A Systematic Review of the Effectiveness of Buprenorphine for Opioid Use Disorder Compared to Other Treatments: Implications for Research and Practice
Received: 21-Jan-2019 / Accepted Date: 29-Mar-2019 / Published Date: 05-Apr-2019 DOI: 10.4172/2155-6105.1000379
Abstract
Background: Prior systematic reviews have compared the relative effectiveness of buprenorphine (BUP), methadone (MET) and other medications and treatments for opioid use disorder (OUD). The results suggest BUP is highly effective for reducing illicit opioid use and retaining people in treatment. The current review extends these prior reviews by synthesizing research, which compares BUP and buprenorphine and naloxone (BUP/NX) to several treatments in addition to MET on several primary and secondary outcomes.
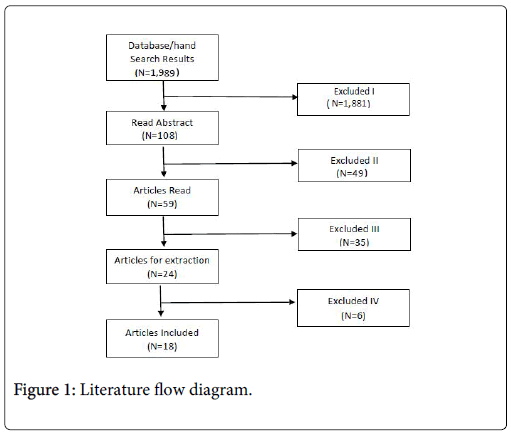
Method: Literature searches were conducted using nine databases. Articles were limited to quantitative reports of studies conducted with adult human subjects in an outpatient, non-residential treatment settings in the United States, in peer-reviewed journals between January 1, 2001 and May 31, 2017, and written in English. Search strategies returned 1,981 articles, an additional eight articles were added through hand searching. Ninety-nine articles met inclusion criteria. After reading abstracts, 48 articles were excluded from the review. After reading the remaining 59 articles, another 36 were excluded. A total of 18 studies were included in the final analyses.
Results: MET was found to be superior to buprenorphine (BUP) in helping patients adhere to and remain in treatment, while BUP was superior to MET for achieving abstinence from opioids. BUP was found to be superior to behavioral treatment alone, extended release naltrexone (XR-NTX), an absence of any treatment, and placebo. Given the range of study designs and quality, populations, and outcomes examined, a meta-analysis was not feasible. The heterogeneity of included studies, however, permitted close examination of both the benefits and barriers of medication treatment for OUD in a range of patient populations and clinical settings, as well as the identification of gaps in both the research and treatment of OUD across a body of available literature.
Conclusion: Buprenorphine (BUP) is an effective treatment option for achieving abstinence from opioids, and with emerging treatment guidelines, may be easier to access than other forms of treatment. The review underscores much of the available research utilized protocols that are inconsistent with current clinical practice guidelines. Further, flaws in research designs make it difficult for providers to determine the best medication treatment in order to improve outcomes. Future research is necessary to determine the effectiveness of BUP when administered According to the most current protocols.
Keywords: Systematic review; Buprenorphine; Methadone; Treatment; Research; Practice
Introduction
Over five million individuals in the United States (US) have opioid use disorder (OUD), a chronic illness with devastating consequences for individuals, their families, and society [1]. Of the 63,000 US drug overdose deaths in 2016, 42,000 were attributable to opioids [2,3]. OUD has also escalated the spread of infectious diseases due to highrisk behaviors such as sharing injection materials. For example, hepatitis C increased from 2004 to 2014 (400% among 18-29 year-olds and 325% among 30 to 39 year-olds) and hepatitis B also increased (20,000 new cases in the US among persons who inject drugs) [4]. Additionally, the rates of HIV, endocarditis, and abscesses have also increased among persons who inject drugs [5]. The economic burden of prescription opioid abuse and fatal overdoses among Americans was an estimated $78.5 billion in 2013; due primarily to healthcare expenditures (38%), non-fatal and fatal lost productivity costs (53%), criminal justice costs (10%) and substance treatment (4%) [6].
Treatment options for opioid use disorder (OUD) include medications and psychologically based approaches, used either alone or in combination with other strategies [7,8]. Three medications, which have FDA approval, are used to treat OUD: buprenorphine (BUP) alone and combined with naloxone (BUP/NX), methadone (MET), and naltrexone (NTX) [8-14]. Many substance abuse treatment providers recommend that psychologically based approaches such as individual, group, and family counseling, self-support groups and residential care be used in combination with medication for the treatment of OUD [12-14]. Opioid antagonists such as NTX prevent opioids from binding at the receptors, while opioid agonists (BUP and MET) act on opioid receptors and have been deemed to be safer and to reduce harmful behaviors associated with addiction when used as prescribed [12,13]. Finally, research findings suggest that all these medications are effective for improving engagement and adherence to treatment, reducing illicit drug use, improving brain function, and overall health [14].
Prior systematic review has compared the relative effectiveness of BUP compared to other medications and treatments for helping patients abstain from opioids and remain in treatment. Ling et al. [15] compared BUP versus counseling on abstinence from illicit opioids, and Mattick et al. [16] compared the effectiveness of BUP versus MET maintenance and placebo on treatment retention and illicit opioid use. Similarly, Timko et al. [17] compared BUP versus MET, an absence of medication treatment, counseling and placebo on retention in treatment. All three reviews confirmed that BUP in high doses is highly effective for OUD reducing illicit opioid use, and retaining people in treatment.
The current review extends these prior reviews by synthesizing research, which compares BUP and BUP/NX to several treatments in addition to MET. These include levo-alpha acetyl methadol (LAAM), extended-release naltrexone (XR-NTX)}, behavioral approaches (BA), placebo, BUP as a maintenance therapy versus detoxification, and an absence of treatment. Additionally, a wide range of primary outcomes (abstinence from illicit opioids and other substances, treatment adherence, retention, and duration) and secondary outcomes (withdrawal symptoms, treatment safety, high-risk sex and needlesharing practices, criminality, employment, treatment cost, and utilization), are evaluated.
Given the range of study designs and quality, populations, and outcomes included, a meta-analysis was not feasible. The heterogeneity of included studies, and lack of emphasis on specific level of evidence, permitted an examination of both the benefits and barriers of MT in a range of patient populations and clinical settings, as well as the identification of gaps in present research on OUD across a body of available literature.
Method
Literature search
Strategy: Literature searches were conducted using nine databases, which included Cochrane Clinical Trials, Cumulative Index of Nursing and Allied Health Literature (CINAHL) Complete, Ovid, ProQuest, PsycArticles, Psych Info, PubMed, PubMed Central, and Web of Science. Additional articles were identified from reference lists of included studies.
A typical search string in each of the databases was:
((effect* OR efficac* OR treat* OR interven*) AND (opiate OR opioid OR heroin) AND (depend* OR addict* OR abuse OR use disorder) AND (buprenor* OR suboxone) NOT pregnan* NOT pain) NOT cancer) NOT smok*) NOT (cocai* OR crack OR stimulant)).
Eligibility criteria: Articles were limited to quantitative reports of research studies conducted with adult human subjects in an outpatient, non-residential treatment setting in the United States, published in peer-reviewed publications between January 1, 2001 and May 31, 2017, and written in English. Given the aim of examining treatment rather than tapering or “detox” with buprenorphine (BUP), only studies that administered BUP for four weeks or longer were included. Studies comparing short (detox) versus longer (maintenance) courses of BUP were only included if there were long-term outcomes with which to compare the effects of BUP versus another treatment.
