A Bioremediation Approach to Mercury Removal in a Shake Flask Culture Using Pseudomonas putida (ATCC 49128)
Received: 14-Mar-2016 / Accepted Date: 08-Apr-2016 / Published Date: 15-Apr-2016 DOI: 10.4172/2155-9872.1000312
Abstract
Mercury is one of the most poisonous elements found on earth bonded to sulfhydryl groups of enzymes and proteins, thereby inactivating vital cell functions. Indeed this has drawn the attention of many environmental researchers who have been attempting through various mean to expunging mercury from these contaminated medium. Biological approach provided a satisfactory outcome in the clean-up of mercury contaminated soil and water due to its high potential for greater performance, environment friendliness and cost effectiveness. Mercuryresistant bacterial strain (P. putida ATCC 49128), was experimented on its potential to grow and reduce mercury to a permissible level under optimum conditions of nutrient, pH and other related physical factors in an incubator shake flask. It was observed that P. putida displayed a usual growth pattern when tried at low level mercury concentration of 1.0 μM, 6.0 μM and 19.0 μM, by exponentially growing during the first 4 hours of inoculation, but drastically decreased by the end of 24 hours’ time. This was indicated by the mercury removal rate of 99.0%, 99.83% and 98.58% in the three mercury concentrations used. Also, under the same optimum condition of growth, mercury concentration of 1000 μM was reduced by 92.0% after the first initial 1 hour to 98.0% at the end of 28 hour study. Comparably, similar trend was also observed when P. putida was used as bioaugmented organism to treat mercury contaminated samples from two petroleum industry based wastewater. A reduction rate of 84% was observed at the initial first 4 hours to about 90.5% after 96 hour experiment for plant P1 wastewater. While results from wastewater plant P2 indicated reduction rate of 97.2%, followed by 94.09% and lastly 56.8% respectively. The result affirmed the ability of this strain to optimally utilize the optimal conditional factors to grow and reduce mercury concentration overwhelmingly within a shortest time of less than 30 hours.
Keywords: Bioremediation; Pseudomonas putida; Mercury concentration; Mercury removal; Orbital shaker
303972Introduction
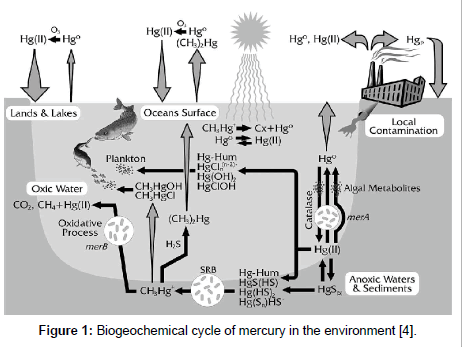
Mercury is one of the most poisonous elements found on earth in which after being discharged into the environment (soil and water) is bonded to the sulfhydryl groups of enzymes and proteins, thereby inactivating vital cell functions where it remains for many decades [1-3]. It is then taken up by aquatic organisms in the form of highly toxic methylmercury and subsequently biomagnified through the food chain which can pose a debilitating effect on birds, fish, seals, and man (Figure 1) [4-6]. Aziz et al. [7] reported that at high mercury concentrations through vapor inhalation produces an acute necrotizing bronchitis and pneumonitis which could lead to death from respiratory and central nervous system failure mainly due to neuronal disorders (i.e., inability to talk, see, smell, move), as well as damage to the cardiovascular system, kidney, bones, etc. [6]. The most common anthropogenic sources of mercury generation and discharge include petrochemical, electronic and equipment (measurement, inks and chlorine-soda). Others include industrial activities, such as extraction of gold and amalgam [3]. Mercury is one of the most strictly regulated elements, often restricted to less than 1 mM and 5 mM or less in Malaysia [2,7].
