Research Article Open Access
Histopathological Study of Acute Toxicity of Adonis Aestivalis (Summer Pheasant's Eye) in Rabbits
Rahim Hobbenaghi1, Javad Javanbakht2*, Moharam Kamrani3, Ali Bashiri Dezfouli4, Mehdi Aghamohammad Hassan5 and Mohammad Zamani21Departement of Pathology, Faculty of Veterinary Medicine, Urmia University, Urmia, Iran
2Department of Pathology, Faculty of Veterinary Medicine, Tehran University, Tehran, Iran
3Graduate student, Faculty Veterinary Medicine Urmia University, Urmia, Iran
4Graduate student, Faculty Veterinary Medicine Tehran University, Tehran, Iran
5Department of Clinical Science, Faculty of Veterinary Medicine, Tehran University, Tehran, Iran
- *Corresponding Author:
- Dr. Javad Javanbakht
Department of Pathology
Faculty of Veterinary Medicine
Tehran University, Tehran, Iran
Tel: +989372512581
E-mail: javadjavanbakht@ut.ac.ir
Received Date: June 12, 2012; Accepted Date: August 14, 2012; Published Date: August 18, 2012
Citation: Hobbenaghi R, Javanbakht J, Kamrani M, Dezfouli AB, Hassan MA, et al. (2012) Histopathological Study of Acute Toxicity of Adonis Aestivalis (Summer Pheasant’s Eye) in Rabbits. J Clin Exp Pathol 2:124.doi: 10.4172/2161-0681.1000124
Copyright: © 2012 Hobbenaghi R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Clinical & Experimental Pathology
Abstract
Abstract
The aim of this study was to evaluate the acute toxicity of the hydroalcolic extract obtained from flowers and
leaves of A. aestivalis in New Zealand White rabbits. In this study Sixteen New Zealand White rabbits (weighing 1700-
2200 g) were tested for 14 days. The animals were randomly divided into 2 groups (treatment and control) which every
group contained 8 rabbits. A. aestivalis (summer pheasant’s eye) was collected over multiple years, under different
growing conditions, and at various stages of maturity, dried, and administered to rabbits to evaluate the toxicity of A.
aestivalis. On microscopic examination, Severe hemorrhage, myocardial degeneration and necrosis in cardiac cells,
severe congestion and swelling of hepatocytes in liver, spread interstitial alveolar edema, congestion of pulmonary
arteries, medial hyperplasia and emphysema in lungs, PVC (pre-vascular cuffing), chromatolysis, neurophagy and
focal gliosis in brain, severe swelling of tubular endothelial cells, congestion of glomeruli, hyaline casts and severe
tubular necrosis in kidney, papillary hyperplasia of mucus layer, and mucous secretions into the intestine lumen were
observed. No pathological were found in other organs as well. Although Adonis spp. contain cardiac glycosides,
cardiac lesions have not previously been described in laboratory animals associated with consumption of adonis, and
this is the first report of adonis toxicosis in Iran. Effectively, these results demonstrate the presence of acute toxicity
due to ip treatment with A. aestivalis for 14 days in New Zealand White rabbits. However, other toxicological studies
are necessary to evaluate the total.