Exclusion criteria: Articles were excluded if samples were comprised primarily of minors, pregnant women, subjects who were incarcerated or recently incarcerated, had severe co-occurring mental illness (e.g., schizophrenia), or who treated for a disorder other than opioid use disorder (OUD) as the primary outcome of the study. Articles were also excluded if the focus was on provider knowledge or attitudes about medication assisted treatment (MAT) or patients with substance use disorder (SUD) or on patient characteristics (e.g., gender) or attitudes as the primary predictor of treatment success. Articles that were editorial or commentary or focused on scale development, medication pharmacokinetics, or educational interventions to improve patient or provider knowledge or attitudes about MAT were also excluded. The protocol was not formally registered. Study selection. Two authors (TM and JB) conducted the literatures searches and independently coded articles for inclusion or exclusion. A third author, (LC) compared the codes for inclusion and exclusion and retained all items that were agreed upon by the first two authors. In the event of a disagreement, the item was reviewed (LC) and a determination made as to whether the item met inclusion criteria and decided whether it met inclusion criteria. A fourth author (AW) resolved differences as to whether to exclude studies from the final inclusion list. Once the initial list was agreed upon and data extraction began, any decision about whether a study should be excluded was determined by consensus.
Data collection: Two authors independently assessed the quality of the studies (high, good, or poor/major flaws) utilizing the Johns Hopkins Nursing Evidence-Based Practice Rating Scale [18]. Another individual (AC) compared the quality ratings, and discrepancies were discussed until consensus was reached. Another author (AW) made two final determinations. Data on the population, methods, treatment protocol, operationalization of concepts (e.g. treatment success), and study results were extracted by three of the authors (TM, JB, AC).
Data extraction: Data were extracted from each of the included studies based upon a priori criteria determined by four of the authors (TM, JB, LC, and AW). Study authors were also contacted for additional information or clarification.
Variables: Data items included funding source, study type and duration, population, sample, methods, design, analysis, outcomes, strengths, limitations, and risk of bias. Study type and duration included: length of study, length of follow-up period, and years in which the data were collected. Sample description data included sample size, mean age, gender, race, and whether significant differences exist between study groups on demographic information. Study methods data extracted included recruitment, study settings, measures and measure reliability, type of laboratory test used to detect substances, whether urine samples were observed, and type of buprenorphine (BUP) and comparison treatment. Study protocol considerations included whether individual, group counseling, and 12- Step program meetings were required; the use of adjunctive treatment for breakthrough symptoms; individualization of treatment; comparison treatments; and treatment medication information (mean dose, frequency of administration, number of weeks received, taper start, length, and taper duration).
Data were extracted on outcomes related to abstinence from opiates and other substances, relapse with opiates and other substance; treatment length; adherence; retention; Human Immunodeficiency Virus (HIV) and hepatitis C virus (HCV) risk behaviors; medication safety and side effects; cravings for substances and symptom management; use and cost of substance abuse treatment; use and cost of other health services; physical and mental health; quality of life; and functional outcomes such as employment and legal involvement. Study analysis data included handling of missing lab samples and other data and year of US dollar value utilized in reports, where applicable. When specifically noted by study authors, operationalization of variables such as treatment compliance, treatment retention, and treatment success were recorded, as was US dollar value of any financial analyses.
Data analyses: Data were evaluated descriptively as not enough studies comparing the same treatments and outcomes were identified that would allow for a formal meta-analysis.
Results
Study selection
The search strategies returned 1,981 articles, and an additional eight articles were added through hand searching of references. A total of 18 studies were included in the final analyses. Inter-rater agreement on whether studies met inclusion criteria was 92.4%. Figure 1 shows the literature flow for the articles in this study.
As demonstrated in Figure 1, approximately 99% of the articles identified in our searches were excluded. Nearly a quarter of the studies that could have been included ultimately had to be excluded because it was not possible to determine the effect of compared to another treatment on the outcomes of interest. These included: the outcome of being in treatment considered being in any treatment compared to no treatment at all [19-22]; the study compared buprenorphine (BUP) to BUP plus other treatments, such as an additional medication [23], or different types of counseling [15,24,25]; different lengths of BUP taper as the treatment groups [26-30]; and not reporting BUP data [31,32]. A detailed list of all studies excluded is provided in Table 1.
| N | % | |
|---|---|---|
| Included | 18 | 0.9 |
| Not a research study (e.g., policy, educational) | 291 | 14.6 |
| Animal studies/studies not focused on OUD | 30 | 1.5 |
| Qualitative studies | 44 | 2.2 |
| No comparison to buprenorphine | 384 | 19.3 |
| Detox only | 48 | 2.4 |
| Predicting attitudes about addiction/MAT | 45 | 2.3 |
| Predicting patient/provider characteristics | 144 | 7.2 |
| Primary population comorbid major disorder | 2 | 0.1 |
| Primary population is incarcerated/recently released | 53 | 2.7 |
| Primary population is pregnant women | 21 | 1.1 |
| Primary population is youth | 31 | 1.6 |
| Focus is pharmacokinetics of medications | 142 | 7.1 |
| Scale/instrument development | 9 | 0.5 |
| Educational Interventions for providers | 19 | 1.0 |
| Duplicate study | 189 | 9.5 |
| Not conducted in US | 519 | 26.0 |
| Total | 1989 |
Table 1: Description of excluded studies.
Study quality
The majority of the included studies were rated as having “good” quality (n=12). One study was rated as high quality, and five studies were rated to be of poor quality or had major flaws.
Study characteristics
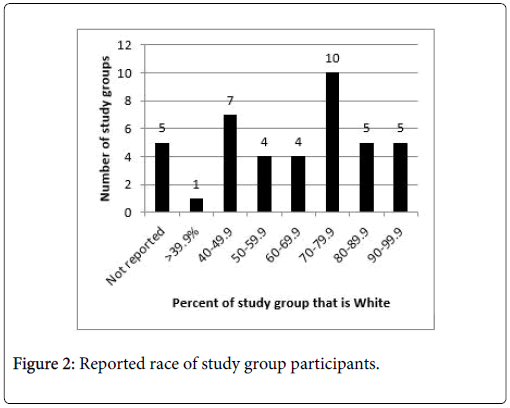
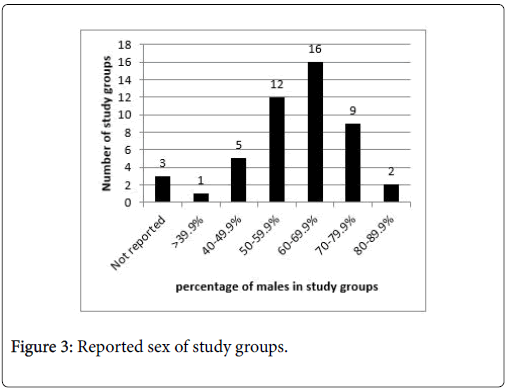
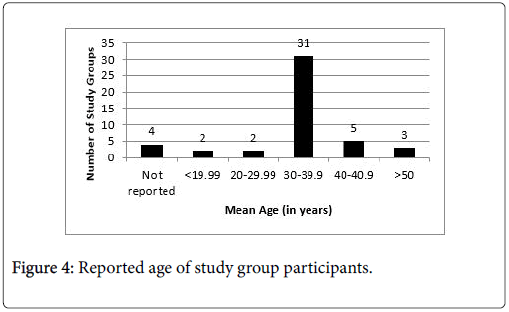
Study sample sizes for experimental studies ranged from 152 to 287. Samples from analyses of medical record data or insurance claims were larger and ranged from 56 to 56,278 patients (Table 1 highlights study characteristics). Samples included subjects that were predominantly white, male, and in their mid-30 s. Figures 2-4 show the race, sex, and mean age of each study group. Studies frequently (n=11) reported significant differences between study groups on major characteristics (race, ethnicity, insurance status, use history, etc.) [Table 2].