Purification of mercury-polluted areas appears very difficult task because they cannot be converted to innocuous elements [8]. Mercury is unique because of the combination of the extreme toxicity with no known biological function as well has low vapor pressure of elemental mercury, which is a liquid at room temperature [6,9]. Several chemical processes have been utilized for removal of mercury from mercurycontaminated industrial wastewater, but it is difficult to apply chemical processes to cleansing mercury contaminated soils and water because of enormous chemicals required, and usually leads to secondary pollution [8]. Various types of technologies were experimented for removing mercury from industrially discharged contaminated water and wastewater. Such processes include chemical precipitation, conventional coagulation, reverse osmosis, ultrafiltration, magnetic filtration, ion exchange and activated carbon adsorption and chemical reduction [2,4,6]. Mercury sequestration through common physicochemical technologies proven to be inefficient, time consuming, expensive and also generates extra waste, used only to treat highly mercury concentrated medium [3,4,7]. Application biotechnological (i.e., bioremediation) system of mercury removing from polluted water has the potential of achieving greater performance at lower cost when compared to the non-biological wastewater treatment [10]. Developments in the field of environmental biotechnology revealed that bacteria, fungi, yeasts and algae can remove heavy metals from aqueous solution through biosorption and bioaccumulation [8,11]. Comparatively, there are three main principle advantages of biological technologies for the removal of pollutants: firstly, biological processes can be carried out in-situ at the contaminated site, secondly, bioprocess technologies are usually environmentally benign (no secondary pollution) and thirdly, they are cost effective [7,8,12].
Reduction of mercury ion by mercury-resistant microorganisms (i.e., P. putida), is due to its genetic determinant mer-operon which consists of some genes of merR, T, P, A. While many molecular biological studies on reduction of mercury ion have been reported, there are only a few studies aimed at purification of the actual mercury polluted water or soil [8]. Although micro-organism cannot destroy metals but they can alter their chemical properties via a surprising array of mechanisms involving highly specific biochemical pathways [13]. The resistance of mercury to microbial transformation has therefore been divided into two categories; reduced accumulation of the metal ions by cells as a result of excretion of metal chelating substances or by the breakdown of the specific transport system and intracellular distribution of the ions by the binding to specific intracellular molecules [14]. Several studies on bioremediation by mercury-resistant bacteria have been reported, but investigations dealing with recovery of volatilized elemental mercury by biological reduction are few and inconclusive. In order to reuse and prevent release to the atmosphere, all the volatilized mercury should be collected [4,15]. There have been many investigations into removal of mercury from medium, but removal of mercury by growing cells that require carbohydrates or nutrients is difficult. Therefore, it seems that the use of resting cells is necessary for biological removal purification of actual contaminated sites [8]. Microorganisms that are highly resistant to mercury are extremely important in detoxifying the mercury compounds by NADPH-linked enzyme called mercuric reductase and organo-mercurial lyase [6]. Microorganisms are known to mediate four typed of enzymatic transformations of mercury which include:
1. Reduction of mercury ion (Hg2+) to mercury metal (Hg0)
2. Breakdown of organomercury compounds (including MeHg+) to Hg0
3. Methylation of Hg2+
4. Oxidation of Hg0 to Hg2+.
The reaction involving mercury ion (Hg2+) reduction and breakdown of organomercury compounds (1 and 2), are performed by mercury reductase enzyme and proteins of the microbial mercury resistance (mer). The mercury metal (Hg0) can volatilize out the system and subsequently recovered either as mercury vapor sorption onto various materials or through sorption to packed column. Thus, the resistant Pseudomonas putida can contribute to mercury removal.
Moreover, researchers have used such mercury resistant bacteria in many bioremediation processes.
Previously, mercury has traditionally been treated by the alteration of the pH value using lime or caustic soda in precipitating hydrated metal oxides. In addition, at the same time sulfide compound and other materials were also added which resulted in a production of heavy metal compounds with lower solubility products [3,16]. According to Aziz et al. [7] the microbial reduction of mercury is a detoxification reaction that requires energy rather than producing it. Thus, in any treatment medium, the bacteria have to be supplied with nutrients, which are their most essential response to their physiochemical environment [17]. Growth is a result of both replication and change in cell size. P. putida can grow and adapt under a variety of physical, chemical, and nutritional conditions. In suitable nutrient medium, organisms extract nutrients from the medium and convert them into biological compounds. Parts of these nutrients are used for energy production and other parts for biosynthesis and product formation. As a result of nutrient utilization, microbial mass increase with time [17]. When a liquid nutrient medium is inoculated with a seed culture, the organism selectively take up dissolved nutrients from the medium and convert them into biomass. Besides meeting requirements for growth and product formation; the length of time required for the removal of this substance in an environment depends on various factors that affect the growth kinetics of the participating microorganisms. These include the techniques of culture, acclimatization, substrate concentration, pH variation, and temperature, chemistry of metal ions, metal concentration, aeration, agitation and presence of toxic intermediates. Any one of these factors or a combination of them can play a role in limiting the rate of biomass growth and substrate biodegradation.
