Acute Toxicity Studies of Methanol Leaf Extract of Chrysophyllum albidum in Swiss Albino Rats
Received: 09-Jul-2021 / Accepted Date: 23-Jul-2021 / Published Date: 30-Jul-2021 DOI: 10.4172/2155-9872.1000001
Abstract
The acute toxicity of methanol leaf extract of Chrysophyllum albidum was evaluated in Swiss albino rats. The rats were randomly distributed into four groups of three animals each for the first phase. The groups were respectively administered both intraperitoneally and orally methanol leaf extract of Chrysophyllum albidum at 0, 10, 100 and 1000 mg/kg body weight in a single dose. In the second phase of the experiment for intraperitoneal administration, the albino rats were randomly distributed into five groups of three animals each and the groups were administered single doses of the extract at 0, 600, 1000, 1600 and 2900 mg/kg body weight while for oral administration, the rats were divided into four groups of 3 animals each and were administered single doses of the extract at 0, 1600, 2900 and 5000mg/kg body weight and monitored frequently for 24 hours and 14 days respectively in both phases. The number of deaths in each group was recorded. The LD50 of the methanol leaf extract of Chrysophyllum albidum was calculated to be 244.95 mg/kg. The results indicate that the extract may be very toxic at a high dose and short term exposure when administered intraperitoneally. For the oral intubation, no mortality was observed in the albino rats. All the animals showed a positive gain in body weight throughout the study. The oral LD50 of methanol leaf extract of Chrysophyllum albidum was estimated to be above 5000mg/kg body weight and the plant extract was concluded to be safe and non-toxic when taken through the oral route.
Keywords: Chrysophyllum; Appetite; Immobility; Convulsion; Toxicity; Albino Rats
Introduction
Toxicity is the relative ability of a substance to cause adverse effects on living organisms, it describes the degree to which bioactive substances cause harm to living organisms as well as their symptoms, mechanisms and treatments [1]. Toxicology is the scientific study of the undesirable effects of chemical, physical or biological agents on living organisms; it involves observing and reporting symptoms that arise following exposure to toxic substances [2].
In Africa, plants are used in traditional medicine to treat different infectious and non-infectious diseases due to their assumed acceptability, effectiveness, affordability, safety and low cost [3]. Due to the large chemical diversity among natural products, many research groups screen plant extracts for new promising therapeutic candidates for infectious diseases [4]. Herbal preparations assumed to be safe may contain contaminants such as heavy metals, aflatoxins and pathogenic microbes due to the manner in which they are prepared or as a result of acquisition of metals (e.g.cadmium) from the soil [5]. However, for proper and documented herbal medicinal products, the toxicity should be explored as in the case with conventional orthodox drugs that are properly researched and developed; the toxicity of traditional herbal medications is not often assessed [6].
The preparation and use of medicinal plants can be harmful to human health, in spite of the benefits of traditional herbal medicine, cases of adverse effects to some plant based herbal preparation have been reported either when used singly or concurrently with conventional orthodox medicine [7]. This has encouraged many researchers to investigate medicinal plants. It has been shown that the toxicity of a given plant depends on various factors, including the strength of secondary metabolites, the quantity consumed, the time of exposure, different parts of the plant (root, oil, leaves, stem bark and seeds), individual body chemistry, climate and soil, and genetic differences within the species. Medicinal plants should be used with precautions and toxicology studies should be conducted to increase the knowledge on the plant or plants preparation given to populations [8].
Chrysophyllum albidum commonly called white star apple belonging to the family of Sapotaceae is a lowland rain forest tree species and very useful medicinal plant common in the tropical and subtropical regions of the world [9]. In folklore medicine, Chrysophyllum albidum bark is employed for the treatment of yellow fever and malaria [10]. The leaf is used as an emollient and for the treatment of stomach ache and diarrhea. The plant could also be employed as sources of natural antioxidant boosters for the treatment of free radical implicated oxidative stress disorders [11]. Information on the safety potential of this plant is lacking, thus there will be need to evaluate the toxicity potential of this popularly used medicinal plant.
Acute toxicity is defined as the harmful effects produced by single exposure of drugs by any route for a short period of time (usually 24 hours) which could alter the functioning of the organism in general or individual organs. Acute toxicity studies in animals are considered necessary for any pharmaceutical intended for human use, results from acute toxicity test serve as a guide in dosage selection for long term toxicity studies as well as other studies that involve the use of animals [12]. However, the safety of Chrysophyllum albidum is important in relation to its therapeutic actions, therefore, this study was aimed at determining the possible acute toxicity of methanol extract of Chrysophyllum albidum in Swiss albino rats..