| N | % | |
|---|---|---|
| Funding source | ||
| Government (e.g., NIH)a | 6 | 33.3 |
| Multiple (including pharmaceutical) | 4 | 22.3 |
| Multiple (no pharmaceutical) | 2 | 11.1 |
| Pharmaceutical Industry | 2 | 11.1 |
| Private/Internal | 2 | 11.1 |
| None | 2 | 11.1 |
| Study type | ||
| Effectiveness | 8 | 44.4 |
| Efficacy | 5 | 27.8 |
| Cost Effectiveness | 3 | 16.7 |
| Safety | 2 | 11.1 |
| Data source | ||
| Randomized Controlled Trial | 6 | 33.3 |
| Medical Record/Charts | 6 | 33.3 |
| Secondary Analysis | 6 | 33.3 |
| Number of comparison treatments | ||
| Compared to 1 med/treatment | 8 | 44.4 |
| Compared to 2 meds/treatments | 6 | 33.3 |
| Compared to 3 meds/treatments | 4 | 22.3 |
| Comparison treatments/groupsb | ||
| Methadone (any) | 9 | 50.0 |
| Location (office/clinic) | 1 | 11.1 |
| Specific doses | 3 | 16.6 |
| Naltrexone (incl. XR-NTX) | 1 | 11.1 |
| LAAM | 2 | 22.3 |
| Placebo | 2 | 22.3 |
| BUP detox | 2 | 22.3 |
| BUP/NX (sublingual) | 2 | 22.3 |
| Naltrexone (XR-NTX) | 1 | 11.1 |
| No treatment | 1 | 11.1 |
| Behavioral only treatment | 1 | 11.1 |
| Patients without OUD | 1 | 11.1 |
| Study protocols | ||
| Included urine drug screen (UDS) | 11 | 61.1 |
| Required counseling (any) | 61.1 | |
| Reduced frequency visits over time | 11 | 16.7 |
| Different treatment protocols between groups | 27.8 | |
| Handling of missing UDS data | ||
| Treated as positive | 5 | 27.8 |
| Not reported | 6 | 33.3 |
aOne study with government funding only had authors affiliated with pharmaceutical company.
bStudies may fall in more than one category.
Table 2: Study characteristics.
Data sources and study locations
Six studies used retrospective chart review of data from primary or secondary sources, and six studies conducted secondary analyses from insurance, episode treatment, WHO and FDA data. Six additional studies presented primary or secondary data from experimental designs, most of which were from randomized controlled trials ([RCTs] n=6). Two of the included studies [33,34], evaluated different outcomes on the same sample. Lott et al. [35] conducted a secondary analysis of a study by Johnson et al. [36] which did not include patients who were on low-dose methadone and then ‘rescued’ with higher doses in their analyses.
Data were primarily from one or more outpatient addiction treatment settings including methadone clinics (n=6) or office-based methadone programs (n=1), youth specific treatment programs (n=2), or intensive outpatient program ([IOP] n=1). Data were also collected from primary care (n=3), federally licensed opioid treatment programs (n=1), state-subsidized treatment programs (n=1), FDA reports (n=1), and the Veteran’s Health Administration ([VHA] n=1).
Study protocols
More than half of the studies (n=11) include urine drug screen (UDS) results as at least one of their measures (Table 3). Some reported using random drug screens (n=6) and some used observed urine drug screens (n=6). Five studies reported temperature of urine when subjects were not observed providing samples.
| Differences in treatment protocols | |
|---|---|
| Random drug screens used | 6 |
| Both/all groups | 4 |
| Comparison group Only | 2 |
| Observed drug screens used | 6 |
| Both/All groups | 3 |
| Comparison group Only | 3 |
| Required group counseling | 5 |
| Both/All groups | 3 |
| Comparison group only | 2 |
| Required individual counseling | 9 |
| Both/All groups | 7 |
| Comparison group only | 2 |
| Required 12-step attendance | 1 |
| Comparison group only | 1 |
| Reasons for study withdrawal | |
| Missing counseling | 3 |
| Missing meds/appts | 2 |
| Missing UDS | 2 |
| Provider discretion | 1 |
Table 3: Differences in treatment protocols.
Counseling
Half of the studies (n=9) required individual counseling of at least some study participants, fewer (n=5) required group counseling for at least some study participants. In one study, participants’ medication was withheld until participants attended counseling [37]. In three studies, participants who struggled with continued opioid use received additional counseling [33,34,38]. One study required subjects who received MET to attend a 12-Step program as part of the treatment protocol [34]. Participants in one study that received medication also received psychosocial treatment; it is unclear if the psychosocial treatment was required [39].
Medication dosing
The majority of the studies required frequent visits to the study site to obtain treatment. Kamien et al. [37] required all participants to attend seven days a week. Two studies required daily attendance except for Sundays and holidays [40,41], and another required attendance five to seven days a week [38]. Participants in an adult intensive outpatient program (IOP) attended sessions for three hours a day, four days a week [42]. In another study following daily visits for medication induction, participants had to come to the site Monday, Wednesday, and Friday [35,36]. Adolescent IOP patients could earn up to one week’s supply of medication as they progressed in treatment [43]. Three of the retrospective chart reviews reported patients who received BUP were seen weekly and MET patients were seen daily or near daily [33,44,45]. Five studies reported different protocols for different study groups, generally with less strict requirements, (e.g., no counseling, less frequent visits to receive medication, fewer urine drug screens) for subjects receiving buprenorphine.
Study outcomes
Study outcomes were grouped into primary and secondary outcomes. Treatment adherence, retention, abstinence from substances, and medication safety were considered primary outcomes. The most common study outcome was relapse with opiates (n=12) or other substances (n=9). Studies also evaluated length of time in treatment (n=7), treatment adherence (n=6) continuing treatment after the study ended (n=3), and medication safety (n=2). Secondary outcomes considered included: cost of substance abuse treatment (n=3), all medical treatment (n=3), crime and arrests (n=3), HIV and Hepatitis C risk reduction (n=2), symptom management (n=2), employment (n=1), cost of mental health treatment (n=1) and treatment utilization (n=1).
Risk of bias with within studies: Six of the studies received some or all of their funding from the pharmaceutical industry [38-40,46-48]; and another study that did not receive pharmaceutical funding included authors who were affiliated with a pharmaceutical company [37]. Response bias is present in two studies [38,41], which relied only on self-reported data for some or all of the outcomes (including recent use of substances, criminality, etc.). Self-selection bias is present in three studies [33,43,49], as they allowed clients to choose their own treatment.
In the sections that follows, the results are presented based on the treatment that buprenorphine is being compared to: MET, levo-alpha acetyl methadol (LAAM), naltrexone (NTX), and other treatments (e.g., counseling only, placebo). A summary of these findings is presented in Table 4.