In this study, effect of mercury concentration was experimented with all other operational parameters kept at optimum; nutrient 8 g/l, pH 7.0, shaker speed 180 rpm, temperature 37°C and acclimatization time of 24 hours [1].
Materials and Methods
Inoculum and growth media
A bacterium (P. putida ATCC 49128-freeze dried) used in this study was obtained from Merck (Malaysia) Sdn. Bhd as local agent dealing with the bacteria, sourced from Microbiologic, 217 Osseo Ave. North, St. Cloud, USA together with the ingredients growth/nutrient broth (5 g of peptone meat and 3 g of extract meat). Enriched culture media was prepared in accordance with the manufacturer’s guidelines. Typically, 8 g of nutrient broth was dissolved in 1000 ml of deionized water (DI) in Schott bottles and shaken vigorously until it dissolved. The solution was then heated on a hot plate and sterilized in an autoclave at 121°C for 15 minutes; the sterilized media was then placed in a water bath to cool the media to 47°C before pouring into various 20 ml sampling bottles.
Seeding of P. putida into prepared media
Inoculation of bacterial strain was done by suspending 1-3 loops from the stock culture into a 20 ml freshly prepared nutrient broth 10% (w v-1). The seeded culture was incubated at 37°C for 24 hours at a vigorous shaking of 180 rpm. After 24 hours, the inoculum was transferred into a 500 ml Erlenmeyer flask containing 150 ml nutrient broth which is 30% of the original volume of the shake flask. The process of inoculum transfer was performed inside a laminar flow to avoid any contamination; as well the flask was passed over a Bunsen burner flame before seeding and after. This inoculation was done three times each to ensure proper bacterial growth [1,18].
Equipment (for experiment and analysis)
Equipment used for this research studies were auto-clave H+PVarioklav Steam Sterilizer ESCO, Shaker (B. Braun, German model), microbiological incubator (Mermmert-Germany/BE 600), mercury analyzer, (RA-300 Mercury, NIC), UV-Visible Spectrophotometer (U- 1800, Hitachi), pH Meter (Mettler Toledo), vacuum pump (HACH) and analytical Balance (Mettler Toledo).
Experimental procedure
Effect of mercury concentration on P. putida growth: To prepare the organism for the task of removing mercury from actual petroleum industry wastewater; a series of experiments using different concentration of Hg were conducted to study the effect of Hg on growth of P. putida. Samples used in the experiments were as follows: Sample A - P. putida in nutrient broth (NB) were mixed with fresh NB (8 g/L) as control; Sample B - P. putida in NB were grown in NB with 6.00 μM Hg solution (medium concentration); Sample C - P. putida in NB were grown in NB with 1.00 μM Hg solution (low concentration), while sample D, P. putida in NB were grown in NB with 19.00 μM Hg solution (higher concentration). The study was performed at optimum operating condition in shake flask within 24 hours acclimatization time, orbital shaker speed of 180 rpm, temperature 37°C, pH 7 and nutrient concentration 8 g/L.
Mercury removal by P. putida in orbital shaker at optimum operating condition: The experiment was conducted for 28 hour, employing the yield of optimum operating condition in a shake flask with 24 hours acclimatization, orbital shaker speed 180 rpm, temperature 37°C, pH 7, nutrient concentration 8 g/L and 1000 μM Hg solution (prepared from Hg(NO3)2). This also was indeed to further prepare the organism for mercury removal from actual wastewater.
