A Giant Primary Clear Cell Hepatocellular Carcinoma in a Young Woman without Cirrhosis
Received: 18-Feb-2015 / Accepted Date: 21-Apr-2015 / Published Date: 28-Apr-2015 DOI: 10.4172/2161-0681.1000224
Abstract
Primary clear cell carcinoma of the liver (PCCCL) is an uncommon variant of hepatocellular carcinoma, usually occurring in older patients with longstanding cirrhosis. We report a case of a giant PCCCL rupturing in a young woman without cirrhosis. A 29 year-old female who presented with a three month history of abdominal pain, nausea, vomiting, food intolerance, and unintentional weight loss, was found to have gastric varices and a large liver mass measuring 22.8cm × 17.8 cm × 13.8 cm. The tumor was comprised of sheets of clear cells with delicate vesicular architecture, and was positive for Hepar 1, Glypican 3, and CD34 in a diffuse staining pattern and CD10 in a canalicular staining pattern, establishing the diagnosis of PCCCL. The patient died of hemorrhagic shock secondary to tumor rupture within two weeks of diagnosis. Conclusion: Although PCCCL most often occurs in older patients with cirrhosis, this uncommon variant of HCC can arise in younger patients without a history of liver disease. It is important to diagnose PCCCL early due to the potentially favorable prognosis of this specific cancer and in order to prevent catastrophic complications.
Keywords: Clear cell hepatocellular carcinoma; Immunohistochemistry; Histology
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common cancer in men and the seventh most common cancer in women, but because of its poor prognosis, it is the third most common cause of cancer death worldwide [1]. The vast majority of HCC patients are above the age of 45 years in the United States, and it is much more common in men than women [2]. Risk factors for developing HCC primarily involve longstanding liver disease, such as cirrhosis secondary to hepatitis B virus (HBV), hepatitis C virus (HCV), alcohol abuse, and non-alcoholic steato-hepatitis (NASH) [2]. Non-cirrhotic patients are estimated to account for less than 20% of HCC cases [3].
Primary clear cell carcinoma of the liver (PCCCL) is an uncommon variant of HCC, representing less than 10% of all HCC cases [4,5]. Most patients with PCCCL are cirrhotic and over the age of 50 years, although PCCCL does proportionately affect more women than common-type HCC (2.30:1 in PCCCL verses 4.38:1 for conventional HCC) [4]. The strongest risk factor for PCCCL is HCV infection, with no significant associations with HBV, alcohol abuse, NASH, hemochromatosis, autoimmune hepatitis (AIH), or primary biliary cirrhosis (PBC) [4,5]. Establishing the diagnosis of PCCCL is important due to the overall better prognosis of this lesion versus common-type HCC [4,6]. In this report, we describe a young female with no known history of liver disease who was diagnosed with a giant PCCCL that spontaneously ruptured within two weeks of presentation, ultimately leading to her death from hemorrhagic shock. We also discuss related cases and literature regarding the diagnosis and reported outcomes of PCCCL.
Case Report
A 29-year-old female presented with a three month history of abdominal pain, nausea, vomiting, food intolerance, and unintentional weight loss of 40 lbs. An EGD performed several days prior to admission to evaluate her gastrointestinal symptoms and weight loss revealed type 1 gastric varices without bleeding. Past medical history was significant for obesity, type II diabetes mellitus, hyperlipidemia, schizoaffective disorder, fibromyalgia, and polymenorrhea managed with oral contraceptives for over a decade. Social history was significant for a 7.5 pack-year history of cigarette smoking; the patient denied alcohol or illicit drug use. Family history was significant for NASH in the patient’s mother and pancreatic cancer in a maternal uncle. Physical exam was remarkable for scleral icterus, mild jaundice, abdominal tenderness, and a large, firm abdominal mass on palpation. On admission, the patient weighed 262 lbs. (107 kg) with a height of 5’8” (1.73 m); her body mass index (BMI) was 39.8.