| First author (year) | Study design | Primary outcome | Secondary outcomes | Protocol Problems/Bias | Overall finding | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | Setting | Req Couns. | Fundb | TX Adhere | TX Retent | TX Length | Abstain from Opiates | Abstain from other | Safety | HIV/HCV risk | TX Cost/Util | |||||
| N | Treatment groups | |||||||||||||||
| Buprenorphinea vs. Other treatments | ||||||||||||||||
| BUP/NX vs. MET | ||||||||||||||||
| Johnson (2000) | S220 | BUP (55) | Outpatient TX | Y | G | NS | NS | BOTH | BOTH | - | NS | - | - | Small/unmatched samples; generalizability | Both | |
| MET dose (55) | ||||||||||||||||
| MET ¯dose (55) | ||||||||||||||||
| Lott (2006) | 137 | BUP (47) | Secondary analysis | - | - | - | NS | NS | - | NS | - | Small samples; generalizability | NS | |||
| MET (51) | ||||||||||||||||
| Kamien (2008) | 268 | BUP (140) | Outpatient TX | Y | G | NS | NS | NS | BUP | NS | - | - | - | Potential conflict of interest bias; unequal sample sizes; generalizability | BUP | |
| MET (128) | ||||||||||||||||
| Barnett (2009) | 8,673 | Only BUP (482) | Secondary analysis of VA EMR | G | - | - | BUP | - | - | BUP | - | BUP | Generalizability | BUP | ||
| Only MET (8191) | ||||||||||||||||
| Jones (2009) | 78 | BUP OBT (34) | Office & Clinic | Y | - | NS | - | - | NS | - | - | MET | Small and unmatched samples; generalizability | NS Primary | ||
| MET OBT (21) | ||||||||||||||||
| MET clinic (23) | MET Secondary | |||||||||||||||
| Fingerhood (2014) | 504 | BUP OBT (252) | Office & Clinic | Y | MET | MET | MET | MET | - | - | - | - | Unmatched samples; generalizability | MET | ||
| MET clinic (252) | (MET) | |||||||||||||||
| Hser (2014) | 1267 | BUP (738) | Secondary analysis | N | MET | MET | MET | BUP | - | - | - | - | Generalizability; inadequate treatment protocol info | Both | ||
| MET (529) | ||||||||||||||||
| Proctor (2014) | BUP (102) | Retrospective review; Outpatient TX | NR | I | - | MET | MET | NS | NS | - | - | - | Inadequate treatment protocol info | MET | ||
| MET (2,738) | ||||||||||||||||
| Woody (2014) | 731 | BUP (340) | Secondary analysis | NR | G | - | MET | - | BOTH | MET | - | BOTH | - | Generalizability; inadequate treatment protocol info | Both | |
| MET (391) | Methadone clinic | |||||||||||||||
| Clark (2015) | 43,175 | BUP (18,866) | Secondary analysis | NA | I | - | - | BOTH | - | - | - | - | BOTH | Generalizability | Both | |
| MET (24,309) | Medicaid claims | |||||||||||||||
| Rastegar (2016) | 504 | BUP (252) | Retrospective chart Review | Y (MET) | N | - | NS | - | - | - | - | - | - | Generalizability | NS | |
| MET (252) | Office & clinic | |||||||||||||||
| Sessler (2017) | 60,179 | BUP trans (22,454) | Secondary analysis FDA database | NA | I | - | - | - | - | - | BUP | - | - | Generalizability | BUP | |
| BUP/NX (11,206) | ||||||||||||||||
| MET (26,519) | ||||||||||||||||
| Johnson (2000) | 220 | BUP (55) | Outpatient TX | Y | G | - | NS | NS | BOTH | BOTH | NS | - | - | Small/unmatched samples; generalizability | Both | |
| LAAM (55) | ||||||||||||||||
| Lott (2006) | 86 | BUP (47) | Secondary analysis | Y | G | - | - | - | NS | NS | NS | Small samples; generalizability | NS | |||
| LAAM (39) | ||||||||||||||||
| BUP/NX vs. XR-NTX | ||||||||||||||||
| Crits-Christoph (2016) | 548 | BUP (394) | Outpatient TX | N | MI | BUP | BUP | - | - | - | - | - | - | Potential COI bias; unequal sample sizes | BUP | |
| XR-NTX (154) | ||||||||||||||||
| Vo (2016) | 56 | BUP (43) | Community-based IOP | Y | S | NS | NS | - | NS | NS | - | - | - | Small/unequal sample sizes; generalizability | NS | |
| XR-NTX (13) | ||||||||||||||||
| BUP/NX vs. BA treatment only | ||||||||||||||||
| Clark (2015) | 50,086 | BUP (18,866) | Secondary analysis of medicaid claims | NA | I | - | - | - | BUP | - | - | - | BUP | Generalizability | BUP | |
| BA Only (31,220) | ||||||||||||||||
| Buprenorphine vs. No buprenorphine | ||||||||||||||||
| BUP/NX implant vs Placebo | ||||||||||||||||
| Ling (2010) | 163 | Implant (108) | Multiple outpatient | Y | I | BUP | BUP | - | BUP | BUP | - | - | - | Unequal sample sizes; generalizability | BUP | |
| Placebo (55) | ||||||||||||||||
| Rosenthal (2013) | 168 | Implant (114) | Outpatient TX | Y | MO* | - | BUP | - | BUP | - | - | - | - | Unequal sample sizes; generalizability | BUP | |
| Placebo (54) | ||||||||||||||||
| BUP/NX maintenance vs. BUP/NX detox | ||||||||||||||||
| Caldiero (2006) | 60 | Maint (30) | Retrospective chart review; Hospital-based IOP | Y | MO | Maint | Maint | Maint | NS | NS | - | - | - | Small sample; generalizability; potential bias | Maint | |
| Detox (30) | ||||||||||||||||
| Woody (2008) | 152 | Maint (74) | Outpatient TX (adol) | Y | MI | Maint | Maint | - | Maint | NS | - | - | - | Generalizability | Maint | |
| Detox (78) | ||||||||||||||||
| BUP/NX vs. no treatment | ||||||||||||||||
| Barnett (2009) | 19,642 | Only BUP (482) | Secondary analysis of VA EMR | NA | G | - | - | BUP | - | - | BUP | - | BUP | Generalizability | BUP | |
| No TX (19,160) | ||||||||||||||||
| Buprenorphine forms & doses compared | ||||||||||||||||
| BUP/NX vs. BUP | ||||||||||||||||
| Proctor (2014) | 495 | BUP (393) BUP/NX (102) | Retrospective chart review; Outpatient TX | NR | I | - | BUP | BUP | NS | NS | - | - | - | Inadequate treatment protocol info | BUP | |
| BUP/NX 16 mg/day vs. BUP/NX 8 mg/day | ||||||||||||||||
| BUP/NX 16 mg/day vs. BUP/NX 8 mg/day | ||||||||||||||||
| Kamien (2008) | 140 | BUP 8/2 (82) | Outpatient TX | Y | G* | - | - | - | 16 mg | - | - | - | - | Potential conflict of interest bias; unequal sample sizes; generalizability | 16 mg | |
| BUP 16/4 (58) | ||||||||||||||||
aExcept where noted, BUP and BUP/NX refer to sublingual forms
bFunding: F: Foundation; G: Government, I: Industry, MI: Multiple, incl. industry, MO: Multiple, No industry, N: None, NR: Not Reported, S: Internal
*Not funded by pharmaceutical industry, but PI or author is affiliated with pharmaceutical industry
Abbreviations: Adhere: Adherence; BUP: Buprenorphine; BUP/NX: Buprenorphine/Naloxone; detox: Short:term treatment with buprenorphine; HCV: Hepatitis C Virus; HIV: Human Immunodeficiency Virus; I: Implant; IOP: Intensive Outpatient Program, LAAM: Levo Alpha Acetyl Methadol; Maint: Buprenorphine maintenance treatment; MET: Methadone; NA: Not Applicable; NTX: Naltrexone; NR: Not Reported; NS: Non Significant findings; Retent: Retention; Subs: Substances; Sx: Symptoms; TX: Treatment; Util: Utilization; XR-NTX: Extended release naltrexone; BOTH-BUP & Comparison treatment
Table 4: Primary and secondary outcomes and favored treatment by comparison treatment.
Methadone
Twelve studies compared the relative effectiveness of buprenorphine (BUP) versus MET and mainly examined primary outcomes. Of these, ten studies examined their relative effectiveness on treatment adherence and retention; seven of the twelve examined their effectiveness in helping subjects remain abstinent from opioids. Several studies examined secondary outcomes such as medication safety and treatment costs. Overall, MET performed better than BUP on both treatment adherence and retention, while BUP was superior than MET in assisting subjects to abstain from opioids.
Two studies reported MET outperformed BUP in helping subjects adhere to treatment [33,40] and four studies that compared treatment retention also found MET was superior [33,40,41,49]. On the outcome of treatment length, one study found BUP to perform better [50], while two studies found the medications comparably effective [36,41], and three others found that MET outperformed BUP [33,40,49].
One study found MET to be better in preventing subsequent opioid use [33], two studies reported that BUP was superior to MET [37,40], and two others found BUP and MET to be comparable [36,41]. BUP was also found to be superior to MET on the outcome of medication safety in two studies [48,50], while no studies reported MET to be safer than BUP. Several studies reported no significant differences between MET and BUP on treatment adherence [36,37], treatment retention [34,36,37,45], abstinence from opioids [35,49], and abstinence from other drugs [35,37,45,49].
Medication safety and symptom management: Among patients with opioid use disorder (OUD) who were treated at the Veterans Health Administration (VHA) over the course of one year, significantly fewer patients on buprenorphine (BUP) died in the study period compared to patients receiving methadone (MET) or no treatment at all [50]. Another study compared adverse events for BUP and MET reported in the Food and Drug Administration Database and identified disproportionately higher rates of broad and narrow cardiac arrhythmia among patients on MET compared to all forms of BUP [48]. Johnson et al. [36] compared low- dose BUP (20 mg) and a variable higher dose (60 mg-100 mg) MET and found subjects who received either BUP or variable higher dose MET rated the severity of their symptoms significantly lower than patients who received lowdose MET. They found no significant differences in symptom severity between BUP and high dose MET treatment groups.