Mercury removal from actual petroleum industry wastewater:
Plant 1: Based on the preliminary analysis of a sample collected during normal production, it was found that the mercury concentration was very low (0.01 μM Hg). Therefore, another 1000 μM mercury solution has to be added into the sample wastewater in order to study on mercury removal at pH 7 with temperature of 37°C and in orbital shaker at 180 rpm for 4 hours. The solution containing mercury was adjusted at the desired pH and three types of samples were prepared as follows:
- Sample A: 20 ml (Nutrient broth, NB+P. putida) added in to 180 ml NB
- Sample B: 20 ml (Nutrient broth, NB+P. putida) added in to 180 ml wastewater
- Sample C: 20 ml (Nutrient broth, NB+P. putida) added in to 180 ml wastewater with 1000 μg/l Hg
Plant 2: Based on petroleum based industries wastewater analysis, the wastewater here was found to contain 22.0 μM mercury with pH 6.09 and temperature of 25°C. The observed difference in the concentration with the first location was indeed due to the variation in refinery configurations and as well the processing techniques. The mercury removal experiments were conducted in a shake flask at optimum operating condition as previously described for 52 hours at optimum condition. Composition of wastewater samples from the petroleum based industrial Plant 2 prepared are as follow:
- P. putida+Wastewater
- P. putida+Nutrient Broth (NB)
- Wastewater only
- Wastewater+Nutrient Broth (NB)
- Wastewater+Nutrient Broth (NB)+P. putida
Analysis of samples
Optical density (growth rate) determination: Bacterial cell growth was determined by measuring optical density (OD) at 600 nm using UV-visible spectrophotometer (U-1800, Hitachi) and this method is based on the absorption of light by suspended cells in media of the sample culture at a specified wavelength. The extent of light transmission in a sample chamber is a function of cell density and modulated by broth absorption and scattering. Aliquots of 2.5 ml were drawn at an interval for growth analysis until a decay phase occurred. During sampling for all concentration, the shake flasks were detached from the orbital shaker.
Mercury reduction rate (bioremediation) determination: The mercury compounds in the sample were first pretreated with strong acid and an oxidizing agent to change the compound into divalent mercury ions (Hg2+). The maximum concentration allowable mercury that can be measured by the mercury analyzer is 15 μM. And hence before analyzing, the sample was first diluted to reduce the mercury concentration in the sample. A solution containing 40 ml hydrogen sulfate (97%) and 40 ml ultrapure water with 1:1 ratio was then prepared using a measuring cylinder. The solution was then poured into a clean glass bottle. In furtherance to this, a 2 g of Stanum chloride was weighed on digital balance and mixture of Stanum chloride and hydrogen sulfate then prepared. A 19 ml ultrapure water and 1 ml of hydrogen sulfate (97%) were poured into a beaker and the mixture then stirred until the solid Stanum chloride was completely dissolved. 10 ml of the sample was poured into a test tube and both measured solution added into the sample. The test tube was then plugged into a test tube socket of the mercury analyzer for three minutes. Samples were labeled and the start button within the software clicked. At 180 seconds, the concentration of sample result was finally recorded in μM unit.
