preliminary screening. The most common type of assay uses radiolabeled TNFα [10,11,6,8] or radio-labeled inhibitors [13]. The special facilities and training needed to utilize these assay conditions imposes a safety and financial constraint. Biotin-labeled TNFα is also used [6,14]; although this technique does not require the facilities and training of radiolabeling, biotin labeling of TNFα is expensive and results in low yields. A kit targeting this interaction from PerkinElmer™ became available in 2003, but requires the use of a special plate reader with AlphaScreenTM technology [15].
Compared to currently available techniques, the assay described here provides a more accessible format, is time and cost effective, and utilizes commercially available reagents. The technique is a sandwich enzyme-linked immunosorbant assay (ELISA) which is modified from the techniques used by Corti et al. [16] for elucidation of the binding of TNFα with TNFR1 and the TNFα quantification by Alzani et al. and Mancini et al. [6,14]. The method used by Corti et al. [16] uses sandwich ELISA formats, where plates are coated with anti- TNFα which are then treated with TNFα containing solutions (either with or without TNFR1). Ideally only free TNFα binds to the antibody; however, it was found that both free and TNFR1 bound TNFα are detected. The scope of effectiveness depended upon varying assay conditions. Based upon this result, it would be ideal to eliminate the ambiguity between bound and unbound TNFα by eliminating the primary antibody step. The technique used by Alzani et al. and Mancini et al. [6,14] utilizes a similar sandwich ELISA technique; however, the plates are coated with sTNFR1 initially and then treated with biotin-labeled TNFα. This process directly measures the binding of TNFα to sTNFR1. Nevertheless, the required biotin-labeled TNFα imposes a time, cost, and yield constraint on the availability of this assay protocol. The technique de-scribed here-in uses sTNFR1 coated plates, unaltered TNFα, and detects TNFα binding using a commercially available biotin labeled antibody with a streptavidin-enzyme. This technique eliminates any ambiguity by directly measuring TNFα binding and does not require the use of biotin- or radio-labeled- TNFα.
Materials and Methods
Materials
Tumor necrosis factor soluble receptor 1 (TNF sR1)/Fc Chimera Human, Recombinant, expressed in mouse NSO cells (sTNFR1), sura min, phosphate buffered saline (PBS), bovine serum albumin (BSA), carbonate-bicarbonate buffer, Greiner® high-binding well plates, and TWEEN-20 was purchased from Sigma-Aldrich, Inc. Recombinant human TNFα (expressed in Escherichia coli), biotinylated anti-human TNF-a/TNFSF1A antibody, streptavidin-horseradish peroxidase (HRP), HRP substrate solution (3,3’, 5,5’-tetramethylbenzidine (TMB)), and HRP stop solution (0.16 M sulfuric acid) were purchased from Thermo Scientific. The peptide WP9QY (sequence YCWSQYLCY with disulfide bond constraint) was purchased through PierceNet. The plate reader used is a Biotek® Epoch microplate reader and all data was processed with SigmaPlot® [17].
sTNFR1/TNFα sandwich ELISA protocol
Using a 384 well high-binding plate, 50 μL of a 25 ng/well sTNFR1 solution in 0.05 M carbonate-bicarbonate buffer (pH 9.6, 25°C) was incubated overnight (16 hours) at room temperature. The plate was aspirated and washed three times with wash solution (PBS/1% TWEEN-20, pH 7.3, 25°C) for 10 minutes each. Unbound sites were blocked using a 10% BSA in PBS (pH 7.3, 25°C) solution (140 μL/well) and incubated for 1 hour at 37°C. The plate was aspirated and washed three times with wash solution for 10 minutes. Plates can be stored up to two weeks with 140 μL/well PBS/4% BSA. Wells were treated with TNFα and with inhibitor as described in the procedures below. The plate was aspirated and washed three times with wash solution for 10 minutes each. 50 μL of 0.25 mM anti- TNFα (biotin) was added to each well and incubated at 25°C for 1 hour. The plate was aspirated and washed three times with wash solution for 10 minutes each. 50 μL of 2.5 μM streptavidin-HRP was added to each well and incubated for 30 minutes at room temperature. The plate was aspirated and washed three times with wash solution for 10 minutes each. 40 μL of HRP-substrate solution (TMB) was added in the dark and incubated for 30 minutes at room temperature, followed by 40 μL of HRP stop solution. After shaking for 20 minutes, the plate was read at 450 nm minus 550 nm (Table 1).