Secondary outcomes: In a retrospective chart review comparing patients enrolled in methadone (MET) maintenance compared to buprenorphine (BUP), only MET patients reported a significant decline in number of criminal cases, including drug charges, at 12 and 24 months of treatment, both treatment groups, however, had significantly reduced odds of criminal charges at 12 months [51].
HIV and hepatitis C risk behaviors: Two studies evaluated whether BUP or MET reduced high-risk behaviors associated with HIV and Hepatitis C infection. Woody et al. [41] found injection risk behaviors were significantly reduced among females in both treatment groups; however, there was no significant differences between groups. Sexual risk behaviors were comparable and were significantly reduced among females in both treatment groups, and for males who received MET. Such behaviors were increased for males on BUP and reduced in males on MET [41]. In another study, patients receiving BUP and MET demonstrated a significant reduction in injection-related risks over time compared to baseline (time effect), but there was no significant difference between groups [35].
Treatment Cost and Utilization: Patients in the VHA system with opioid use disorder (OUD) who were prescribed buprenorphine (BUP) had significantly fewer ambulatory care visits after MAT initiation compared to patients who received methadone (MET) [50]. The cost of ambulatory care treatment was significantly reduced for patients on either BUP (18%) or MET (11.8%) [50]. Further, making BUP available through providers in the VHA system did not result in a significant increase in visits in the system overall [50]. A study of Medicaid claims also found that treatment with BUP costs significantly less than MET [44]. Additionally, the researchers found that providing either BUP or MET costs significantly less than behavioral-only (e.g. counseling) treatment [44]. In a comparison of the cost of MAT that considered both medication and location in which it was administered, MET administered in a clinic was least expensive and BUP was the most expensive for both providers and patients [45].
Levomethadyl acetate
Of the two studies which compared buprenorphine (BUP) to levoalpha acetyl methadol (LAAM), Johnson et al. [36] reported both medications were comparably effective for sustaining abstinence from opioids and other drugs, however, they found no significant differences in length of time in treatment, treatment retention and medication safety [36]. Lott et al. [35] reported no significant differences between the two medications in maintaining abstinence from opioids and other drugs, and for HIV/HCVI risk behaviors.
Naltrexone
In the two studies that compared buprenorphine (BUP) and extended release naltrexone (XR-NTX), Crits-Christoph et al. [39] reported BUP was more effective for treatment adherence and retention [39], however, Vo et al. [43] reported no significant differences between the two medications for treatment adherence, treatment retention, and remaining abstinent from opioids and other drugs. Subjects in the study by Crits-Christoph et al. [39], who received XR-NTX showed a significant increase in employment from baseline compared to BUP patients. Conversely, BUP patients had a significant decrease in employment at the end of the study compared to baseline [39]. Neither group reported significant changes in arrests or self-help group attendance over time [39].
Other treatments
Clark et al. [44], found buprenorphine (BUP) superior to counseling alone in maintaining abstinence from opioids, and for treatment cost and utilization. In studies that compared BUP and placebo, BUP was found more effective for maintaining treatment adherence and treatment retention [46,47], abstinence from opioids [46,47] and abstinence from other drugs [46]. Barnett [50] found that BUP was superior to an absence of any treatment for lengthening time in treatment, medication safety, and treatment cost and utilization. In a study that compared BUP alone versus BUP combined with naloxone, the results suggest BUP was more effective for improving treatment retention and lengthening time in treatment [49], however, no significant differences were found for remaining abstinent from opioids and other drugs [49].
In two studies, the findings suggest BUP administered as a maintenance medication was more effective than detoxification on several outcomes including treatment adherence [38,42], treatment retention [38,42], abstinence from opioids [42], and abstinence from other drugs [38]. In at least one study, no significant differences were noted in abstinence from opioids [42], and both studies found no significant difference between the two methods of delivery for sustaining abstinence from other drugs [38,42]. In a study that compared high dosages (16 mg or greater) versus low dosages (8 mg) of BUP, high dosages was found more effective for maintaining abstinence from opioids than low dosages [37].
Predictors of relapse
An analysis of six years of Medicaid claims for patients with opioid use disorder (OUD) demonstrated that the risk of relapse increased by 4.32 if patients had a co-occurring addiction to alcohol and 2.33 if patients were addicted to other substances [44]. Rastegar et al. [34] identified that being in any treatment at six months predicted being in MAT at twelve months. Longer periods of treatment with MAT (either MET or BUP) significantly reduced the risk of relapse [44].
Strengths of reviewed studies
Six of the included studies were RCTs, which in some instances used blind treatments [35-38,46,47], and six studies were secondary analyses of data collected in other studies, RCTs or databases [40,41,44,45,48,50]. Two studies focused on opioid use disorder (OUD) treatment among young adults [38,43], an age group that is disproportionately impacted by opioids, particularly prescription drug misuse [52], and is also underrepresented in research studies. Six additional studies were retrospective chart reviews of patients in treatment for opioid use disorder [33,34,39,42,43,49], and improved the quality of data by conducting rigorous analyses such as using only cases that had complete demographic information available [49]; matching patients for comparison on age and gender [42]; and including the first episode of treatment in their analyses [39]. Among other studies, additional strategies included triangulating data with other sources (e.g. billing claims) [44], including patients with OUD receiving no MAT when evaluating primary outcomes [39], and including patients without OUD in cost and utilization studies [50]. One design that included searching databases for reports of adverse events utilized standardized terminology in the queries [48].
Limitations of reviewed studies
Many of the studies had strict exclusion criteria, which limits the generalizability of the findings. For example, two studies did not include subjects with another substance disorder, including nicotine [38,46]. Ling et al. [46] also excluded participants who had moderatesevere withdrawal symptoms, which could overestimate the effectiveness of medications on symptom management. Another analysis used secondary data from a study that required participants to be stable on medication for 12 months prior to entering the study [45], in contrast to most other studies. Rastegar et al. [34] compared outcomes for patients who received treatment at two different programs. Two other samples that might limit generalizability of the findings based on the population studied are a sample in which 25-30% of each group had a history of anti-social personality disorder [35], and Medicaid patients receiving treatment in Massachusetts [44]. Some studies had small sample sizes and may have lacked statistical power to identify small effects [37,39,42,43,45]. One study using medical record data reported a great deal of missing or incomplete data, making it difficult to interpret results [39]. The measurement of outcomes was poorly done in some studies. For example, Woody et al. [38] relied on self-report only at 6, 9, and 12-month follow up from their study; response bias could be part of the reason they did not find significant differences between groups. In a few studies, treatment groups were not mutually exclusive, so patients may have received more than one type of MAT or other treatment in the time period being evaluated [44,50]. Key variables were not clearly identified in some studies such as duration of treatment with medication and frequency and duration of counseling [39]. Finally, many of the studies used protocols in which the delivery and requirements of care do not emulate current best practice models, such as requiring daily or neardaily dosing of medication with buprenorphine (BUP) [35,40,43]. Others mandated counseling [36-38,42,45,46], and some went so far as to withhold treatment for missed counseling [37], or withdraw clients from the study for missing counseling [38,46].
Discussion
The purpose of this review was to determine the effectiveness of buprenorphine (BUP) compared to other treatments on several primary and secondary outcomes for treatment of opioid use disorder (OUD). Overall, methadone (MET) performed better than BUP in helping subjects adhere to and remain in treatment, while BUP was found to be superior to MET in helping patients abstain from opioids. BUP was found to be superior to behavioral treatment alone, extended release naltrexone (XR-NTX), an absence of any treatment, and placebo. Given the range of study designs and quality, populations, and outcomes examined, a meta-analysis was not feasible. The heterogeneity of included studies, however, permitted close examination of both the benefits and barriers of MT for OUD in a range of patient populations and clinical settings, as well as the identification of gaps in both the research and treatment of OUD across a body of available literature. Following is a discussion of the results of this analysis.
