A Novel Approach: Vesicular Drug Delivery System
Received: 01-Jul-2022 / Manuscript No. jabt-22-70525 / Editor assigned: 04-Jul-2022 / PreQC No. jabt-22-70525(PQ) / Reviewed: 18-Jul-2022 / QC No. jabt-22-70525 / Revised: 22-Jul-2022 / Manuscript No. jabt-22-70525(R) / Published Date: 29-Jul-2022 DOI: 10.4172/2155-9872.1000468
Abstract
The vesicular drug delivery system such as liposomes, niosomes, invasomes, transferosomes, and pharmacosomes are used to improve the drug bioavailability by encapsulating an active medicament inside the vesicles. The vesicular drug delivery system can improve drug bioavailability of poorly absorbed drugs, improve solubility, reduce the cost of therapy, reduce the dose of drug administered, and reduce side effects. Both hydrophilic, as well as lipophilic drugs can be incorporated into vesicles. Vesicles can be incorporated into the different pharmaceutical formulations as oral, topical, and transdermal. In recent, Nikkosome® PV-PF is the natural vesicles forming agent with the excellent promotion of transdermal absorption efficacy.
Keywords
Vesicular drug delivery; Bioavailability enhancement; Encapsulation efficiency; Liposomes; Niosomes
Introduction
Vesicles are tiny sacs that transport material within or outside the cell. There are several types of vesicles, including transport vesicles, secretory vesicles, and lysosomes [1]. Vesicles have become the vehicle of choice in a drug delivery system called the vesicular drug delivery system. E.g.: Liposomes, Invasomes, Niosomes, Cubosomes [2]. The biologic origin of these vesicles was first reported in 1965 by ‘Bingham’ and has been given the name ‘Bingham bodies’. The first vesicular drug delivery system is liposomes [3]. Vesicular drug delivery reduces the cost of therapy by improving the bioavailability of medication, especially in the case of poorly soluble drugs [4, 5]. They can incorporate both hydrophilic as well as lipophilic drugs [6].
Why Vesicular Drug Delivery System
1. Hydrophilic-Lipophilic drugs can be incorporated.
2. Improves bioavailability of poorly absorbed drugs
3. Reduce the cost of therapy
4. They are osmotically active and stable
5. Easy to be formulated
6. Effective permeation of drugs into cells
7. Reduce harmful side effects of controlled release drug delivery systems
8. Vesicles are used in many preparations such as pharmaceuticals (Oral, transdermal, topical, ocular, parenteral.) as well as cosmetic preparations
9. The vesicles are nano-range particles so they can easily penetrate the skin
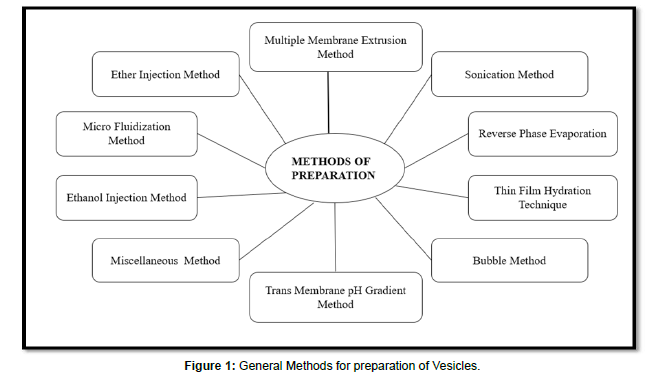
Methods of Preparation of Vesicles
The general different methods of preparation of vesicles as shown below in Figure 1. (Figure 1)
Classification of Vesicles
Classification of vesicles according to size2. As shown in Table 1. (Table 1)
| Sr. no. | Type of vesicles | Description | Size |
|---|---|---|---|
| UV | Unilamellar vesicles | All size range | |
| OLV | Oligolamellar vesicles | 0.1-1 µm | |
| MLV | Multilamellar large vesicles | ˃0.5 µm | |
| SUV | Small unilamellar vesicles | 20-100 nm | |
| MUV | Medium unilamellar vesicles | - | |
| LUV | Large unilamellar vesicles | ˃100 µm | |
| GUV | Giant unilamellar vesicles | ˃1 µm | |
| MV | Multivesicular vesicles | 1 µm |
Types of Vesicles
There are different types of vesicles like Liposomes, Niosomes, Invasomes, Pharmacosomes, Ufosomes, and many more as shown in Tables 2 to 6. (TableS 2-6)
Sr.no. |
Vesicles | Description |
|---|---|---|
| Liposomes | A liposome is a spherical vesicle having at least one lipid bilayer. It can be used as a drug delivery vehicle for the administration of nutrients and pharmaceutical drugs. | |
| Niosomes | Niosomes are non-ionic surfactant-based unilamellar or multilamellar bilayer vesicles upon hydration of non-ionic surfactants with or without incorporation of cholesterol. They are biodegradable, biocompatible, do not require any special storage conditions, and are available at a low cost. | |