Results and Discussion
Effect of mercury concentration on the growth of pseudomonas putida ATCC 49128
The results from Table 1, showed the initial effect of mercury concentration on the growth of P. putida in the range of 1 μM Hg, 6 μM Hg and 19 μM Hg at optimal growth parameters of nutrient (8 g/L), pH (7.0), temperature (37°C), acclimatization time (24 hours/1 day) and shaker speed/agitation (180 rpm). It can be seen that the optical density (OD) decreased from 0.53 after 4 hours to 0.05 after 24 hours for the control sample with no mercury added. This shows the normal behavior of P. putida growth in a batch system when the nutrient is introduced at an early stage. Cell density also increases for the first 4 hours and after which it starts to reduce due to the decrease in nutrient concentration. And study conducted earlier showed that the growth rate of P. putida is dependent upon nutrient concentration. Termination of this growth may be associated with the exhaustion of an essential nutrients or accumulation of toxic products. If an inhibitory product is produced and accumulated in the medium, the growth rate will slow depending on inhibitor production at a certain level of concentration. For culture with mercury concentration of 1.00 μM after 4 hours of experiment, the maximum optical density (ODmax) obtained was is 0.50 and the cell density further decreased to 0.08 after 24 hours. As a result, the mercury concentration has decreased from 1.00 μM to 0.01 μM and the percentage mercury removal stood at 99% and the ratio of mercury mass over cell mass is 1.00 μM Hg/ gcell. In mercury concentration of 6.00 μM, the maximum optical density ODmax obtained was 0.37 and the cell density decreased to 0.03 after 24 hours of experiment. And it can be seen that, mercury concentration decreased from 6.00 μM to 0.01 μM with percentage mercury removal of 99.58%. In this case, the ratio of mercury mass over cell mass is 0.33 μM Hg /gcell. While for culture with 19.00 μM mercury concentration, the results showed that the maximum optical density, ODmax is 0.30 but cell density dropped to 0.12 after 24 hours. In this case, the percentage mercury removal was 98.50% which is just slightly lower than the above two experiments carried out earlier. As a result the ratio of mercury mass over cell mass increased dramatically i.e., 6.75 μM Hg/gcell. Overall growth was higher in medium with 1.00 μM Hg concentration, while the culture with 19.00 μM Hg recorded the least value. This indeed affirmed the earlier reported negative effect of metabolites accumulation (detoxification), despite the resistivity of this strain to mercury concentration. And agrees with the findings of Dhandayuthapani [19] on the effect of Selenium on P. putida growth.
| Initial Hg Concentration (μM) | ODo Initial | ODmax (4 hr) | OD (24 hr) | Biomass Concentration (μM) | Final Hg Concentration (μM) | Hg%Removal | Hg (μM/gcell) |
|---|---|---|---|---|---|---|---|
| 0.00 | 0.00 | 0.53 | 0.39 | 0.02 | 0.00 | 0.00 | 0.00 |
| 1.00 | 0.00 | 0.50 | 0.08 | 0.03 | 0.01 | 99.00 | 0.33 |
| 6.00 | 0.00 | 0.37 | 0.03 | 0.01 | 0.01 | 99.83 | 1.00 |
| 19.00 | 0.00 | 0.30 | 0.12 | 0.04 | 0.27 | 98.58 | 6.75 |
Table 1: Effect of Low Mercury Concentration (μM) on P. putida growth behavior for 24 hours.
Mercury removal at optimal operating conditions by P. putida
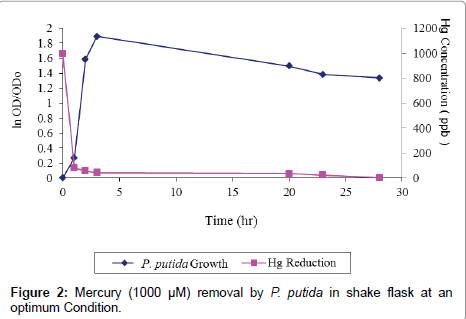
Mercury removal at concentration of 1000 μM was conducted at optimum operating conditions when the growth of P. putida is high (exponential phase). The experiments were conducted for 28 hour, employing the yield of optimum operating conditions in a shake flask with 24 hours acclimatization time, orbital shaker speed 180 rpm, temperature 37°C, pH 7 and nutrient concentration 8 g/L. It was observed that, P. putida grew immediately after inoculation because an exponentially growing culture was transferred into the medium under the same condition of growth. Usually the interval may be brief or extended, depending on the history of culture and growth condition. A constant mercury concentration of 79 μM was obtained after 1 hour and the ratio of mercury mass over cell mass increased drastically (1215.00 μM Hg/gcell) and mercury removal of 92.0% within this period of exponential growth phase. With decreasing mercury concentration, P. putida showed an increased growth rate. And the cell density increased in the first three hours, i.e., 0.33; with maximum OD and exponential cell growth of 0.86 and 1.89 respectively. Both OD and cell density maintained low values up to the end of the experiment, while mercury removal rate contrastingly continue to increase with highest value of 98% after 28 days. However, cell growth remained constant and slightly decreased over time after this period (Figure 2 and Tables 2 and 3).