Materials and Methods
Plant material and extract preparation
Fresh leaves of African star apple (Chrysophyllum albidum) were collected from Eziobodo Elu, Owerri, Imo state, Nigeria in the month of Febuary, 2021 and identified by a taxonomist, Dr. Faruwa Francis of the Department of Forestry and wildlife Technology, School of Agriculture and Agricultural Technology (SAAT) Federal University of technology Owerri FUTO. The plant was prepared and kept in the University herbarium with voucher number 2021059.
The leaves of Chrysophyllum a lbidum were air dried under the shade, inside the laboratory for four weeks and ground into powdered form. This powdered sample was stored in airtight polythene bags to protect the sample from sunlight. The methanol extract of the leaf powder was prepared by quantitative extraction i.e 100 g of this powder was soaked in 800 ml of methanol in a 1000 ml conical flask sealed air tightly with cotton wool and foil for 72 hours and thereafter filtered with whatman no 1 filter paper. The decoction liquid obtained was concentrated in a water bath at 450 C to produce a semi solid residue. The extract was stored in the refrigerator at 40oc until it was used for evaluation.
Experimental animals
The female albino rats used for the study were purchased from the department of pharmacology and physiology, college of veterinary medicine, Michael Okpara University of Agriculture Umudike, Abia state. They were kept in standard cages in the animal house of the Department of Biochemistry, Federal university of Technology Owerri, Imo state. The animals were about 8-9 weeks old and weighed between 80-110 g initially. They were allowed to acclimatize for two weeks before the commencement of the study. They were fed growers pellet feeds with tap water ad libitum. The animals were starved for some hours before the commencement of the experiment.
Acute toxicity evaluation/determination of median lethal dose
The acute toxicity of methanol leaf extract of Chrysophyllum albidum was conducted in accordance with Lorke’s method (Lorke, 1983). Two different routes of administration were used for this study, the intraperitoneal route of administration and the oral intubation. The method involved two phases for each route of administration.
For the first phase, 12 rats were randomly distributed into four groups of three animals each. The groups were respectively administered both intraperitoneally and orally single doses of methanol leaf extract of Chrysophyllum albidum at 10, 100 and 1000 mg/kg body weight, the fourth group which was the control was administered normal saline only. The animals were observed closely for the first one hour and then every 30 minutes for the first 24 hours for the onset of any immediate signs of toxicity and daily during the 14 day observation period to record any delayed acute effects. During this period, their weights were recorded daily and clinical signs of toxicity such as loss of appetite, immobility, convulsion, etc., were observed. The mortality in each group were also recorded
In the second phase of the experiment for intraperitoneal administration, 15 animals were divided into 5 groups of 3 animals each and further specific doses of 600 mg, 1000 mg, 1600 mg and 2900 mg/ kg body weight were administered to the animals, the fifth group which was the control received normal saline only. For oral administration, 12 rats were divided into four groups of 3 animals each and were administered single doses of the extract at 1600, 2900 and 5000 mg/kg body weight, the fourth group which was the control received normal saline only. The second phase doses were chosen based on the result of the first phase treatment. The animals were observed as frequently as previously described in the first phase and the mortality in each group were equally recorded. The surviving animals were weighed daily and monitored for two weeks for clinical signs of acute toxicity such as; writhing, gasping, palpitation increased or decreased respiration rate. The result of the first and second phase of the experiment was used to calculate the LD50 of the plant extract.
The LD50 was calculated according to the method outlined by Lorke (1983) as:
√Geometric mean of the maximal dose without mortality × Geometric mean of the minimal dose with mortality = Xmg/kg.
Statistical analysis
All data generated during the cause of the research were expressed as mean ± standard deviation (SD) and analyzed statistically by analysis of variance (ANOVA). Means were compared using LSD post hoc test and differences between treatment and control groups accepted as significant at p ≤ 0.05
Results
Results o quantitative extraction

Acute toxicity evaluation of the methanol leaf extract of Chrysophyllum albidum
For the animals that were administered the extract via the intraperitoneal route. In the first phase of treatment, the rats developed clinical signs of toxicity (loss of appetite, loss of stimuli sensitivity, loss of agility and immobility) after 30 minutes of the post treatment period with 100 mg/kg and 1000 mg/kg of the extract. There were no clinical signs of toxicity observed with the rats in the 10 mg/kg and control groups either immediately or during the post treatment period. Onethird of the rats in 1000 mg/kg group died after 48 hours of treatment.