| Invasomes | Invasomes are liposomal vesicles embodying small amounts of ethanol and terpenes mixtures, which act as the potential carrier with increased skin penetration. Invasomes have a higher penetration rate through the skin compared to liposomes and ethosomes. | |
| Pharmacosomes | Pharmacosomes are colloidal dispersion, drugs covalently bound to the lipids, nanometric size micelles, vesicles, or hexagonal aggregates, based on the chemical structure of the drug-lipid complex. The system is composed of linking a drug (pharmakon) to a carrier (soma), so they are termed “Pharmacosomes”. | |
| Transferosomes | The term is derived from the Latin word ‘transfer’ meaning ‘to carry across’ and the Greek word ‘soma’ for a ‘body’.Transferosomes are a special type of liposomes, consisting of phosphatidylcholine and an edge activator. Transferosomes are self aggregates, with an ultra-flexible membrane that delivers the drug reproducibly into or through the skin. | |
| Cubosomes | Cubosomes are distinct, sub-micron, the square, and round-shaped particles with internally visible cubic lattices. Cubosomes are composed of polymers, lipids, and surfactants with polar and non-polar constituents hence said to be amphiphilic. Cubosomes are bicontinuous cubic liquid phases surrounded by two separate regions of water divided by surfactant-controlled bilayers. | |
| Erythrosomes | Erythrosomes are a modified version of the liposome system, in which chemically cross-linked human erythrocyte cytoskeletons are used as a support, upon which a lipid bilayer is coated. |
Sr.no. |
Vesicles | Description |
|---|---|---|
| Glycerosomes | Glycerosomes are bilayer vesicles used for dermal and transdermal drug delivery. These vesicles differ from conventional liposomes in bilayer fluidity. Glycerosomes are mainly composed of phospholipids and glycerol. | |
| Ethosomes | Ethosomes are phospholipid nanovesicles used for dermal and transdermal delivery of molecules. They are reported to improve the skin delivery of various drugs. Ethanol is an efficient permeation enhancer that is believed to act by affecting the intercellular region of the stratum corneum. Ethosomes are soft malleable vesicles composed mainly of phospholipids, ethanol (relatively high concentration), and water. | |
| Bilosomes | Bilosomes are the novel innovative drug delivery carriers that consist of deoxycholic acid incorporated into the membrane of niosomes. | |
| Aquasomes | Aquasomes are three-layered structures (i.e. core, coating, and drug) that are self-assembled through non-covalent bonds, ionic bonds, and van der Waals forces. They consist of tin oxide, ceramics carbon nanocrystalline particulate core coated with, glassy cellobiose specific targeting, and molecular shielding. | |
| Emulsomes | Emulsomes are a novel lipoidal vesicular system with an inner solid fat core surrounded by a phospholipid bilayer. Emulsomal formulations are composed of solid lipid core material and stimulated by cholesterol and soya lecithin. The drug is loaded accompanied by sonication to produce emulsomes of small size. | |
| Enzymosomes | Enzymosomes utilize the specific nature of an enzyme, which is binding to a specific substrate at a controlled rate and catalyzes the product production step. Enzymes are covalently immobilized or coupled to the surface of liposomes. | |
| Proliposomes | Proliposomes are defined as dry, free-flowing particles with a dispersed system that can immediately form a liposomal suspension when in contact with water. Compared with conventional liposomes, proliposomes exhibit more advantages in promoting drug absorption. |
Sr.no. |
Vesicles | Description |
|---|---|---|
| Sphingosomes | Sphingosomes are concentric, bilayered vesicles where an aqueous volume is entirely enclosed by a membranous lipid bilayer mainly constituted of natural or synthetic sphingolipid. Sphingosomes are mainly composed of cholesterol and sphingolipid. | |