| Step 1 | Parameter | Amount/Well (µL) | Concentration | Buffer* | Incubation |
| 1 | Coat wells STNFR1 | 50 | 0.25 µg/mL | A | 16 hours 25°C |
| 2 | Wash TWEEN-20 | 140 | 1% | B | 10 minutes 3 times, 25°C |
| 3 | Block wells BSA | 140 | 10% | B | 1 hour, 37°C |
| 4 | Wash TWEEN-20 | 140 | 1% | B | 10 minutes 3 times, 25°C |
| 5a | Saturation TNFα | 50 | Serial dilutions from 100nM | C | 2 hours, 25°C |
| 5b | Inhibition | Serial dilutions from 100 nM | C | 2 hours, 25°C | |
| TNFa | 25 | 14 nM (7 nM final) | D | 2 hours | |
| inhibitor | 25 | Serial Dilution | D | 25°C | |
| 6 | Wash TWEEN-20 | 140 | 1% | B | 10 minutes 3 times, 25°C |
| 7 | Anti- TNFα(biotin) | 50 | 0.25 mM | C | 1 hour, 25°C |
| 8 | Wash TWEEN-20 | 140 | 1% | B | 10 minutes 3 times, 25°C |
| 9 | TMB | 40 | 30 minutes, 25°C | ||
| 10 | Sulfuric acid | 40 | 0.16 M | 20 minutes, 25°C | |
| 11 | Read at 450 nm minus 550 nm | ||||
*Buffer A: 50 mM Carbonate-bicarbonate (pH 9.6), B: PBS, C: 4% (v/v) BSA in PBS (pH 7.3), D: 4% BSA (v/v) in PBS, 2.5% (v/v) DMSO (pH 7.3).
Table 1: Sandwich ELISA.
sTNFR1/TNFα sandwich ELISA concentration studies
The assay protocol described above was tested for minimal concentrations of all the proteins utilized. sTNFR1 concentration was tested with serial dilutions from 2.5 μg/mL sTNFR1 with 5.0 nM TNFα. Concentrations for anti- TNFα -biotin were tested from 1:10 dilutions starting from 50 μg/mL. Concentrations for streptavidin-HRP were tested from 1:10 dilutions starting from 1:750 dilution of stock solution. Minimum acceptable detection of Bmax was assessed through maximum absorbance values and calculation of the z-factor from Bmin.
sTNFR1/TNFα sandwich ELISA saturation studies
Using a 384 well high-binding plate bound with sTNFR1 and blocked with BSA as described previously, wells were treated with 50 μL pre-prepared serial dilutions of TNFα from a starting concentration of 100 nM in PBS/4% BSA (pH 7.3, 25°C) for 2 hours at room temperature. Incubations of TNFα with sTNFR1 were tested with cona centrations of DMSO from 1 – 10% (v/v), varying the pH of the buffer from 5 to 7 with 0.1 M HCl and 8 – 9 with 0.1 M NaOH, and incubation times from 1 – 8 hours. Detection of TNFα was carried out as described previously.
sTNF1/TNFα sandwich ELISA inhibition studies
Using a 384 well high-binding plate bound with sTNFR1 and blocked with BSA as described previously, the wells are treated with 25 μL of serial dilutions of inhibitor in (4% BSA, 5% DMSO in PBS) with 25 μL of 14 nM TNFα in PBS/4% BSA (resulting in 7 nM final concentration), preincubated for 15 minutes, and then for two hours at room temperature. Detection of TNFα is carried out as described previously.
Plate uniformity and z-factor study
Using two 384 well, wells in columns 3, 9. 12, 15, 18, 21, and 24 of three high binding plates were bound with sTNFR1 and blocked with BSA as described previously. Wells of the three plates were treated with 50 μL of either 25nM, 6.25 nM, or 0.01 nM TNFα PBS/4% BSA (pH 7.3, 25°C) and incubated for 2 hours at room temperature. Detection of TNFα was carried out as described previously.
Data analysis
Data was analyzed using SigmaPlot®. For KD or EC50 calculations, the macro for ligand binding single site saturation (y= (Bmaxx)/(KD+x)) or sigmoidal dose response (y= (max-min)/(1+10(logEC50-x)) curves were used, respectively. For IC50 calculations, the macro for ligand binding one site competition (y= (max-min)/(1+10(logEC50-x)) was used. The zfactor was calculated using 1-(3(σmax-σmin))/|averagemax-averagemin|.
Results
The assay conditions described were optimized over a series of experiments to obtain the ideal concentrations for both saturation and inhibition curves. Both cost and detection levels were considered in deciding on the final conditions. In addition, various experimental conditions, including varying the percent DMSO and pH were tested to ensure consistency of the results.