In the second phase of the treatment, rats administered with the extract developed clinical signs of toxicity (loss of appetite, loss of stimuli sensitivity, loss of agility and immobility) after 15 minutes of the post treatment period with 600 mg/kg, 1000 mg/kg, 1600 mg/kg, 2900 mg/kg of the extract. One third of the rats in the 600 mg/kg group died after 4 hours of treatment, two third of the rats in the 1000 mg/kg and1600 mg/kg group died after 3 hours of treatment and all the rats in the 2900 mg/kg group after 1 hour of treatment. No mortality occurred in the control group during the 14- day observation period (Table 1).
| Plant | Experiment | Dose (mg/kg) | Proportion of death | |
|---|---|---|---|---|
| After 24 hours | After 2 weeks | |||
| Chrysophyllum albidum (methanol leaf extract) | Phase 1 | 10 | 0/3 | 0/3 |
| 100 | 0/3 | 0/3 | ||
| 1000 | 0/3 | 1/3 | ||
| Control | 0/3 | 0/3 | ||
| Chrysophyllum albidum (methanol leaf extract) | Phase 2 | 600 | 1/3 | 1/3 |
| 1000 | 2/3 | 2/3 | ||
| 1600 | 2/3 | 2/3 | ||
| 2900 | 3/3 | 3/3 | ||
| Control | 0/3 | 0/3 | ||
Table 1: Acute toxicity studies (Ld50) of methanol leaf extract of Chrysophyllum albidum.
From the result the LD50 of the chloroform extract of Chrysophyllum albidum was calculated to be 244.95 mg/kg (Table 1).
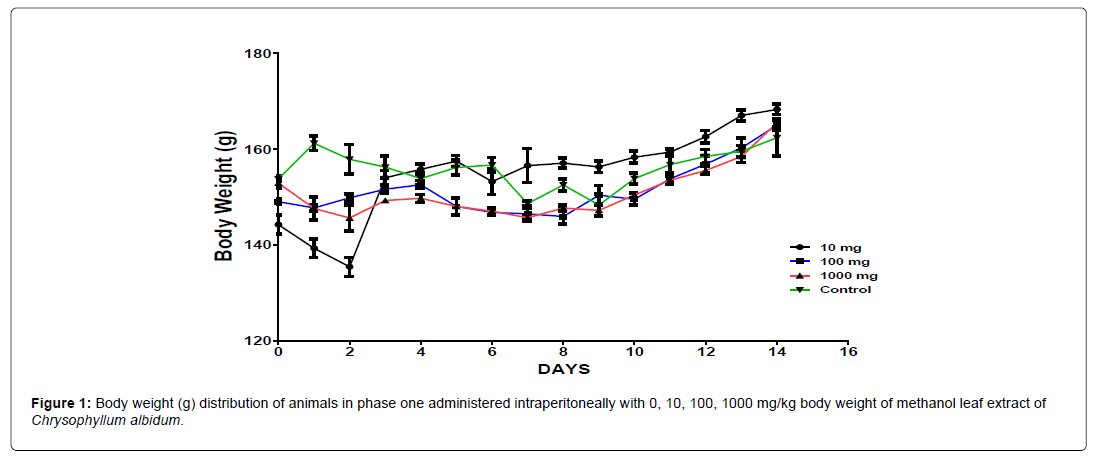
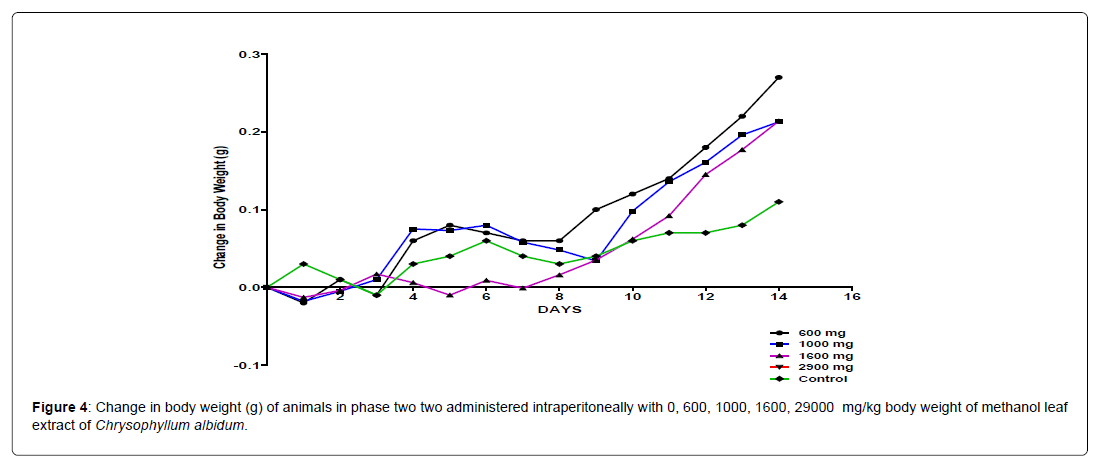
The body weight distribution and changes of the animals treated with methanol leaf extract of Chrysophyllum albidum and the control groups treated with normal saline only (Figures 1-4).