| Time (hr) | OD600 nm | Biomass Concentration (g/L) | μMHg/ gcell | % Hg removal | |
|---|---|---|---|---|---|
| 0.00 | 0.00 | 0.00 | 1000 | 0.00 | 0.00 |
| 1.00 | 0.17 | 0.06 | 79 | 1215.00 | 92.10 |
| 2.00 | 0.63 | 0.24 | 56 | 230.00 | 94.40 |
| 3.00 | 0.86 | 0.33 | 43 | 130.00 | 95.70 |
| 20.00 | 0.58 | 0.22 | 34 | 153.00 | 96.60 |
| 23.00 | 0.51 | 0.2 | 24 | 121.00 | 97.60 |
| 28.00 | 0.5 | 0.19 | 20 | 105.00 | 98.0 |
Table 2: The Growth Kinetics of P. putida in mercury removal at 1000 μM Concentration.
| Growth Parameters | Min | Max |
|---|---|---|
| Specific growth rate, µ (hr-1) | 0.70 | - |
| Optical Density (600 nm) | 0.13 | 0.86 |
| Exponential cell growth (ln OD/OD0) | 0.27 | 1.89 |
| Number of Generation, n | 0.39 | 1.98 |
| Generation time, g (hr) | 0.88 | 14.64 |
| Growth rate constant, k(hr-1) | 0.06 | 0.78 |
| Mercury removal (%) | - | 98 |
Table 3: Mercury (1000 µM) removal by P. putida in orbital shaker at optimum operating Condition.
Mercury removal form petroleum refinery wastewater in an orbital shaker
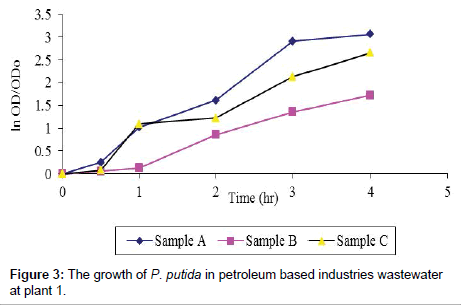
Plant 1: It took P. putida quite sometimes to grow after inoculation especially in the petroleum based industries wastewater as indicated in sample B and C. This was further indicated by highest OD of 1.52 in sample A, followed by sample C with 0.86 and lastly sample B having 0.46 optical density values. This as well correspond to exponential growth of 3.01 for sample A, 2.66 for sample C and 1.73 for sample B. It is evident that the presence of mercury in the sample wastewater affects the kinetics of P. putida, with higher value of specific growth rate, μ of 0.67 hr-1, for sample C and 0.47 hr-1 for sample B. In this case, sample A, as the control experimental, has the highest specific growth rate, μ, of 0.83 hr-1. The efficiency of mercury removal increased from 84% after 4.00 hours of experiment to 90.5% after 96 hours (Table 4; Figure 3). These agree with the results by Green-Ruiz, where he observed that the removal of mercury from wastewater appeared to be more efficient at a lower metal concentration (92% for 250 ppb) than at the highest (68.5% for 10 000 ppb). In addition, Volesky [17] reported that alternative conventional techniques may be ineffective for low mercury concentration in the wastewater that is less than 100 000 ppb.
| Growth Parameter | Min | Max | ||||
|---|---|---|---|---|---|---|
| Sample | A | B | C | A | B | C |
| Specific growth rate, µ (hr-1) | 0.83 | 0.47 | 0.68 | |||
| Optical Density (600 nm) | 0.07 | 0.08 | 0.06 | 1.52 | 0.46 | 0.86 |
| Exponential cell growth (ln OD/OD0) | 0.25 | 0.06 | 0.08 | 3.07 | 1.73 | 2.06 |
| Number of Generation, n | 0.36 | 0.08 | 0.11 | 4.4 | 2.48 | 3.81 |
| Generation time, g (hr) | 0.68 | 1.54 | 0.64 | 1.41 | 5.9 | 1.14 |
| Growth rate constant , k (hr-1) | 0.46 | 0.11 | 0.16 | 1.01 | 0.45 | 1.09 |
| Hg Removal (%) | 84 (4 hr) | 90.5 (96 hr) | ||||
A one-tailed test was used to test the unidirectional hypothesis 0
*p < 0.001
Table 4: Mercury removal from petroleum based wastewater at plant P1.