Concentration experiments
Standard solutions were tested to make certain that the absorbance was detecting only the adhesion of TNFα to TNFR1. Of the twenty-two permutations tested, only those experiments containing all of the assay components gave significant absorbance values (0.39 ± 0.03, 3.5 nM TNFα, n=4). Using HRP, substrate, and stop solution alone gives a low absorbance value (0.047 ± 0.005, 3.5 nM TNFα, n=4) and is recommended for use as a blank.
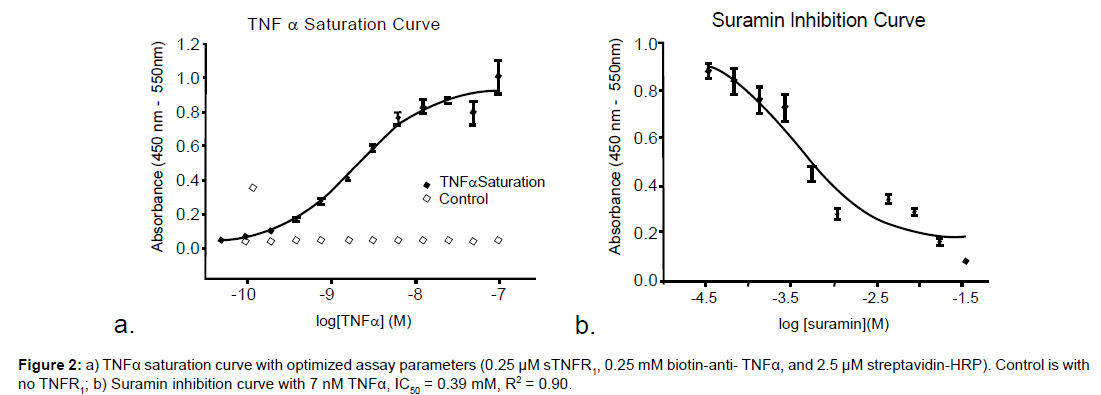
Exploration of minimal concentrations of all the proteins and antibodies was performed and final concentrations were chosen based on effectiveness and cost. The concentrations of TNFR1, antibody, and HRP were tested keeping the concentrations of all other components constant, chosen from the concentrations originally used by Alanzi et al. [5,6] or recommended from the reagent suppliers. The most cost effective concentrations were tested using concentrations that gave an appreciable signal, which were 0.25 μM sTNFR1, 0.25 mM biotin-anti- TNFα, and 2.5 μM streptavidin-HRP (Figure 2a).
For inhibition curves (Figure 2b), a final concentration of 7 nM TNFα can be used with an acceptable change in absorbance (z-factor = 0.62, Figure 4). Any new reagent must be retested for optimal effective concentration.
Condition tests
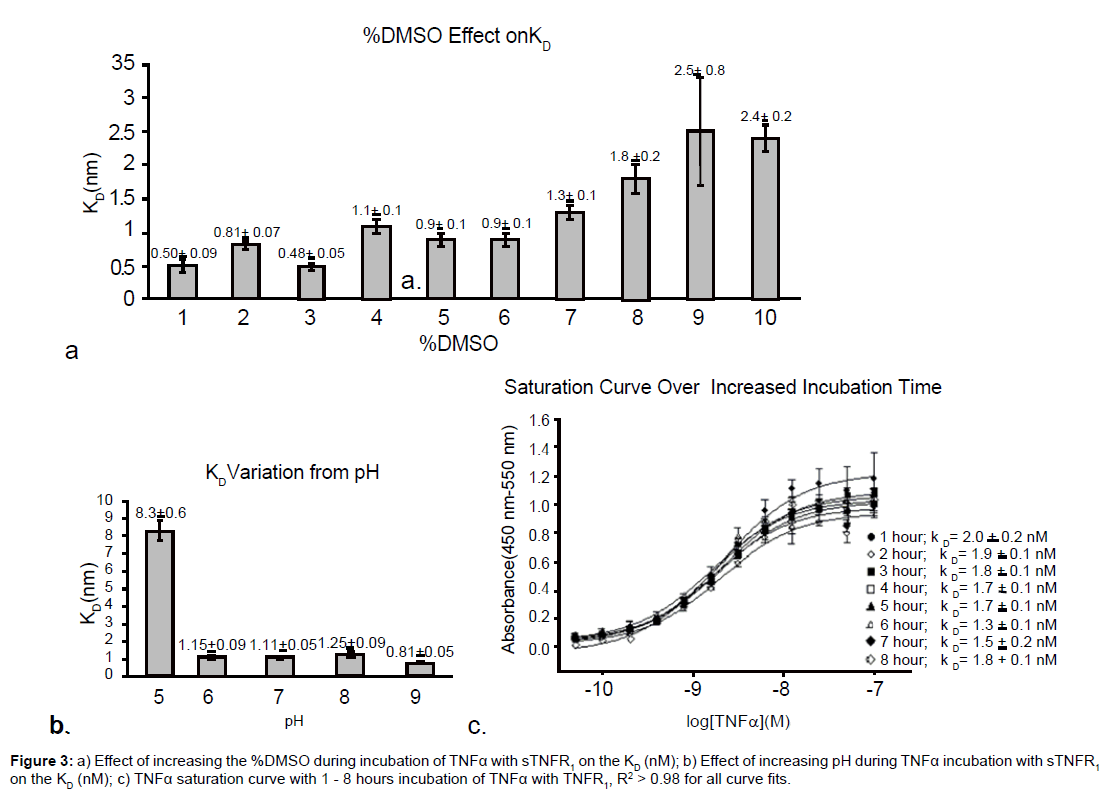
Preference to maintain cell-like conditions make it preferable to maintain low DMSO levels. However, DMSO is often a necessary component to solubilize compounds for inhibitition studies. Tolerance for DMSO is tested in the saturation curve to find how its presence effects the KD. Varying the percent DMSO from 0 to 10% (v/v) during the incubation of TNFα with sTNFR1 showed little variation in the dissociation constant between 4 - 7% DMSO, as seen in Figure 3a. This result is valuable in finding hydrophobic compounds that can disrupt the protein-protein interaction since addition of DMSO is often necessary to dissolve such compounds in water at the concentrations needed for preliminary studies. The increase in the KD with increasing levels of DMSO may indicate increase solubilization of the trimeric structure of TNFα; however, further studies would have to be conducted to verify this hypothesis.
Variations in pH were also tested to ensure that addition of acidic or basic compounds beyond the buffer range will not greatly affect the dissociation constant. Figure 3b shows that the KD is stable between pH 6 to 9. Repeated experimentation confirms that the assay is less stable at lower pH.
Incubation of TNFα with sTNFR1 can be varied from one to eight hours without any significant effect on the KD, as seen in Figure 3c.
Storage tests