| Colloidosomes | Colloidosomes are hollow shell microcapsules involving coagulated or fused particles at the interface of emulsion droplets. Colloidosomes have stimulating potential applications in the controlled release of drugs, proteins, and vitamins as well as in cosmetics and food supplements. | |
| Herbosomes | The term "herbo" means plant, while "some" means cell-like. Over the past century, phytochemical and Phyto‐ pharmacological sciences established the compositions, biological activities, and health-promoting benefits of numerous botanical products. Most of the biologically active constituents of plants are polar or water-soluble molecules. | |
| Layerosomes | Layerosomes are conventional liposomes coated with one or more multiple layers of biocompatible polyelectrolytes to stabilize their structures. The layer-by-layer coating concept is one of the strategies used for the preparation or the stabilization of nanosystems. The formulation is based on an alternative coating procedure of positive poly(lysine) (PLL) and negative poly(glutamic acid) (PGA) polypeptides on initially charged small unilamellar liposomes. | |
| Cryptosomes | Cryptosomes are lipid vesicles with a surface coat composed of pc and suitable polyoxyethylene derivative of phosphotidyl ethanolamine. Capable of Ligand mediated drug targeting. | |
| Discosomes | Discosomes are giant, disc-shaped structures modified from niosomes by arresting the vesicles at the discosome phase. Discomes may act as potential drug delivery carriers as they released drugs in a sustained manner at the ocular site. | |
| Genosomes | Genosomes are artificial macromolecular complexes for functional gene transfer. Cationic lipids are most suitable because they possess high biodegradability and stability in the bloodstream. Cell-specific gene transfer. |
Sr.no. |
Vesicles | Description |
|---|---|---|
| Photosomes | Photolysase encapsulated in liposomes, which release the content photo triggered charges in membrane permeability characteristics. | |
| Virosomes | Virosomes are liposomes spiked with virus glycoprotein, incorporated into the liposomal bilayers based on retro viruses’ derived lipids. | |
| Vesosomes | Vesosomes cuddled bilayer compartment in vitro via the inter-digested bilayer phase formed by adding ethanol to a variety of saturated phospholipids. Multiple compartments of the vesosomes give better protection to the interior contents in serum. | |
| Proteosomes | Proteosomes are high molecular weight multi-submit enzyme complexes with catalytic activity, which is specifically due to the assembly pattern of enzymes. Better catalytic activity turnover than non-associated enzymes. | |
| Archaeosomes | Archaeosomes are a combination of archaea and liposomes. Archaeosomes as liposomes made with one or more ether lipids that are unique to the domain of Archaeobacteria found in Archaea constitute a novel family of the liposome. Achaean-type lipids consist of archaeol (diether) and/or caldarchaeol (tetraether) core structures. | |
| Exosomes | Exosomes are a type of extracellular vesicle that contain constituents (Protein, DNA, and RNA) of the cells that secrete them. They are taken up by distant cells, where they can affect cell function and behavior. Exosomes are membrane-bound extracellular vesicles that are produced in the endosomal compartment of most eukaryotic cells. | |
| Phytosomes | Phytosomes arealso called Phyto-phospholipid complexe. The term “Phyto” means plant, and “some” means cell-like. Phytosomes are one of the lipid-based vesicular delivery systems which can be used for encapsulation of drugs and plant-derived nutraceuticals such as polyphenolic compounds. A phytosome is a complex made between herbal extracts and dietary phospholipid and shows improved bioavailability of phytoconstituents. | |