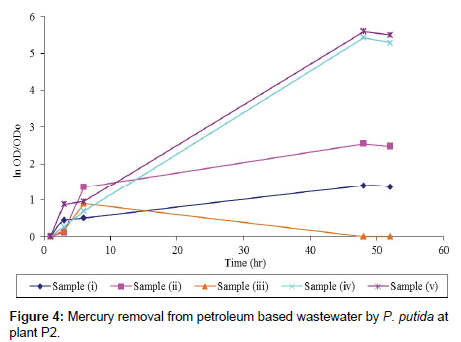
Plant 2: This experiment was also conducted under optimum growth conditions and the results obtained indicated the specific growth rate, μ of 0.02 hr-1 for sample (i), 0.05 hr-1 for sample (ii), 0.02 hr-1 for sample (iii), 0.12 hr-1 for sample (iv) and 0.11 hr-1 for sample (v). Also from the results samples (ii), (iv) and (v) showed high cell activity with increasing optical density and the exponential growth especially for samples (iv) and (v) where the nutrient was added in wastewater sample. In addition, mercury removal was 97.27% for sample (v), 94.09% for sample (i) and 56.82% for sample (iv) respectively. Highest mercury removal recorded in sample (i) and (v), was indeed due to bioaugmentation of these samples with P. putida strain which complimented the biodegradation capacity of the indigenous microbes in the wastewater. And of course the different between these two samples further confirmed the effect of substrate interaction during the growth and mercury detoxification by this bacterial strain (Table 5; Figure 4).
| Growth Parameter | Min | Max | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sample | i | ii | iii | iv | v | i | ii | iii | iv | v |
| Specific growth rate, μ (hr-1) | 0.02 | 0.05 | 0.02 | 0.11 | 0.11 | |||||
| OD | 0.14 | 0.18 | 0.01 | 0.01 | 0.01 | 0.55 | 2.34 | 0.03 | 2.28 | 2.68 |
| Exponential cell growth (ln OD/OD0) | 0.45 | 0.11 | 0.00 | 0.26 | 0.88 | 1.40 | 2.55 | 0.9 | 5.43 | 5.59 |
| Number of Generation, n | 0.64 | 0.16 | 0.00 | 0.38 | 1.25 | 2.00 | 3.65 | 1.29 | 7.78 | 8.01 |
| Generation time, g (hr) | 0.08 | 0.05 | 0.00 | 0.10 | 0.04 | 26.63 | 18.44 | 4.46 | 7.98 | 6.6 |
| Growth rate constant , k (hr-1) | 1.56 | 2.26 | 0.00 | 0.52 | 6.30 | 8.86 | 1.35 | 0.15 | 6.88 | 17.88 |
| Hg Removal (%) | 94.09 | 12.73 | 56.82 | 97.27 | ||||||
Table 5: Association on Pre-test and Post-test between Levels of Mental Health and Age of Old Age Home Residents in Experimental Group.
Conclusion
Mercury-resistant Pseudomonas putida was successfully grown and tested for mercury removal from petroleum refinery wastewater. The effect of initial mercury concentration, nutrient, pH, temperature, shaker speed and acclimatization time were also tested for P. Putida growth and mercury removal. Growth was overwhelmingly high in all the different ranges of mercury in the presence under optimum growth parameters, especially nutrient. And the decrease in growth observed at the later stage of the growth was rather not due to the effect of mercury concentration, but due to nutrient depletion and accumulation of detoxified metabolites. Based on the results obtained, it further confirmed P. putida as a reliable candidate for mercury removal from contaminated waters especially petroleum refinery effluents.
Acknowledgements
The authors wish to acknowledge the financial and technical support by Faculty of Chemical and Natural Resources Engineering, University Malaysia Pahang. Also, the efforts of technical and laboratory supporting staff is quite recognized. And all those whom time and space will not allow me to mention, your efforts are all appreciated.
References
- Azoddein AAM, Yunus RM, Sulaiman NM, Bustary AB, Sabar K (2015) Mercury Removal Using Pseudomonas putida (ATTC 49128): Effect of Acclimatization Time, Speed and Temperature of Incubator Shaker. International Journal of Biological, Biomolecular, Agricultural, Food and Biotechnological Engineering 9: 204-209.