Treatment cost and service utilization
Jones et al. [45] reported buprenorphine (BUP) and BUP/naloxone (NX) cost more than other medications, accounting for 77% of the cost associated with providing medication treatment. Cost of medications, especially BUP and BUP/NX, to providers and patients may be overestimated in the included studies as they were conducted before generic forms of BUP and BUP/NX were available. In addition, other studies highlighted that even when BUP cost more than the comparison treatment, the overall cost of providing healthcare to patients on BUP was significantly lowered as it reduces the number of ambulatory care and other visits required [44,50].
Criminal behavior
Though we excluded studies whose primary population was incarcerated or recently incarcerated individuals, Rastegar et al. [34] examined the association between treatment and criminal behavior and identified that methadone (MET) is better at reducing drugrelated arrests [34, 53]. One possible reason for this finding is that there were significantly more men than women in the buprenorphine (BUP) group, and men are more likely to have criminal issues and legal involvement related to OUD opioid use disorder (OUD) than women [26,53]. Arrest and formal charges may also be influenced by other factors (e.g., racism), and race of participants is not reported. Limited evidence with populations a history of criminal justice involvement suggests an increased willingness to use BUP over MET [54] and reduced rates of recidivism [55].
Unclear study outcomes and methodological challenges
For some of the outcomes of interest (e.g., relapse) results are less clear. Among the 12 studies that included relapse with opiates as an outcome, three favored buprenorphine (BUP), two favored the comparison treatment, four found no difference between groups, and three reported significant reduction in relapse for both BUP and at least one other comparison treatment. One possible explanation is that patient characteristics (e.g., use history, insurance status) varied widely within and between studies, as did study protocols. Similarly, strict definitions of relapse (e.g., seeking care in ER) [44] and permissive definitions of who is receiving medication (e.g. a single prescription for BUP) [33,34] could also influence results.
Both included and excluded studies had methodological challenges that make it difficult to answer our initial research question. For example, some studies excluded participants with co-occurring mental disorders, but evidence suggests that a high proportion of patients with OUD also have co-morbid conditions such as depression or are addicted to multiple substances [52]. It is widely known that cooccurring substance abuse disorder and other mental illnesses are known to increase risk of relapse and cost for treatment [44]. Many studies used protocols that are not consistent with current practice such as requiring daily or near-daily dosing of medications [40,41], administering different doses on different days [35,36], and higher than usual doses of methadone (MET) [35-37], which could lead to an overestimate of the effectiveness of comparison treatments (usually MET). Patients in standard outpatient BUP treatment are generally not be expected to have observed daily dosing of medication. As such, it would be understandable that a subject participating in a study examining treatment with BUP, may elect to seek treatment elsewhere if they are required to come to a clinic on a daily basis. A deviation from practice such as this, may explain a high attrition rate in subjects in receiving BUP, and therefore makes it difficult to assess which of the two medications would have improved retention rates.
Barriers to treatment
In our practice experience, many patients never return to office – based treatment after being prescribed buprenorphine (BUP). This may be due in part to requirements for frequent office visits or random drug screening which present a significant burden on patients in early recovery, making continued drug use easier than participation in care. Practice models that present significant barriers to treatment for many patients were frequently used in the included studies and may have influenced the outcomes as well.
Required counseling: In some of the studies that required counseling, missing counseling was the most common reason for people being withdrawn from the study [38]. Though counseling is a common addition to treatment programs, there are few if any studies to date that demonstrate that counseling is an essential component of outpatient opioid use disorder (OUD) treatment for all patients. In fact, the few studies that have examined the effects of types of counseling combined with BUP have identified few if any additional benefits [15,25,56,57]. Similarly, lower-barrier counseling options (e.g., phone-based) have had little impact on treatment outcomes, mostly due to patients’ lack of interest in participation. In one study, more than half of the intervention group didn’t complete a single phonebased counseling session [58]. Based on the lack of evidence in support of counseling, In fact, the American Society of Addiction Medicine [59] has recently updated their treatment guidelines to suggest counseling should not be mandatory; instead, patient care should be individualized and reassessed often to determine if any additional supports beyond medication may be necessary to continue treatment.
Subjects withdrawn from treatment: A significant concern about study approaches was the practice of withholding medication [37] or withdrawing patients from a study for noncompliance, or treatment ‘failure’ [46]. For example, participants whose withdrawal symptoms were not adequately reduced by treatment with a combination of either 1) buprenorphine (BUP) or placebo implant and 2) supplemental sublingual BUP were withdrawn from the study conducted by Ling et al. [46]. While such practices may make sense in a research setting, the approach of withholding treatment is not consistent with the treatment of a chronic, relapsing condition. Patients with OUD often require years of treatment before they stabilize and may require lifelong maintenance therapy to avoid relapse. Remaining in treatment and receiving either BUP or MET significantly reduces risk of mortality from overdose [60], suggesting a harm reduction approach is beneficial to patients [61]. Studies included in the review also highlighted significant benefits of actively receiving any treatment, including reductions in relapse, HIV and HCV risk behaviors, costs associated with all treatment (including substance abuse treatment), and number of arrests, as well as improved compliance, retention and predicted being in treatment in the future [34-37,44].
Expanding definitions of successful treatment
Initially, we set out to include any outcomes of treatment, with an interest in quality of life and other markers of recovery [62]. However, none of the included studies (and few of the studies excluded after reading) evaluated addiction treatment success beyond abstinence. A review focused on functional outcomes in medication assisted treatment published after our search was conducted and identified the existing literature was both sparse and of low or very low quality. There have been a growing number of calls for a different approach to the measurement of success in treatment. For example, SAMHSA’s Recovery Support Strategic Initiative [63] recognized the need for a new definition of recovery that includes four dimensions: 1) Health [being healthy physically and emotionally], 2) Home [having a stable place to live], 3) Purpose [having a purpose in life and the ability to participate in society], and 4) Community [maintaining relationships]. Similarly, Dupont [64] argues that patient outcomes worth considering include substance-related illness, injury, accidents, arrests, selfevaluation of recovery, employment and education status, and overall physical and mental health. The National Institute of Drug Abuse [65] recognized that use of opioids while in treatment is common, suggesting the need to consider opioid use disorder (OUD) treatment outcomes other than abstinence. Martin et al. [61] remind that shifting the focus to include other outcomes does not mean we should ignore intermittent or continued opioid use; instead, it can be seen as an opportunity to identify patient-centered augmentations to the treatment plan (e.g. family supervision of BUP administration) to support recovery
Suggestions for future research
The findings of the review and limitations of the included studies provide many questions to be answered in future research. First, given the urgent need and lack of access to treatment, future research should examine the effectiveness of the use of buprenorphine (BUP) as a standalone treatment, as well as the effectiveness of reducing barriers to treatment protocols presently suggested by current practice guidelines. Studies should also examine the application of current treatment models with the aforementioned expanded measures of treatment “success”. This aligns more closely with the present view that opioid use disorder (OUD) is chronic illness from which one can expect to “recover”. Despite periods of relapse, many individuals with OUD continue to function effectively in many areas and achieve a good quality of life [13].
It is also important that treatment protocols and outcome measures be standardized in order to permit direct comparison of treatment effectiveness [64]. Also, including measures of patient satisfaction with treatment may help clinicians and researchers further understand what is and is not effective for patients. Although research findings suggest there is a wide range of effective treatments, a principle of opioid abuse treatment put forth by NIDA [65] suggests that treatment plans should be individualized, and that no one treatment plan works for everyone. Studies evaluating the effectiveness of medications should be adequately powered and include matched samples. Study designs should account for between-group differences or other confounding variables in statistical analyses. Since methadone (MET) and BUP demonstrate comparable effectiveness on many outcomes, research in pinpointing characteristics of persons who respond better to one medication over the other would be highly useful. For instance, we may learn more about individuals who benefit from continuous monitoring often required by treatment facilities that dispense MET.