The total time required to complete the assay is 20 hours. To reduce the consecutive time commitment, plates coated with TNFR1 and BSA were stored for up to 4 weeks in PBS/4% BSA buffer at 4°C. Using plates thus stored allows for a number of plates to be prepared and saved, then continuing the assay from the point of addition of TNFα and inhibitor. This is ideal when screening a large number of compounds. After two weeks storage there is a 10 fold change in KD. Addition of a preservative, such as sodium azide, may increase the longevity of the plates, but this has not been tested.
Inhibition
Three known inhibitors were tested, suramin, saponin, and the constrained peptide WP9QY. These inhibitors were chosen because of their commercial availability. The inhibitors gave comparable results to the literature values, which further validates the assay design. Suramin, saponin, and the constrained peptide WP9QY, giving 63%, 41%, and 67% inhibition, respectively, with 1 mM inhibitor and 7.0 nM TNFα. Takasaki et al. [10] report 75% inhibition at 1 mM WP9QY. Suramin was further tested, giving an IC50 of 0.39 mM, which is comparable to the value reported by Alzani et al. [5] of 0.65 mM using radiolabeled- TNFα assay since both methods use a direct detection technique for TNFα binding. Investigation of inhibitors for the TNFα interaction with TNFR1 may require increased incubation time. Incubation of TNFα with sTNFR1 can be varied from one to eight hours without any significant effect on the KD, as seen in Figure 3c.
The difference in the proposed mechanism of these inhibitors, suramin disrupting the TNFα trimer and WP9QY mimicking a binding region of TNFR1, validates the versatility of the assay. Only the direct detection of TNFα bound to sTNFR1 is measured, allowing for a variety of antagonist mechanisms.
Conclusion
A cost effective, safe assay has been developed for testing inhibitors of the TNFα interaction with TNFR1. The assay is based on a sandwich ELISA format and has been optimized for low concentrations of most proteins. The total time required to complete an experiment is 20 hours; however, plates coated with TNFR1 and BSA can be stored for up to two weeks without diminishing the reliability of the results. The assay is also effective between 4-7% DMSO and between pH 6-9. Extending these assay conditions to the TNFα -TNFR1 interaction should be a relatively simple exchange of the protein in the first step and warrant further investigation. The hope is that this assay will provide researchers with more accessible conditions and at a lower cost than what is currently available for preliminary screening of inhibitors of this important protein-protein interaction.