| Aspasomes | Aspasomes are multilayered vesicles formed by amphiphiles molecules, Ascorbyl palmitate (ASP), in combination with cholesterol and charged lipids for drug encapsulation. |
Sr.no. |
Vesicles | Description |
|---|---|---|
| Hemosomes | Haem means hemoglobin and some means cell-like. Hemoglobin, also spelled as hemoglobin, iron-containing protein in the red blood cells of many animals. Encapsulation of haem or hemoglobin within lipid vesicles or liposomes is called “Hemosomes” or is also called Liposome encapsulated hemoglobin. | |
| Ultrasomes | Ultrasomes are UV-endonuclease enzymes encapsulated in multilamellar liposomes. The endonuclease is prepared from Micrococcus luteus. Ultrasomes are the advanced type of lipid particles that acts as a hybrid system between lipid nanoparticles and oil-in-water (o/w) emulsions. | |
| Ufasomes | The formations of fatty acid vesicles are named "ufasomes," Ufosomes are unsaturated fatty acid liposomes. Fatty acid vesicles are colloidal suspensions of closed lipid bilayers that are composed of fatty acids and their ionized species (soap). | |
| Marinosomes | Marinosomes are liposomes based on a natural marine lipid extract containing a high polyunsaturated fatty acid (PUFA) ratio. | |
| Subtilosomes | Subtilosomes are liposomes made from phospholipids separated from Bacillus subtilis (B. subtilis), a Gram-positive, catalase-positive bacterium. | |
| Proniosomes | Proniosomes are a novel vesicular system for transdermal and topical delivery of drugs. Proniosomes are a dry formulation of water-soluble carrier particles that are coated with surfactants. | |
| Carbohydrosomes | Carbohydrosome is also known as a carbohydrate-based liposome. Carbohydrosome is a new lipid-based vesicular system with 3-dimensional structures formed from zwitterionic, cationic, or anionic carbohydrate-based lipid molecules. | |
| Immunoliposomes | Immunoliposomes combine antibody-mediated tumor recognition with liposomal delivery which are designed for target cell internalization and intracellular drug release. Immunoliposomes are generated by the coupling of antibodies to the liposomal surface and allow for an active tissue targeting through binding to tumor cell-specific receptors. |
Liposomes
Composition: Phospholipids, Cholesterol
Description: A liposome is a spherical vesicle having at least one lipid bilayer. It can be used as a drug delivery vehicle for the administration of nutrients and pharmaceutical drugs.
Applications: Liposomes are one of the unique drug delivery systems, which use in controlling and targeting drug delivery [7]. They are administrated orally, parenteral, and topically, sustained-release formulations, and carriers in gene delivery of various drugs. Nowadays, liposomes are used as versatile carriers for the targeted delivery of the drug [8].
Niosomes
Composition: Non-ionic surfactant (Span 20,40,60,80) (Tween 20,40,60,80) (Brij 30,52,72, 76,78).
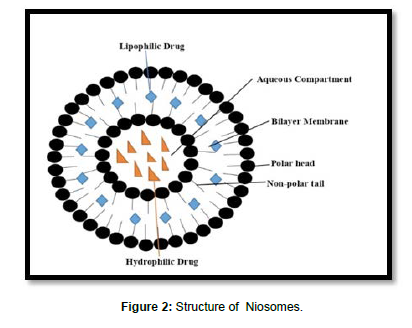
Description: Niosomes are non-ionic surfactant-based unilamellar or multilamellar bilayer vesicles upon hydration of non-ionic surfactants with or without incorporation of cholesterol [9]. They are biodegradable, biocompatible, do not require any special storage conditions, and are available at low cost [10].
Structure: Structure of Niosomes as shown in Figure 2. (Figure 2)
Applications: Controlled release formulations are often prepared to permit the establishment and maintenance of any concentration at the target site for a longer period. They are administrated orally, parenterally, and topically, controlled release formulations [11, 12].
Vesicles Loaded Dosage Forms
The vesicular drug delivery system of site-specific targeting of drugs by loading vesicles into suitable formulations. Drugs can be directly targeted to a specific site of action to prevent toxic and undesired effects on other sites. The vesicles are loaded into different formulations such as patches, films, gels, suspension, ocular formulations, and cosmetic formulations [13].