In the first phase of the treatment, a decrease in body weight was observed during the first few days in the experimental animals treated with 10 mg/kg, 100 mg/kg and 1000 mg/kg of the extract when compared with the control which was seen as a clinical sign of toxicity. Subsequently there was a significant increase in body weight after the body weight decrease was observed in the surviving animals treated with 10 mg/kg, 100 mg/kg and 1000 mg/kg of the extract from the phase one experiment when compared to the control, which was seen as a sign of recovery. This shows that methanol leaf extract of Chrysophyllum albidum has potentials of supporting weight gain at the dosages given.
In the second phase of the treatment, a decrease in body weight was observed during the first few days in the experimental animals treated with 600 mg/kg, 1000 mg/kg, 1600 mg/kg and 2900 mg/kg of the extract when compared with the control. This was seen as a clinical sign of toxicity. Subsequently there was a significant increase in body weight after the body weight decrease was observed in the surviving animals treated with 600 mg/kg, 1000 mg/kg and 1600 mg/kg and 2900 mg/kg of the extract from the phase one experiment when compared to the control, which was seen as a sign of recovery. This shows that methanol leaf extract of Chrysophyllum albidum has potentials of supporting weight gain at the dosages given.
For the animals administered the extract via the oral route
The results of the oral LD50 determination of methanol leaf extract of Chrysophyllum albidum is presented on Table 2 below. No mortality was recorded in both the control and the treated groups during the two phases of treatments. No clinical sign of toxicity were observed in all the animal groups during the phase one. During the phase two, the animals treated with 2900 mg/kg and 5000 mg/kg body weight of the plant extract showed signs of irritability and weakness after 1 hour of administration. However, the animals recovered within two hours. The lack of mortality during the study indicates that the LD50 is above 5000 mg/kg body weight.
| Experiment | Dose (mg/kg) | No of mortality |
|---|---|---|
| Phase 1 | Control | 0/3 |
| 10 | 0/3 | |
| 100 | 0/3 | |
| 1000 | 0/3 | |
| Phase 2 | Control | 0/3 |
| 1600 | 0/3 | |
| 2900 | 0/3 | |
| 5000 | 0/3 |
Table 2: Ld50 determination of methanol leaf extract of Chrysophyllum Albidum.