- Cabral L, Giovanella P, Gianello C, Bento FM, Andreazza R, et al. (2013) Isolation and characterization of bacteria from mercury contaminated sites in Rio Grande do Sul, Brazil, and assessment of methylmercury removal capability of a Pseudomonas putida V1 strain. Biodegradation 24: 319-331.
- Giovanella P, Cabral L, Bento FM, Gianello C, Camargo FA (2016) Mercury (II) removal by resistant bacterial isolates and mercuric (II) reductase activity in a new strain of Pseudomonas sp. B50A. N Biotechnol 33: 216-223.
- Barkay T, Miller SM, Summers AO (2003) Bacterial mercury resistance from atoms to ecosystems. FEMS Microbiology Reviews 27: 355-384.
- Braune B, Muir D, DeMarch B, Gamberg M, Poole K, et al. (1999) Spatial and temporal trends of contaminants in Canadian Arctic freshwater and terrestrial ecosystems: a review. Sci Total Environ 230: 145-207.
- Wagner-Döbler (2003) Pilot plant for bioremediation of mercury-containing industrial wastewater. Applied Microbiology and Biotechnology 62: 124-133.
- Aziz A, Azoddein M, Yunus RM, Meriam N, Sulaiman N (2015) Mercury Removal from Actual Petroleum Based Industries Wastewater by P. putida in Membrane Bioreactor. UMP 2: 313-330.
- Okino S, Iwasaki K, Yagi O, Tanaka H (2000) Development of a biological mercury removal-recovery system. Biotechnology Letters 22: 783-788.
- Mukherjee AK, Bordoloi NK (2011) Bioremediation and reclamation of soil contaminated with petroleum oil hydrocarbons by exogenously seeded bacterial consortium: A pilot-scale study. Environ Sci Pollut Res Int 18: 471-478.
- Manikandaraja P, Senthilkumaran R (2014) A Study on degradation and characterization of heavy metals in industrial effluents waste using Pseudomonas sp. isolated from soil samples. International Journal of Advanced Multidisciplinary Research 1: 63-72.
- Mortula M, Shabani S (2012) Removal of TDS and BOD from Synthetic Industrial Wastewater via Adsorption. IPCBEE 41: 166-170.
- Vijayaraghavan K, Yun YS (2008) Bacterial biosorbents and biosorption. Biotechnol Adv 26: 266-291.
- Lloyd JR, Lovley DR (2001) Microbial detoxification of metals and radionuclides. Curr Opin Biotechnol 12: 248-253.
- Joho M, Inouhe M, Tohoyama H, Murayama T (1995) Nickel resistance mechanisms in yeasts and other fungi. Journal of Industrial Microbiology 14: 164-168.
- Fortunato R, Crespo JG, Reis MA (2005) Biodegradation of thiomersal containing effluents by a mercury resistant Pseudomonas putida strain. Water Res 39: 3511-3522.
- Ledin M, Pedersen K, Allard B (1997) Effects of pH and ionic strength on the adsorption of Cs, Sr, Eu, Zn, Cd and Hg by Pseudomonas putida. Water Air Soil Pollution 93: 367-381.
- Shea M, Litvin S, Chirnside A (2013) The Effect of Nitrogen, Sulfur , and Phosphorus Compounds on Bioremediation of Oil Spills by Pseudomonas fluorescens and Bacillus subtilis. University of Delaware 30-37.
- Dhandayuthapani S (2013) Optimization of Phenol Biodegradation by Pseudomonas Putida Isolated from Industrial Effluent. International Journal of Pharma and Bio Sciences 4: 405-413.
Citation: Azoddein AABM, Ahmad MM, Yunus RM, Sulaiman NMN (2016) A Bioremediation Approach to Mercury Removal in a Shake Flask Culture Using Pseudomonas putida (ATCC 49128). J Anal Bioanal Tech 7:312. DOI: 10.4172/2155-9872.1000312
Copyright: © 2016 Azoddein AABM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 13255
- [From(publication date): 6-2016 - Aug 18, 2025]
- Breakdown by view type
- HTML page views: 12191
- PDF downloads: 1064