Keywords
Adonis aestivalis; Body tissues; Rabbit; Histopathology; Acute toxicity
Introduction
Adonis spp. including Adonis aestivalis (summer pheasant’s eye, summer adonis) have long been utilized in European folk medicine for their cardiac-enhancing effects [1,2]. Summer pheasant’s eye growths in vast areas of Europe, northern Africa and western and central Asia and grows extensively around Tehran, Khoram Abad, Isfahan, Azarbaiejan in Iran [3]. Adonis spp. is members of the buttercup family (Ranunculaceae), one of 11 plant families with 34 genera known to contain cardiac glycosides [4,5]. Cardiac glycosides are well absorbed by the digestive system as mostly accumulated in liver, intestines, kidney, moderately in lungs, spleen, heart and slightly in blood, skeletal muscles and nervous system [6,7]. Acute poisonings with Adonis spp. have been reported in a variety of animal species [7,8,9]. Reports of toxicoses in horses [7,10], pigs [8] sheep [11] and calves [12] have been based on cases of natural exposure to Adonis sp. In horses, Gastrointestinal stasis and myocardial necrosis were reported in horses after consuming hay contaminated with Adonis aestivalis [7], pigs showed feed refusal, vomiting, and dyspnoea just before death after consuming a diet containing seeds of Adonis microcarpa 8 sheeps indicated the lethargy, and no lesions were noted on gross or microscopic examination of selected tissues of the sheeps. Also no microscopic lesions were seen in the sections of heart tissue examined from each of the ewes [11] and calves revealed that no lesions were noted on gross or microscopic examination of tissues from all 4 Holstein calves and the 2 Jersey calves after consuming a diet containing seeds of Adonis aestivalis [12]. Information on the susceptibility of ruminants to Adonis species toxicosis is incomplete. In laboratory studies in mice [13] and cats [14], intravenous administrations of Adonis-like glycosides and the aglycone strophanthidin resulted in acute toxicoses. In fact, reports of toxicosis in livestock associated with consumption of Adonis spp. are rare throughout the world [15]. In addition, although Adonis spp. contains cardiac glycosides, myocardial degeneration has not been described in livestock associated with consumption of adonis. Respectively, a comprehensive histopathological examination of different tissues affected with A. aestivalis was not done and at previous studies pathology and histopathology were a minor part of investigation. Purpose of the current study was a histopathological inspection of toxic effects by Adonis in a laboratory animal model (rabbit).
Materials and Methods
The Swiss albino mice model has been used according to Lorke [16] method in order to indicating, obtaining and carrying out the tests on rabbit model. Furthermore, this research is correspondent to other studies with the same methods which aimed to determine the drug dose. In addition, the histopathology reference is Adonis toxic effects evaluation on large animals by wood [11,12]. The present study is the first one that was on laboratory animals, and the mouse method description was completely written in materials and methods.
Sampling and preparation of the plants
During May and April (2009-2011), flowers and leaves of the Adonis aestivalis plant have been collected from the province of Urmia and brought to the hospital of Faculty of Veterinary Medicine. The extraction provision method of toxins has previously been demonstrated by wood [11].
Animals
New Zealand White rabbits (1700-2200 g) and Swiss albino mice (20–30 g) of either sex, maintained at Animal Facility Centre were used for the acute toxicity. All experimental protocols were approved by the local animal care committee in accordance with Faculty of Urmia Veterinary Medicine office regulations. In current study, 16 New Zealand White rabbits of both sexes, with averaging (1700-2200 g) 2 weeks old for the acute toxicity were utilized. The animals were kept in individual propylene cages under standard laboratory conditions by the dimensions of 30×50×25 cm3 two by two. Rats were maintained on a 12 hour light/dark cycle at 22 ± 1°C and 50 ± 10% humidity. The animals were kept in standard room conditions and fed with standard rabbit diet and water ad libitum.
Acute toxicity study (LD50)
The intraperitoneal (i.p.) acute toxicity lethal doses (LD50) profile of the extract was evaluated in Swiss albino mice according to the method of Lorke [16]. The LD50 was then calculated based on the pattern of death observed in the second step. Briefly, this method involved the determination of LD50 value in biphasic manner. The animals were starved of feed but allowed access to water 24 h prior to the study. In the initial investigatory step (phase 1), a range of doses of the extract producing the toxic effects was established. This was done by intraperitoneal administration of geometric doses of the extract (10, 100, 1000 mg/kg body weight respectively for phase I test) to 2 groups of mice (n=8). Based on the results obtained, a phase 2 investigatory step was done by giving more specific doses (200, 400, 600 mg/kg i.p, body weight respectively for phase II test) to 2 other groups of mice (n=8). The mice were observed for 24 h for such behavioral signs as, excitement, dullness, ataxia or death. The LD50 was estimated from the geometric mean of the dose that caused 100% mortality and the dose which caused no lethality. The same procedure was used in rabbits which received (10, 100, 1000 mg/kg oral) in phase 1 and (1500, 3000, 5000 mg/kg oral) in the second phase. Heart beat and respiration frequency were measured by electrocardiograms (model YM-102, SANYO, Japan) and stethoscope. During the following 14 days the rabbits which survived were observed for the signs of toxicity. The surviving rabbits were then sacrificed by decapitation under ketamine anesthesia. The following organs were collected for histopathological examination both from animals which died and those which survived: brain, pituitary gland, thyroid gland, suprarenal gland, tongue (skeletal muscles), heart, lung, stomach, duodenum, ileum, colon, liver, pancreas, kidney, urinary bladder, thymus, spleen, lymph node and testis or ovary. The tissues were fixed in Bouin’s solution for 24 h and processed for paraffin embedding by standard methods. Finally, 5 μm sections of the tissues were cut, stained with hematoxylin and eosin and examined by light microscopy.