A final limitation of the current review is that many of the studies included other medications in their OUD treatment protocols, for example comfort medications for withdrawal and related symptoms. These reports did not provide information regarding the impact of such medications on outcomes. Thus, it is not clear the extent to which these medications might have impacted the results.
Implications for policy and practice
Policy makers, researchers, and clinicians will need to be cautious and must not be misled by studies with short treatment schedules and those with many barriers to care. We can begin the paradigm shift in addiction treatment and research by moving away from the abbreviation MAT, as ASAM has suggested, and using medication treatment (MT) instead, which suggests that medication is treatment, rather than a tool that assists other treatment approaches. As such, payers should remove additional treatment requirements and limits on treatment length, so providers and researchers can study the outcomes of treatment when patients can engage in OUD treatment long-term.
Policy makers, providers, and researchers should work together to identify a set of standardized outcomes for the evaluation of treatment for opioid use disorder that allow (OUD) us to understand the benefits of treatment beyond abstinence from opiates and other substances. Standardized outcomes should be required as common data elements in clinical practice (via electronic health record) and research studies, which would facilitate data-sharing and reasonable evaluation of treatment.
Providers need to be aware of all barriers to successful treatment that individuals might face including unidentified and untreated cooccurring substance use disorders or mental illness; given the increased prevalence of co-morbid conditions and risks to health should they remain untreated. In addition, a compassionate approach with an understanding that relapse is an inherent aspect of this chronic illness, and providing additional support options rather than punishing patients, could improve retention in treatment and the possibility for better outcomes.
Conclusion
Buprenorphine (BUP) is an effective treatment option for many individuals with opioid use disorder (OUD) and with emerging treatment guidelines, may be easier to access than other forms of treatment. Previous reviews have found BUP in higher doses is more highly effective than other medications and counselling for reducing the use of illicit opioids and as a standalone treatment. The unique contribution of this review is the synthesis of data from multiple study types (RCT, secondary data, retrospective chart review, and insurance claims) that compare different formulations of BUP for a wide range of outcomes. Further, these review highlights that much of the available literature utilized protocols that are inconsistent with current clinical practice recommendations and what is understood about the needs of patients with OUD. In addition, flaws in research designs make it difficult for providers to determine the best MT and ancillary services to improve outcomes for specific patient populations. Thus, additional research that carefully examines the effectiveness of BUP when administered in accordance with current best practice guidelines and a set of standardized, patient-centered outcomes is needed to help us understand the best approach to mitigate the personal and social consequences of opioid use disorder.
References
- Optum (2014) The Four Steps of Population Health Management. Eden Prarie, MN :9.
- Centers for Disease Control (2017) Increase in hepatitis C infections linked to worsening opioid crisis.
- Department of Health and Human Services (2017) Hidden Casualties: Consequences of Opioid Epidemic on the Spread of Infectious Diseases.
- Florence CS, Zhou C, Luo F, Xu L (2016) The Economic Burden of Prescription Opioid Overdose, Abuse, and Dependence in the United States, 2013. Medical Care 54: 901-906.
- (2017) National Institute on Drug Abuse Effective Treatments for Opioid Addiction.
- Thomas CP, Fullerton CA, Kim M, Montejano L, Lyman DR, et al. (2014) Medication-assisted treatment with buprenorphine: Assessing the evidence. Psychiatric Services 65: 158-170.
- Fullerton CA, Kim M, Thomas CP, Lyman DR, Montejano LB, et al. (2014) Medication-assisted treatment with methadone: assessing the evidence. Psychiatr Services 65: 146-157.
- Lee JD, Nunes EV, Novo P, Bachrach K, Bailey GL, et al. (2018) Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet 391: 309-318.
- (2018) Medications for Opioid Use Disorder. Treatment Improvement Protocol (TIP) Series 63.
- Ling W, Hillhouse M, Ang A, Jenkins J, Fahey J (2013) Comparison of behavioral treatment conditions in buprenorphine maintenance. Addiction 108: 1788-1798.
- Mattick RP, Breen C, Kimber J, Davoli M (2014) Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database of Systematic Reviews : 1-2.
- Timko C, Schultz NR, Cucciare MA, Vittorio L, Garrison-Diehn C (2016) Retention in medication-assisted treatment for opiate dependence: A systematic review. Journal of Addictive Diseases 35: 22-35.
- Newhouse R, Dearholt S, Poe S, Pugh LC, White K (2005) The Johns Hopkins Nursing Evidence-based Practice Rating Scale. The Johns Hopkins University.
- D’Onofrio G, Chawarski MC, O’Connor PG, Pantalon MV, Busch SH, et al. (2017) Emergency Department-Initiated Buprenorphine for Opioid Dependence with Continuation in Primary Care: Outcomes During and After Intervention. JGIM: Journal of General Internal Medicine 32: 660-666.
- Petry NM, Carroll KM (2013) Contingency management is efficacious in opioid-dependent outpatients not maintained on agonist pharmacotherapy. Psychology of Addictive Behaviors 27: 1036-1043.
- Tsui JI, Evans JL, Lum PJ, Hahn JA, Page K, et al. (2014) Association of opioid agonist therapy with lower incidence of hepatitis C virus infection in young adult injection drug users. JAMA Intern Med 174: 1974-1981.
- Weiss RD, Potter JS, Griffin ML, Provost SE, Fitzmaurice GM, et al. (2015) Long-term Outcomes from the National Drug Abuse Treatment Clinical Trials Network Prescription Opioid Addiction Treatment Study. Drug and Alcohol Dependence 150: 112-119.
- Kowalczyk WJ, Furnari MA, Phillips KA, Jobes ML, Ghitza U, et al. (2015) Reducing the cost of free time: Treatment success in a randomized trial of clonidine as adjunct to buprenorphine maintenance is associated with more leisure activities in the clonidine condition. Drug and Alcohol Dependence Drug and Alcohol Dependence 156: e118.
- Stein MD, Herman DS, Moitra E, Hecht J, Lopez R, et al. (2015) A preliminary randomized controlled trial of a distress tolerance treatment for opioid dependent persons initiating buprenorphine. Drug & Alcohol Dependence 147: 243-250.
- Weiss RD, Rao V (2017) The Prescription Opioid Addiction Treatment Study: What have we learned. Drug & Alcohol Dependence 173: S48-S54.
- Back SE, Payne RL, Wahlquist AH, Carter RE, Stroud Z, et al. (2011) Comparative profiles of men and women with opioid dependence: results from a national multisite effectiveness trial. The American Journal Of Drug And Alcohol Abuse 37: 313-323.
- Fiellin D, Cutter CJ, Moore BA, Barry D, Connor PO, et al. (2015) Primary care buprenorphine detoxification vs. maintenance for prescription opioid dependence. DAD Drug Alcohol Depend e 146: e277-e277.
- Gonzalez G, DiGirolamo G, Romero-Gonzalez M, Smelson D, Ziedonis D, et al. (2015) Memantine improves buprenorphine/naloxone treatment for opioid dependent young adults. Drug Alcohol Depend 156: 243-253.
- Polsky D, Glick HA, Yang J, Subramaniam GA, Poole SA, et al. (2010) Cost-effectiveness of extended buprenorphine-naloxone treatment for opioid-dependent youth: data from a randomized trial. Addiction 105: 1616-1624.
- Sigmon SC, Dunn KE, Saulsgiver K, Patrick ME, Badger GJ, et al. (2013) A randomized, double-blind evaluation of buprenorphine taper duration in primary prescription opioid abusers. JAMA Psychiatry 70: 1347-1354.
- Lofwall MR, Stitzer ML, George E Bigelow GE, Eric C Strain (2005) Comparative safety and side effect profiles of buprenorphine and methadone in the outpatient treatment of opioid dependence. Addictive Disorders & Their Treatment 4: 49-64.
- Jackson H, Mandell K, Johnson K, Chatterjee D, Vanness DJ (2015) Cost-effectiveness of injectable extended-release naltrexone compared with methadone maintenance and buprenorphine maintenance treatment for opioid dependence. Substance Abuse 36: 226-231.