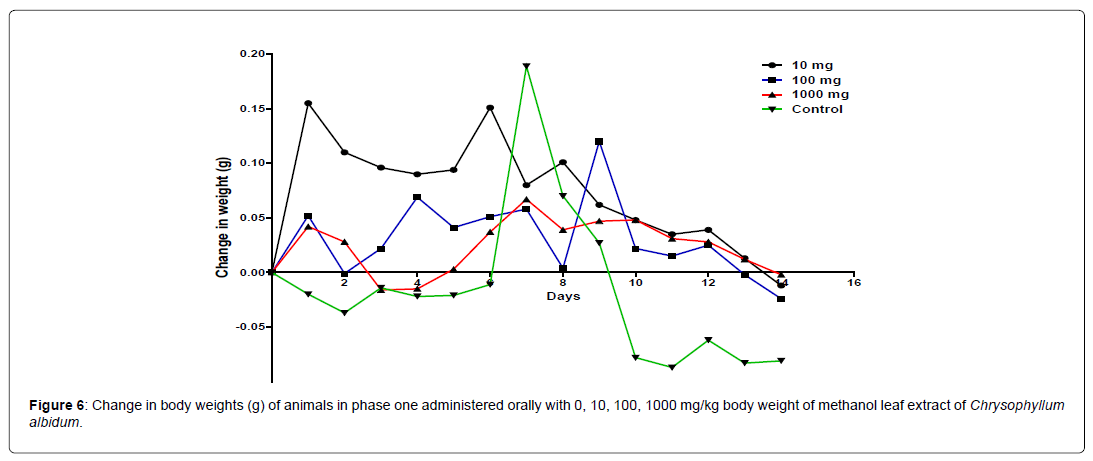
The results of the 14 days weight measurement of the animals during the phase 1 is presented in Figure 5. The change in body weight of the animals during the phase 1 is plotted in Figure 5. No significant difference (p<0.05) in body weight was observed among all the groups except on day 8. A significant difference in the body weight of the animals treated with 100 mg/kg body weight of the extract was observed when compared with the control animals on day 8. All the groups showed an increase and positive change in body weight during the first 8 days of measurement followed by a decline from day 8 to day 14 (Figure 6).
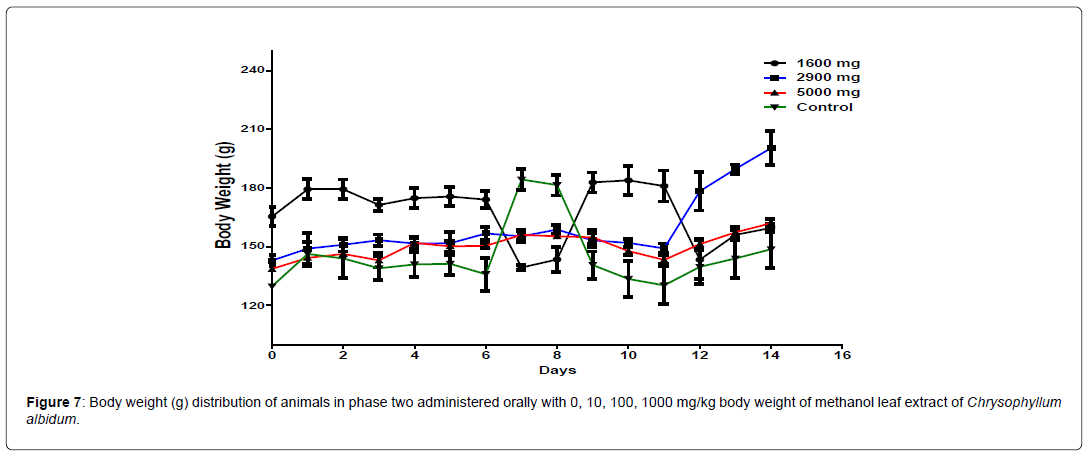
The body weight distribution of the animal in phase 2 is presented in Figure 7. There is a significant difference (p<0.05) in the body weight of the treatment groups from day 6 to day 11 compared to the control animals. From day 12 to day 14, significant difference was only observed between the animals treated with 2900 mg/kg body weight and the control group. The change in body weight of the animals during the phase 2 is shown in Figure 8. The groups treated with 1000 mg/kg, and 5000 mg/kg body weight of the extract and the control group showed a positive change in body weight of the animals throughout the study. The group treated with 2900 mg/kg body weight maintained a positive change in body weight until day 11 before declining to a negative change in body weight from day 11 to day 14 (Figure 8).
Discussion
Toxicity is an expression of being poisonous, indicating the state of adverse effect, led by the interactions between toxicants and cells. The toxicological evaluation of a plant extract seeks to discover its potential collateral effects, to ensure the safety of use. Some factors are capable of interfering with the toxicity of medicinal plant extract. This toxicity can be inherent in the vegetable drug or happens during the process of extract preparation. The toxicity of the drug is related to its compound(s) and could be ascribed to the active principle or not.
The many useful properties of Chrysophyllum albidum is a pointer to its potential as a natural source of drug, but also makes it a subject of potential abuse especially among the local consumers (Medu et al., 2016). Hence, the present study was particularly designed to investigate the acute toxicity of methanol leaf extract of Chrysophyllum albidum by using intraperitoneal and oral toxicity analysis.