Results
Necropsy finding
Red-brown, cloudy peritoneal fluid and the diaphragm had fibrin tags on the peritoneal surface and the lungs were noncollapsable and wet. The soft tissues of the head and neck (particularly the retropharyngeal soft tissue and ligamentum nuchae) had pockets of yellow gelantinous edema. The pericardial sac contained approximately 30 ml of red-brown clear fluid. The stomach was distended by green faecal fluid, and the colon contained scant feces. Ecchymotic hemorrhages were present on the serosa of the large colon and on the parietal pleura adjacent to the column. There were ecchymotic hemorrhages (Almost spindle shaped) along the coronary groove of the heart, and dry, ecchymotic endocardial hemorrhages were observable.
Histopathological results
Observed histopathological lesions in different organs (most lesions observed at high doses).
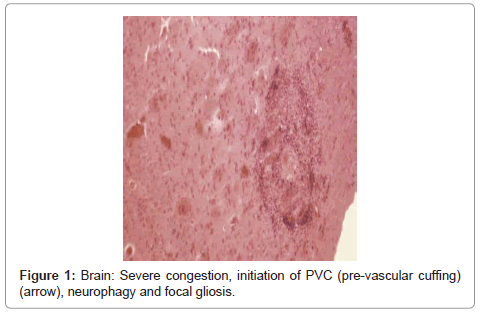
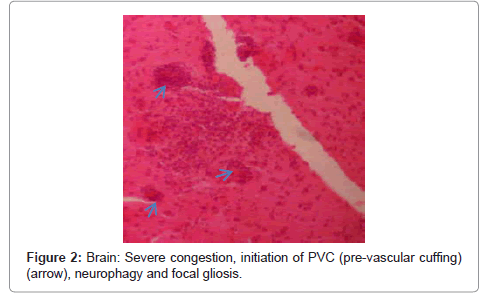
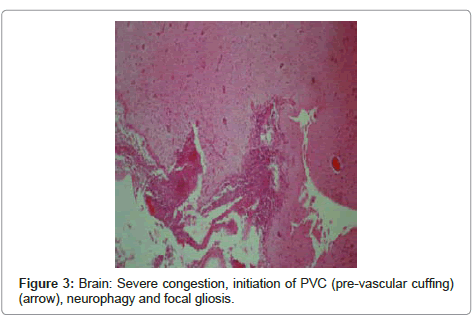
Brain: the lesions included severe congestion, initiation of PVC (pre-vascular cuffing), chromatolysis, neurophagy, focal gliosis and ischemic lesions of neurons (Figure 1, Figure 2 and Figure 3).
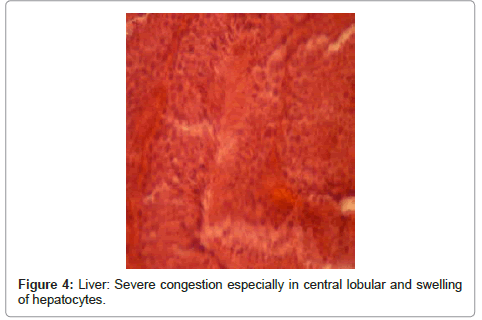
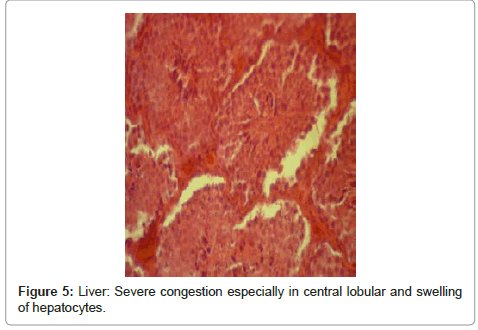
Liver: alterations resulted from cardiac failure, including severe congestion and swelling of hepatocytes, severe congestion especially in central lobular vein and surrounding sinuses and fat degeneration, cardiac failure in liver; centrallobular congestion vein and surrounding sinuses (Figure 4 and Figure 5).