Initial laboratory studies revealed AST 359 u/L, ALT 254 u/L, alkaline phosphatase 1276 u/L, albumin 2.0 g/dL, total bilirubin 5.3 mg/dL, direct bilirubin 4.1 mg/dL, and indirect bilirubin 1.2 mg/dL. On further workup, anti-nuclear antibodies (ANA) were positive at 1:320 in a speckled pattern, anti-mitochondrial M2 antibody (AMA) was measured at 43.4 units (normal range 0.0-20.0 units), and alpha fetoprotein was 47 ng/mL. Serologies for HBV and HCV were non-reactive and iron studies, ceruloplasmin, F-actin IgG, and alpha-1 antitrypsin levels were all within normal limits.
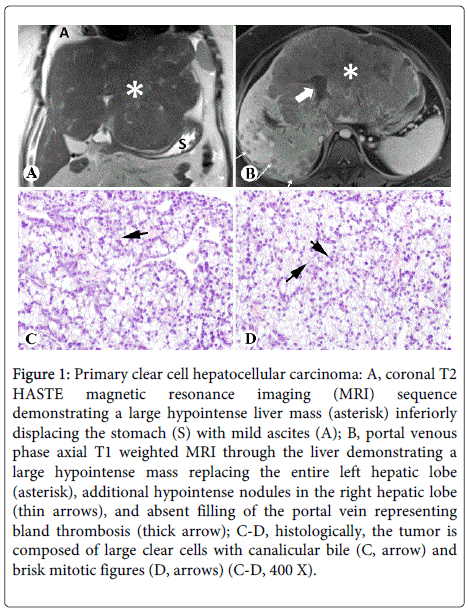
Abdominal MRI revealed a large, enhancing, centrally necrotic mass measuring 22.8 cm × 17.8 cm × 13.8 cm replacing the left hepatic lobe and the anterior right hepatic lobe, with numerous additional arterial enhancing lesions throughout the remaining right hepatic lobe (Figures 1A and 1B). Furthermore, portal vein thrombosis (PVT) and occlusion of the middle and left hepatic veins was noted, as well as sub centimeter nodules along the gastro-hepatic ligament concerning for metastatic deposits. Further imaging did not reveal any mass lesions in other organs.
Figure 1: Primary clear cell hepatocellular carcinoma: A, coronal T2 HASTE magnetic resonance imaging (MRI) sequence demonstrating a large hypointense liver mass (asterisk) inferiorly displacing the stomach (S) with mild ascites (A); B, portal venous phase axial T1 weighted MRI through the liver demonstrating a large hypointense mass replacing the entire left hepatic lobe (asterisk), additional hypointense nodules in the right hepatic lobe (thin arrows), and absent filling of the portal vein representing bland thrombosis (thick arrow); C-D, histologically, the tumor is composed of large clear cells with canalicular bile (C, arrow) and brisk mitotic figures (D, arrows) (C-D, 400 X).
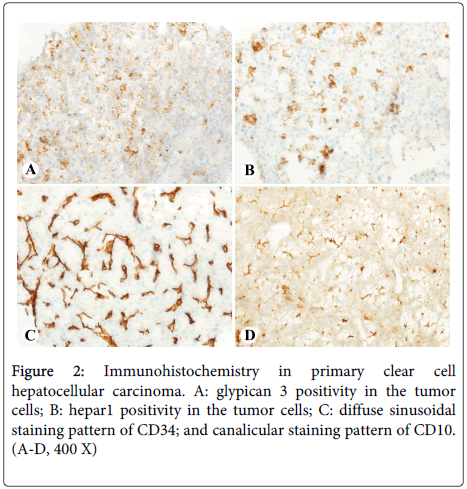
Hematoxylin and eosin (HE) stain of multiple ultrasound-guided core biopsies showed that the liver mass was composed of 60-90% clear cells with canalicular bile plugs (Figure 1C) and brisk mitotic activity (Figure 1D). Minute fragments of non-neoplastic hepatic parenchyma showed mass effect, mild portal inflammation with scattered plasma cells, and no significant steatosis or fibrosis. The tumor was positive for Hepar1 and glypican 3, with a diffuse staining pattern of CD34 and a canalicular staining pattern of CD10 (Figures 2A-2D); rare cells (<5%) were positive for CK19 and CK7. The tissue was otherwise negative for CK20, CDX2, nuclear TTF1, ER, RCC, and GCDFP-15. These features were diagnostic for moderately differentiated hepatocellular carcinoma, clear cell variant.