Acknowledgements
This project was supported by a Research Corporation Cottrell College Research Award ID 7715, NIH-AREA 1R15DK081934-01A1, and start-up support from Fairfield University.
References
- Dinarello CA (1991) Inflammatory Cytokines - Interleukin-1 and Tumor-Necrosis-Factor as Effector Molecules in Autoimmune-Diseases. Curr Opin Immun3: 941-948.
- Kodama S, Davis M, Faustman DL (2005) The therapeutic potential of tumor necrosis factor for autoimmune disease: a mechanistically based hypothesis. Cell Mol Life Sci62: 1850-1862.
- Atzeni F, Turiel M, Capsoni F, Doria A, Meroni P, et al. (2005) Autoimmunity and anti-TNF-alpha agents. Ann N Y Acad Sci 1051: 559-569.
- Sheridan C (2008) Small molecule challenges dominance of TNFα inhibitors. Nat Biotechnol26: 143-144.
- Alzani R, Corti A, Grazioli L, Cozzi E, Ghezzi P, et al. (1993) Suramin induces deoligomerization of human tumor necrosis factor α. J Biol Chem268:12526-12529.
- Alzani R, Cozzi E, Corti A, Temponi M, Trizio D, et al. (1995) Mechanism of suramin-induced deoligomerization of tumor necrosis factor α. Biochemistry 34: 6344-6350.
- Shah B A, Chib R, Gupta P, Sethi VK, Koul S, et al. (2009) Saponins as novel TNF- α inhibitors: Isolation of saponins and nor-pseudoguainanolide from Parthenium hysterophorus. Org Biomol Chem 7: 3230-3235.
- Kruszynski M, Shealy D J, Leone AO, Heavner GA (1999) Identification of TNF- α binding peptides from a D-amino acid hexapeptide library that specifically inhibit TNF- α binding to recombinant p55 receptor. Cytokine11: 37-44.
- Cao Y, Wang Z, Bu X, Tang S, Mei Z, et al. (2009) A Synthetic Peptide Derived from A1 Module in CRD4 of Human TNF Receptor-1 Inhibits Binding and Proinflammatory Effect of Human TNF-α. Inflammation32: 1573-2576.
- Takasaki W, Kajino Y, Kajino K, Murali R, Greene MI (1997) Structure-based design and characterization of exocyclic peptidomimetics that inhibit TNFα binding to its receptor. Nat Biotechnol 15: 1266-1270.
- Carter P H, Scherle PA, Muckelbauer JK, Voss ME, Liu RQ, et al. (2001) Photochemically enhanced binding of small molecules to the tumor necrosis factor receptor-1 inhibits the binding of TNF- α. Proc Natl Acad Sci U S A 98: 11879-11884.
- He MM, Smith AS, Oslob JD, Flanagan WM, Braisted AC, et al. (2005) Small-molecule inhibition of TNF-α. Science 310: 1022-1025.
- Loetscher H, Gentz R, Zulauf M, Lustig A, Tabuchi H, et al. (1991) Recombinant 55-kDa Tumor Necrosis Factor (TNF) Receptor: Stoichiometry of binding to TNF α and TNF β and inhibition of TNF activity. J Biol Chem 266: 18324-18329.
- Mancini F, Toro CM, Mabilia M, Giannangeli M, Pinza M, et al. (1999) Inhibition of tumor necrosis factor-alpha (TNF-alpha)/TNF-alpha receptor binding by structural analogues of suramin. Biochem Pharmacol58: 851-859.
- Bouchard N, Legault M, Wenham D (2003) AlphaScreenTM TNF α binding assay kit: A homogenous, sensitive and high-throughput assay for screening TNFα receptors. PerkinElmer BioSignal.
- Corti A, Poiesi C, Merli S, Cassani G (1994) Tumor necrosis factor (TNF) alpha quantification by ELISA and bioassay: effects of TNF alpha-soluble TNF receptor (p55) complex dissociation during assay incubations. J Immun Meth 177: 191-198.
- SigmaPlot®, Copyright© 2010 Systat Software Inc. - All Rights Reserved.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 16342
- [From(publication date):
March-2012 - Dec 09, 2025] - Breakdown by view type
- HTML page views : 11599
- PDF downloads : 4743