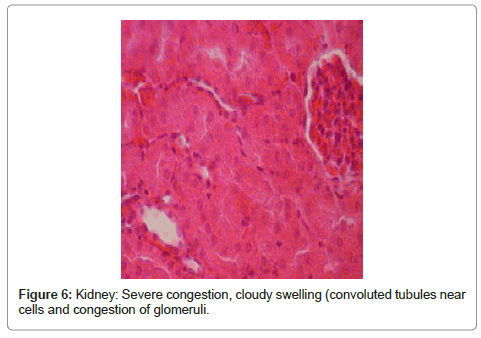
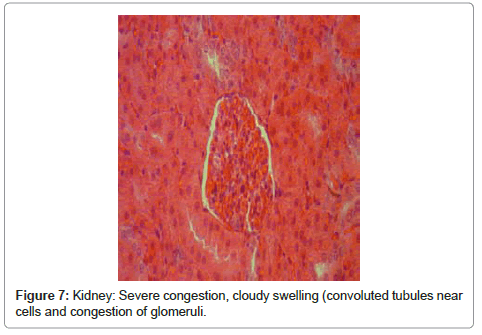
Kidney: included severe congestion, cloudy swelling (convoluted tubules near cells), severe swelling of tubular endothelial cells, congestion of glomeruli, hyaline casts that is a sign for proteinuria and severe tubular necrosis (Figure 6 and Figure 7).
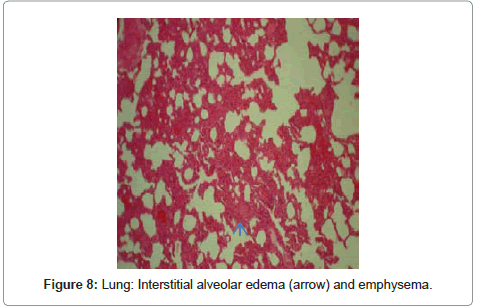
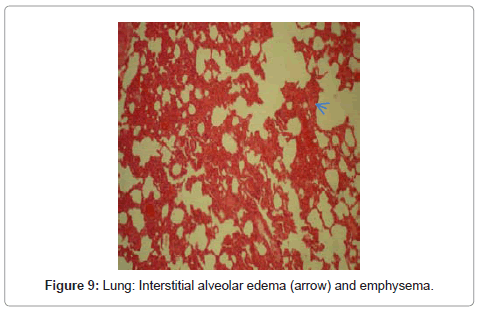
Lungs: In histopathological inspection of intoxicated rabbit lungs revealed that many lesions included spread interstitial alveolar edema, congestion of pulmonary arteries, medial hyperplasia and emphysema were detectable (Figure 8 and Figure 9).
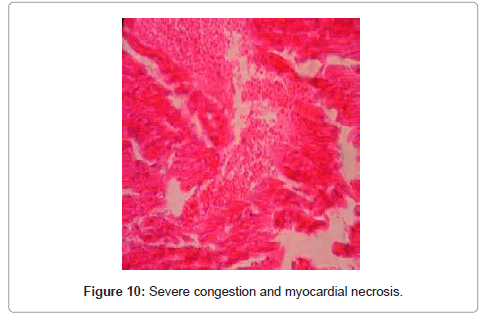
Heart: changes such as severe congestion, myocardial necrosis, edema fibers and necrosis in Purkinje cells were observed. Necrotic myocardial fibers with pyknotic nuclei and eosinophilic cytoplasm, and hyperplasia of Anitschkow cells were visible around or inside the destroyed fibers. Histopathological examination indicated necrosis of myocardial fibers with hyalinization and eosinophilia in some of the peripheral fibers of high dose-injected rabbits. In the treatment group, myocardial lysis, necrosis and proliferation of Anitschkow cells were recorded (Figure 10)., congestion of glomeruli, hyaline casts that is a sign for proteinuria and severe tubular necrosis.