Due to the extremely large size of the tumor and the suggestion of extrahepatic metastasis, the patient was not deemed a candidate for surgical resection or transplant. Due to portal and hepatic vein thrombosis presumably with tumor, large bilobar tumor burden, and lack of variceal bleeding, transjugular intrahepatic protosystemic shunt (TIPS) was not considered a viable treatment for the portal vein thrombosis. Transarterial chemo-embolization (TACE) was also contraindicated due to the advanced hepatocellular carcinoma with vascular invasion or extrahepatic metastasis according to Barcelona clinic liver cancer (BCLC) staging classification (the patient was Child-Pugh class C, and thus automatically BCLC stage D). Yttrium-90 (Y-90) treatment was contraindicated as well due to presence of large tumor mass with bilobar disease and a significantly raised total bilirubin.
After a nine-day hospital course, the patient was discharged with low-dose sorafenib (full-dose could not be recommended due to rising total bilirubin) and instructions for outpatient treatment with palliative external beam radiation. Two days after discharge however, the patient was readmitted for severe generalized abdominal pain and was found to be tachycardic, hypotensive, and intermittently to minimally responsive. The patient was transferred to the intensive care unit, and an abdominal CT revealed a perihepatic hematoma with small to moderate volume hemorrhagic ascites concerning for ruptured hepatocellular carcinoma. After convening, the patient’s family decided to withdraw care, and the patient subsequently died from hemorrhagic shock secondary to her ruptured PCCCL. Per the family’s wishes, an autopsy was not performed on the patient.
Discussion
Primary clear cell carcinoma of the liver is an uncommon variant of hepatocellular carcinoma, usually arising in older patients with cirrhosis [4]. Other cases of PCCCL have been described in non-cirrhotic patients, including a case occurring in a 36 year-old woman with a lesion measuring 6 cm in diameter [7]. Our case is unique in the especially young age of the patient, the enormous size of the lesion on initial presentation, and death from tumor rupture within two weeks of diagnosis.
There are some aspects of this case which potentially lend themselves to liver disease and cancer. Given the patient’s young age, fibrolamellar HCC must be considered; however, the histology of her biopsies does not at all resemble this variant of HCC. Next, the patient is obese and has type II diabetes mellitus and hyperlipidemia, all of which could lead to the development metabolic syndrome and NASH; still, the patient’s specimen did not display any significant steatosis or other evidence of NASH microscopically. Of note, it must be considered that the patient’s near morbid obesity could have led to a delay in diagnosis in that a liver mass of such size may be difficult to palpate due to excess abdominal fat. Her history of oral contraceptive use could suggest hepatic adenoma with subsequent malignant transformation and rupture; a case report has described an instance where a PCCCL was mistaken for a hepatic adenoma [8]. Although the presence of brisk mitotic activity heavily favors the diagnosis of a carcinoma over an adenoma in our case, we cannot definitively rule out adenoma with malignant transformation and rupture due to our usage of core biopsies. Finally, the presence of ANA, AMA, and scattered plasma cell infiltrate could suggest an autoimmune pathology such as AIH or PBC; while an association between such autoimmune pathologies with the PCCCL cannot effectively be ruled out, there is no significant link between PCCCL and AIH or PBC, and the autoimmune liver disease patients that do develop HCC overall tend to be over the age of 50 years and cirrhotic [5,9].