Conclusion
The vesicular drug delivery system has lots of advantages over conventional drug delivery systems because of the vesicular drug delivery system of site-specific targeting of drugs. Drugs can be directly targeted to a specific site of action to prevent toxic and undesired effects on other sites. A vesicular drug delivery system can be used to enhance the bioavailability of poorly absorbed drugs, reduce the dose of drug administered, reduces the cost of therapy by improving the bioavailability of medication, and enhance the pharmacological action of the drug. In recent, the Nikko Chemicals launched a Nikkosome®.
Nikkosome® PV-PF is the natural vesicles forming agent with the excellent promotion of transdermal absorption efficacy.
References
- Jhawat V, Pandit A, Chandrasekar B, (2013) Vesicular Drug Delivery Systems: A Novel Approach for Drug Targeting Related Papers Biomimet Ic Lipid-Based Nanosyst Ems for Enhanced Dermal Delivery of Drugs and Bioact Ive A… Vesicular Drug Delivery Systems: A Novel Approach for Drug Targeting. Int J Drug Deliv 5: 975-1215
- Rao BN, Reddy KR, Mounika B, Fathima SR, Tejaswini A (2019) Vesicular Drug Delivery System: A Review. Int J Chemtech Res 12:39-53.
- Ashara K, Paun JS, Soniwala MM, Chavda JR, Nathawani SV, et al. (2014) vesicular drug delivery system: a novel approach. Mintage j pharm med sci 3: 2320-3315.
- Bansal S, Prasad Kashyap C, Aggarwal G, Harikumar S (2012) A comparative review on vesicular drug delivery system and stability issues. Int J Res Pharm chem 2: 2231-2781.
- Bilia A, Bergonzi M, Boulos J, Efferth T (2020) Nanocarriers to enhance solubility, bioavailability, and efficacy of artemisinins. World J Tradit Chin Med 6:26-38.
- Sreekanth N, Bhargavi S, Padmavati S, Visvavidyalayam M (2013) Vesicular Drug Delivery System-An Over View Validated High Performance Thin Layer Chromatography Method for Simultaneous Estimation of Rofecoxib and Tizanidine Hydrochloride in Pure and Tablet Dosage Forms View Project Ntitumor and Cytotoxic Effects of Phyllanthus Polyphyllus on Ehrlich Ascites Carcinoma and Human Cancer Cell Lines View Project.
- Farooque F, Wasi M, Mughees MM (2021) Liposomes as Drug Delivery System: An Updated Review. J drug deliv ther 11:149-158.
- Shaker S, Rifaat Gardouh A, Ghorab MM (2017) Factors Affecting Liposomes Particle Size Prepared by Ethanol Injection Method. Res Pharm Sci 12:346-352.
- Rathi JC, Tamizharasi S, Dubey A, Rathi V (2009) Development and characterization of niosomal drug delivery of gliclazide. J Young Pharm 1:205.
- Bansal S, Aggarwal G, Chandel P, Harikumar SL (2013) Design and development of cefdinir niosomes for oral delivery. J Pharm Bioallied Sci 5:318-325.
- Maurya K, Singh A, Pandey S, Kumar N, Siddhiqui MA (2021) Niosomes: Classification, Preparation And Application. Int j indig herbs drugs 6:29-32.
- Parmar AM, Sukumaran B (2018) Niosomes as Transdermal Drug Delivery System. Biomed Res J 5: 54-63.
- Prabhu P, Koland M, Vijaynarayan K, Kumar RN, Harish NM, et al. (2010) Preparation And Evaluation Of Niosomes Of Brimonidine Tartrate As Ocular Drug Delivery System. J Young Pharm 2: 356–361.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Khillari GM, Parande BS, Dongaonkar CC, Humbad DN (2022) A Novel Approach: Vesicular Drug Delivery System. J Anal Bioanal Tech 10: 468. DOI: 10.4172/2155-9872.1000468
Copyright: © 2022 Khillari GM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 3439
- [From(publication date): 0-2022 - Nov 06, 2025]
- Breakdown by view type
- HTML page views: 2908
- PDF downloads: 531