Intestine: changes such as congestion, papillary hyperplasia of mucus layer, and outflow of fibrinous/mucous secretions into the intestine lumen, lymphatic expansion and sub-mucus edema were observed in the rabbits of treatment group.
Stomach: Pathological including congestion, an increase in basophilic cells until near the neck glands was observed.
Spleen: No pathological were found in spleen and other organs as well.
Discussion
Since very early ages, medicinal herbs that contain cardiac glycosides have been used by Greeks and Egyptians, as well as African countries in which the extracts of these herbs have been beneficial in poisoning the bullets and tilts [12]. However, it needs to be mentioned that due to the valuable effects of cardiac glycosides in increasing the contraction ability of heart and regulation of some types of dysrhythmias, they are broadly used in medical and veterinary medical compounds [13]. Adonis is one of the valuable plants containing cardiac glycosides. Different types of such plant are being used as medicine in many countries around the world. However, overconsumption leads to intoxication of both humans and animals [14,15]. Collected observations in present study demonstrated that administration of alcoholic extract of Adonis herb results in significant biological effects in rabbit. Clinical signs of poisoning with this plant in rabbits are mostly seen in circulatory system, digestive tract and neuromuscular system. Heart lesions observed in this study could be related to the presence of cardiac glycosides in this herb which is in accordance with wood study on horse. Histopathological Adonis plant showed that most of lesions were found in the heart in terms of hyaline degeneration, myocardial fibril necrosis. Even claimed that the toxin has a direct effect on other organs such as liver and severe congestion in central lobular vein, surrounding sinuses and fat degeneration which might be due to cardiac failure originated from the plant cardiac glycosides. Some histopathological results of this study is similar to Woods [7] study regarding the intoxication effects by Adonis herb on 3 horses; however, have not observed diffuse endocardial and epicardial hemorrhage with pockets of fibrin or infiltration of neutrophils or other inflammatory cells in myocardium [16]. Woods [11,12] reported that calves and sheep intoxicated with Adonis aestival, indicated no microscopic lesions in the lung and kidney. It seems that rumen or other digestive tract parts of ruminant have major role in inactivation of toxic compounds of Adonis plant [17,18]. In another research on a different species of Adonis plant (Adonis microcarpa) by Davis [8] they found that similar heart lesions such as myofibril necrosis have also occurred in the swine [5], whereas they did not evaluated pathologic changes in other organs. According to another study conducted by Hoffman [18] the observed reversible muscular paralysis was not seemingly related to the heart lesions, resulting from intoxication. Many different types of muscular and neural disorders caused by cardiac glycoside intoxication have been reported which many of them occurred in humans (such as headache, double vision, and seeing unnatural colors) that are not evaluative in animals [13]. Two types of motion disorders have been reported as the subject of poisoning with cardiac glycosides: In acute poisoning, muscular tremor, muscular weakness and sometimes hind paralysis. Chronic form of intoxication namely Krimpsiekte or Cotyledonosis is mostly seen in small herbivores but also occasionally reported in equine, dog and fowl. In this sort of intoxication clinical signs are such as renal dysfunction, repetitive sleeping and waking up, paralysis and protruding tongue [14,19]. Another group of dysfunctions caused by cardiac glycosides are digestive disorders which were yellow-colored diarrhea in the current study in rabbits. Clinical signs such as flatulence (gas), ruminal stasis, stomachache, vomiting (swine), diarrhea and dysentery are reported in cases of intoxication in large animals. Although diarrhea has been seen in most of the poisoning cases [20-22], Casteel [23] showed that animals poisoned by cardiac glycosides have very fragile conditions and any kind of stress during the treatment procedure, sampling or transportation could lead to their collapse and death [23]. The present study was carried out on the animal model preliminary, thus there is no significant evidence in order to confirming the obtained results except large animals.
References
- Abduchamidov N, Hammermann A, Sokolov W (1971) Adonis turkestanius--a new promising medicinal plant with cardiovascular effects. Planta Med 20: 272–277.
- Burrows GE, Tyrl RJ, Burrows GE (2001) Adonis L. In: Toxic Plants of North America, pp. 1006–1007. Iowa State University Press, Ames, IA.
- Zargari A (1991) Medicinal herbs. (4thedn) Vol 5, University of Tehran publications.