Evidence from radiography, histology, and immunohistochemistry must be utilized in order to definitively diagnose PCCCL. Radio-graphically it can be difficult to distinguish PCCCL from other types of HCC, though there is evidence that PCCCL has a propensity to form pseudo-capsules visible on CT or MRI [10,11]. In our case the mass did not evince such a pseudo-capsule, though most importantly the imaging did not find evidence of malignancy in other organ systems, which helped to establish that the liver was the origin of the cancer. Histologically, PCCCL appears as sheets of large clear cells that do not stain with HE due to intracytoplasmic fat and glycogen accumulation, and this histology may cause the lesion to be mistaken for other primary liver tumors with similar appearances such as common-type HCC with foamy histiocytes, clear cell cholangiocarcinoma and epithelioid angiomyolipomas, as well as metastatic clear cell cancers from other sites such as the kidneys, adrenal glands, lung, thyroid, and female genitourinary system [12]. The diagnostic criteria for PCCCL is not clearly defined, though in the literature it is commonly accepted that a specimen containing over 30% clear cells can be labeled a clear cell cancer [5]; again, our specimen contained 60-90% clear cells with canalicular bile plugs, lending further support to our diagnosis of PCCCL. The most robust evidence for PCCCL was provided by the immunohistochemical profile of our specimen. Hepar1 was strongly positive, and as reported by Murakata et al., this stain has 90% sensitivity and 100% specificity for establishing the liver as the origin of a clear cell cancer [12]. Moreover, CD10 staining in a canalicular pattern, not cytoplasmic/membranous-staining pattern seen in clear cell renal cell carcinoma, further supports that this mass is hepatic in origin [13]. Finally, positive glypican 3 and diffuse sinusoidal staining pattern of CD34 further established that this was a malignant primary liver tumor [14,15]. Using the combination of radiographic, histologic, and immunohistochemical means, the diagnosis of PCCCL was firmly established for our patient.
The current literature is limited to studies analyzing surgical resection as the primary treatment for PCCCL. In terms of overall survival, the prognosis is generally suggested to be better for PCCCL than for common-type HCC and clear cell cancers from other sites, with some studies linking higher percentages of clear cells to better outcomes [4,6,16]. PCCCL is associated with smaller size, less vascular invasion, and better differentiation than common-type HCC as well [4,6]. The rates of recurrence do not differ between PCCCL and common-type HCC, though PCCCL is associated with later recurrence (over 1 year post resection) and common-type HCC with early recurrence (less than 1 year post resection) [6]. Because the PCCCL literature is dominated by surgical resection series, our understanding of the prognosis of PCCCL may be biased as patients undergoing surgical resection have better outcomes due to a lower tumor burden and lack of vascular invasion [17]. Due to the rarity of PCCCL, no data currently exist on non-surgical treatments utilized first-line, such as TACE [18], radiofrequency ablation (RFA), Y-90, or sorafenib. However, limited data on treating PCCCL recurrences does suggest that surgical resection is superior to treatment with TACE, RFA, and percutaneous ethanol injection [6].
Conclusion
Primary clear cell carcinoma of the liver is an uncommon variant of hepatocellular carcinoma, usually arising in the setting of cirrhosis. However, it must be noted that PCCCL can occur in younger patients without significant history of liver disease. Early and definitive diagnosis of PCCCL is important due to the potentially favorable prognostic implications of this specific cancer and in order to prevent such devastating complications as tumor rupture with subsequent hemorrhagic shock and death.
References
- Bosetti C, Turati F, Vecchia CL (2014) Hepatocellular carcinoma epidemiology. Best Pract Res ClGa 28:753-770.
- El-Serag HB, Kanwal F (2014) Epidemiology of hepatocellular carcinoma in the United States: Where are we? Where do we go? Hepatology 60: 1767-1775.