- Fingerhood MI, King VL, Brooner RK, Rastegar DA (2014) A Comparison of Characteristics and Outcomes of Opioid-Dependent Patients Initiating Office-Based Buprenorphine or Methadone Maintenance Treatment. Substance Abuse 35: 122-126.
- Rastegar DA, Kawasaki SS, King VL, Harris EE, Brooner RK (2016) Criminal charges prior to and after enrollment in opioid agonist treatment: A comparison of methadone maintenance and office-based buprenorphine. Substance Use & Misuse 51: 803-811.
- Lott DC, Strain EC, Brooner RK, Bigelow GE, Johnson RE (2006) HIV risk behaviors during pharmacologic treatment for opioid dependence: A comparison of levomethadyl acetate hydrochloride, buprenorphine, and methadone. Journal of Substance Abuse Treatment 31: 187-194.
- Johnson RE, Chutuape MA, Strain EC, Walsh SL, Stitzer ML, et al. (2000) A Comparison of Levomethadyl Acetate, Buprenorphine, and Methadone for Opioid Dependence. New England Journal of Medicine 343: 1290-1297.
- Kamien JB, Branstetter SA, Amass L (2008) A mass Buprenorphine-naloxone versus methadone maintenance therapy: a randomised double-blind trial with opioid-dependent patients. . Heroin Addict Relat Clin Prob 10: 5-18.
- Woody GE, Poole SA, Subramaniam G, Dugosh K, Bogenschutz M, et al. (2008) Extended vs short-term buprenorphine-naloxone for treatment of opioid-addicted youth: a randomized trial. JAMA: Journal of the American Medical Association 300: 2003-2011.
- Crits-Christoph P, Markell HM, Gibbons MBC, Gallop R, Lundy C, et al. (2016) A naturalistic evaluation of extended-release naltrexone in clinical practice in Missouri. Journal of Substance Abuse Treatment 70: 50-57.
- Hser Y, Saxon AJ, Huang D, Hasson A, Thomas C, et al. (2014) Treatment Retention among Patients Randomized to Buprenorphine/Naloxone Compared to Methadone in A Multi-site Trial. Addiction 109: 79-87.
- Woody GE, Bruce D, Korthuis PT, Chhatre S, Poole S, et al. (2014) HIV risk reduction with buprenorphine-naloxone or methadone: findings from a randomized trial. J Acquir Immune Defic Syndr 66: 288-293.
- Caldiero RM, Parran TV, Adelman CL, Piche B (2006) Inpatient Initiation of Buprenorphine Maintenance vs. Detoxification: Can Retention of Opioid-Dependent Patients in Outpatient Counseling Be Improved? The American Journal on Addictions 15: 1-7.
- Vo HT, Robbins E, Westwood M, Lezama D, Fishman M (2016) Relapse prevention medications in community treatment for young adults with opioid addiction. Substance Abuse 37: 392-397.
- Clark RE, Baxter JD, Aweh G, O’Connell E, Fisher WH, et al. (2015) Risk Factors for Relapse and Higher Costs Among Medicaid Members with Opioid Dependence or Abuse: Opioid Agonists, Comorbidities, and Treatment History. Journal of Substance Abuse Treatment 57: 75-80.
- Jones ES, Moore BA, Sindelar JL, O’Connor PG, Schottenfeld RS, et al. (2009) Cost analysis of clinic and office-based treatment of opioid dependence: Results with methadone and buprenorphine in clinically stable patients. Drug and Alcohol Dependence 99: 132-140.
- Ling W, Casadonte P, Bigelow G, Kampman KM, Patkar A, et al. (2010) Buprenorphine implants for treatment of opioid dependence: A randomized controlled trial. JAMA: Journal of the American Medical Association 304: 1576-1583.
- Rosenthal RN, Ling W, Casadonte P, Vocci F, Bailey GL, et al. (2013) Buprenorphine implants for treatment of opioid dependence: randomized comparison to placebo and sublingual buprenorphine/naloxone. Addiction 108: 2141-2149.
- Sessler NE, Walker E, Chickballapur H, Kacholakalayil J, Coplan PM, et al. (2017) Disproportionality analysis of buprenorphine transdermal system and cardiac arrhythmia using FDA and WHO postmarketing reporting system data. Postgrad Med 129: 62-68.
- Proctor SL, Copeland AL, Kopak AM, Herschman PL, Polukhina N (2014) A naturalistic comparison of the effectiveness of methadone and two sublingual formulations of buprenorphine on maintenance treatment outcomes: Findings from a retrospective multisite study. Experimental and Clinical Psychopharmacology. 22: 424-433.
- Barnett PG (2009) Comparison of costs and utilization among buprenorphine and methadone patients. Addiction 104: 982-992.
- Accurso AJ, Rastegar DA (2016) The Effect of a Payer-Mandated Decrease in Buprenorphine Dose on Aberrant Drug Tests and Treatment Retention Among Patients with Opioid Dependence. Journal of Substance Abuse Treatment 61: 74-79.
- Peter (2016) National Survey on Drug Use and Health. Substance Abuse and Mental Health Services Administration.
- Ohlin L, Fridell M, Nyhlen A (2015) Buprenorphine maintenance program with contracted work/education and low tolerance for non-prescribed drug use: a cohort study of outcome for women and men after seven years. BMC Psychiatry 15: 56.
- Awgu E, Magura S, Rosenblum A (2010) Heroin-Dependent Inmates’ Experiences with Buprenorphine or Methadone Maintenance. J Psychoactive Drugs 42: 339-346.
- Larney S, Toson B, Burns L, Dolan K (2012) Effect of prison-based opioid substitution treatment and post-release retention in treatment on risk of re-incarceration. Addiction 107: 372-380.
- Amato L, Minozzi S, Davoli M, Vecchi S (2011) Psychosocial combined with agonist maintenance treatments versus agonist maintenance treatments alone for treatment of opioid dependence. Cochrane Database of Systematic Reviews 2011: 10.
- Christensen DR, Landes RD, Jackson L, Marsch LA, Mancino MJ (2014) Adding an Internet-delivered treatment to an efficacious treatment package for opioid dependence. Journal of Consulting and Clinical Psychology 82: 964-972.
- Ruetsch C, Tkacz J, McPherson TL, Cacciola J (2012) The effect of telephonic patient support on treatment for opioid dependence: Outcomes at one year follow-up. Addictive Behaviors 37: 686-689.
- Kampman K, Jarvis M (2015) American Society of Addiction Medicine (ASAM) National Practice Guideline for the Use of Medications in the Treatment of Addiction Involving Opioid Use. Journal of Addiction Medicine 9: 358-367.
- Sordo L, Barrio G, Bravo MJ, Indave BC, Degenhardt L, et al. (2017) Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. BMJ j1550.
- Martin SA, Chiodo LM, Bosse JD, Wilson A (2018) The Next Stage of Buprenorphine Care for Opioid Use Disorder. Annals of Internal Medicine 169: 628.
- Maglione MA, Raaen L, Chen C, Azhar G, Shahidinia N, et al. (2018) Effects of medication assisted treatment (MAT) for opioid use disorder on functional outcomes: A systematic review. J Subst Abuse Treat 89: 28-51.
- SAMHSA (2010) Substance Abuse and Mental Health Services Administration Ten Guiding Principles of Recovery. PEP12-RECDEF.
- Dupont RL R (2014) Creating a new standard for addiction treatment outcomes: A report from the Institute for Behavior and Health Inc. 1-52.
- Abuse (2018) Principles of Drug Addiction Treatment: A Research-Based Guide.
Citation: Mariolis T, Bosse J, Martin S, Wilson A, Chiodo L (2019) A Systematic Review of the Effectiveness of Buprenorphine for Opioid Use Disorder Compared to Other Treatments: Implications for Research and Practice. J Addict Res Ther 10:379. DOI: 10.4172/2155-6105.1000379
Copyright: © 2019 Mariolis T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 6592
- [From(publication date): 0-2019 - Nov 11, 2025]
- Breakdown by view type
- HTML page views: 5531
- PDF downloads: 1061