- Joubert JPJ, Cheeke PR, Joubert JPJ (1989) Cardiac glycosides. In: Toxicants of Plant Origin, Glycosides, ed. Cheeke PR, vol. 2, pp. 61–96. CRC Press, Boca Raton, FL.
- Knight AP, Walter RG (2001) Plants affecting the cardiovascular system. In: A Guide to Plant Poisoning of Animals in North America, ed. Knight AP, Walter RG, pp. 57–59. Teton New Media, Jackson, WY
- Pauli CF, Junior P (1995) Phenolic Zargarglycosides from Adonis aleppica , phytochemistry 38: 1245 – 1250.
- Woods LW,Filigenzi MS,Booth MC,Rodger LD,Arnold JS, et al. (2004) Summer pheasant's eye (Adonis aestivalis) poisoning in three horses. Vet Pathol 41: 215–220
- Davies RL,Whyte PB (1989) Adonis microcarpa (pheasant's eye) toxicity in pigs fed field pea screenings. Aust Vet J 66: 141-143.
- Hurst E (1942) Family Ranunculaceae. In: The poison plants of New South Wales, pp. 113–114. The Snelling Printing Works PTY. Ltd., Sydney, Australia.
- Kummer DH (1952) Vergiftungen bei pferden durch Adonis in luzerneheu (poisonings in horses with Adonis-contaminated alfalfa hay). Tierarztl Umsch 7: 430–431.
- Woods WL, Puschner S, Filignezi SM, George W (2011) Evaluation of the toxicity of Adonis aestivalis in sheep. Vet record 168: 49.
- Woods LW,George LW,Anderson ML,Woods DM,Filigenzi MS, et al. (2007) Evaluation of the toxicity of Adonis aestivalis in calves. J Vet Diagn Invest 19: 581–585.
- Greeff K, Kasperat H (1961) Konvulsive und paralytische wirkungen von digitalis-glykosiden und geninen bei intracerebraler und intravenoser injektion an mausen (convulsive and paralytic effects of digitalis glycosides and genins after intracerebral and intravenous injection in mice). Arzeimittel-forschung 11: 908–909
- Chen KK, Hendernson FG, Anderson RC (1951) Comparison of forty-two cardiac glycosides and aglycones. J Pharmacol Exp Ther 103: 420–430.
- Rottinghaus GE, Olesen B, Osweiler GD, Rottinghaus GE, Olesen B (1982) Rapid screening method for aflatoxin B, zearalenone, ochratoxin A, T-2 toxin, diacetoxyscirpenol and vomitoxin. Proc Annu Meet Am Assoc Vet Lab Diagn 25: 477–484.
- Lorke D (1983) A new approach to practical acute toxicity testing. ArchToxical 54: 257 – 287.
- Joubert JPJ (1989) Cardiacglycosides, in Toxicants of Plant origin. vol 2. cheeke PR (eds.), Florida, Boca Raton: CRC press. 61–64.
- Hoffman BF, Bigger JT (1991) Digitalis and allied cardiac glycosides, in Good man & Cilmans The pharmacological Basis of Therapeutics, (7thedn), Gilman AG, Rall T, Nies AS, Taylor P, Maxwell Mac Millan (eds), New York. 814–839.
- Radostits OM, Blood DC, Cay CC (2007) Veterinary Medicine, (9thedn). 545–556, 748, 989.
- Vermeulen N (1998) The complete encyclopedia of herbs, Reb oInter, Nether lands 25.
- KOPP B, Krenn L, Kubelka E, Kubelka W (1992) Cardenolides from Adonis aestivalis. Phytochemistry 31: 3195–3198.
- Kellerman TS, Coetzer JAW, Naude TW (1989) Plant Poisoning and mycotoxicoses of lives to skin southern Africa, oxford university press, Cape Town, South Africa. pp: 83–103.
- Casteel SW, south PJ (1999) Collapse / Sudden death, in Large Animal internal Medicine, edited by Smith, B.P. C.V. Mosby company, st. louis, Missouri. p 288.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 14870
- [From(publication date):
September-2012 - Dec 23, 2025] - Breakdown by view type
- HTML page views : 10134
- PDF downloads : 4736