- Alkofer B, Lepennec V, Chiche L (2011) Hepatocellular cancer in the non-cirrhotic liver. J ViscSurg148: 3-11.
- Liu ZS, Ma WD, Li HK, Li Q (2008) Clinicopathological and prognostic features of primary clear cell carcinoma of the liver. Hepatol Res 38: 291-299.
- Emile JF, Lemoine A, Azoulay D, Debuire B, Bismuth H, et al. (2001) Histological, genomic and clinical heterogeneity of clear cell hepatocellular carcinoma. Histopathology 38: 225-231.
- Li T, Fan J, Qin LX, Zhou J, Sun HC, et al. (2011) Risk Factors, Prognosis, and Management of Early and Late Intrahepatic Recurrence After Resection of Primary Clear Cell Carcinoma of the Liver. Ann SurgOncol18: 1955-1963.
- Takahashi A, Saito H, Kanno Y, Abe K, Yokokawa J, et al. (2008) Case of clear-cell hepatocellular carcinoma that developed in the normal liver of a middle-aged woman. World J Gastroenterol 14: 129-131.
- Sivrioglu AK, Saglam M, Incedayi M, Sonmez G (2013) Clear cell HCC mimicking to hepatic adenoma. BMJ Case Reports.
- Czaja AJ (2013) Hepatocellular Carcinoma and Other Malignancies in Autoimmune Hepatitis. Dig Dis Sci 58: 1459-1476.
- Wang HY, Tan BY, Zhao B, Gong G, Xu ZD. (2014) CT findings of primary clear cell carcinoma of liver: with analysis of 19 cases and review of the literature. Abdom Imaging 39: 736–743
- Liu QY, Li HG, Gao M, Lin XF, Li Y, et al. (2011) Primary clear cell carcinoma in the liver: CT and MRI findings. World J Gastroenterol 17: 946-952.
- Murakata LA, Ishak KG, Nzeako UC (2000) Clear Cell Carcinoma of the Liver: A Comparative Immunohistochemical Study with Renal Clear Cell Carcinoma. ModernPathol 13: 874-881
- Lin F, Abdallah H, Meschter S (2004) Diagnostic utility of CD10 in differentiating hepatocellular carcinoma from metastatic carcinoma in fine-needle aspiration biopsy (FNAB) of the liver. DiagnCytopathol 30: 92-97.
- Coston WM, Loera S, Lau SK, Ishizawa S, Jiang Z, et al. (2008) Distinction of hepatocellular carcinoma from benign hepatic mimickers using Glypican-3 and CD34 immunohistochemistry.Am J SurgPathol32: 433-444.
- Lai JP, Oseini AM, Moser CD, Yu C, Elsawa SF, et al. (2010) The oncogenic effect of sulfatase 2 in human hepatocellular carcinoma is mediated in part by glypican 3-dependent Wnt activation. Hepatology 52: 1680-1689.
- Ji SP, Li Q, Dong H (2010) Therapy and prognostic features of primary clear cell carcinoma of the liver. World J Gastroenterol 16: 764-769.
- Vauthey JN, Klimstra D, Franceschi D, Tao Y, Fortner J, et al. (1995) Factors affecting long-term outcome after hepatic resection for hepatocellular carcinoma.Am J Surg169: 28-34.
- Lai JP, Conley A, Knudsen BS, Guindi M (2014)Hypoxia after transarterial chemoembolization may trigger a progenitor cell phenotype in hepatocellular carcin oma Histopathology.
Citation: ZH Ko J, Chen Y, Nepute J, Goodwill V, Garrett R, et al. (2015) A Giant Primary Clear Cell Hepatocellular Carcinoma in a Young Woman without Cirrhosis. J Clin Exp Pathol 5:224. Doi: 10.4172/2161-0681.1000224
Copyright: © 2015 Jeffrey ZH Ko, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 15590
- [From(publication date): 6-2015 - May 02, 2024]
- Breakdown by view type
- HTML page views: 11141
- PDF downloads: 4449