For the intraperitoneal acute toxicity evaluation , there was a significant (p ≤ 0.05) decline in the body weight of the treated animals both in phase 1 and phase 2 when compared to the untreated control after the first few days of the treatment which was seen as a clinical sign of toxicity. In the subsequent days of observation there was a significant (p ≤ 0.05) increase in the body weight of the treated animals both in phase 1 and phase 2 when compared to the untreated control. This indicates the extract has toxic effect on the rats and also has the ability to stimulate appetite of the rats and support weight gain at the dosages given. In a similar study Ekene and Odigie (2019) reported a significant increase in body weight of rats treated with Chrysophyllum albidum when compared to control as a result of tannins present in the extract, as tannins have been implicated to stimulate increase in body mass [13]. A recent study by Marcus et al., 2003 revealed that tannins present in medicinal plants are potent in increasing body mass. Also, a phytochemical analysis of Chrysophyllum albidum [14] has shown the presence of small quantities of tannins, among other components as one of its active component. This could be responsible for the increased body weight observed in this study.
There were no clinical signs of toxicity observed in the animals treated with 10 mg/kg body weight of the extract. Clinical signs of toxicity were observed in the animals treated 100 mg/kg, 600 mg/kg, 1000 mg/kg, 1600 mg/kg, 2900 mg/kg body weight of the extract which included loss of appetite, loss of stimuli sensitivity, loss of agility and immobility. Also one of the animals in the first phase of the treatment (100 mg/kg group) died while in the second phase, one animal died in the 600 mg/kg group, two animals died in the 1000 mg/kg and 1600 mg/kg group and all three animals died in the 2900 mg/kg group This shows the extract was not toxic at very low doses but was toxic at higher doses when administered intraperitoneally.
The acute toxicity study indicated that the methanol leaf extract of Chrysophyllum albidum is toxic when administered through the intraperitoneal route to the experimental animal at a dose greater than 244.95 mg/kg. In a similar study, the toxic effect of the methanol extract and butanol extract of Chrysophyllum albidum seed cotyledon in albino rats and the LD50 was calculated to be 200 mg/kg body weight for the methanol extract and 760 mg/kg body weight for the butanol extract[15]. In another study by Ene et al., the acute toxicity of the chloroform extract of Artemisia macivera was studied. In their own study, they reported that the chloroform extract of Artemisia macivera has an LD50 value 566 mg/kg intraperitoneally in mice, They showed that the plant extract was moderately toxic to the experimental animals. In the first phase of the treatment, they used the doses of 10, 100 and 1000 mg/kg, and the result of the phase one treatment determined the doses used in phase two treatment. This is in agreement with the methodology of this present study.
For the oral acute toxicity evaluation, The LD50 of the methanol extract of Chrysophyllum albidum was estimated to be more than 5000 mg/kg. The dose produced no mortality after 24 hours and 14 days observation period. It also had no adverse effects o n t he b ehavioral responses of the tested rats after 1 4 days of observation. It has been suggested that any substance with an oral LD50 of above 5000 mg/kg should be regarded as safe [16]. It can therefore be inferred that, the plant under study is non-toxic and safe. Although, the extract can be deduced to be safe, some dose dependent toxic manifestations were observed in the groups treated with 2900 mg/kg and 5000 mg/kg body weight of the extract following oral administration. This may be due to the effect of one or more of the chemical constituents present in the extract. The non-toxic observation that was made in the current studies following the evaluation of the acute toxicity aligns very well with other studies on toxicity of Chrysophyllum albidum. Adewoye et al., reported a nontoxic LD50 of methanol bark extract of Chrysophyllum albidum while Bada reported that the seed extract are non-toxic following both oral and intraperitoneal administration to albino rats [17]. The nontoxic effect of the plant could be due its rich content of nutritional molecules. Adisa (2000) reported that the whole plant is rich in Vitamin C, protein and mineral contents.
Available evidence has shown that body weight changes are important and sensitive indices of toxic effects (Sharma, 2011). The monitoring of body weight of the experimental animals is important while studying the toxicity and safety of a natural product since it hints at the physiological and metabolic status of the animals and gets rid of the researcher from deriving any “false” observations due to nutritional abnormalities of the rats. In this current study, the positive change in body weight shown by all the rats was comparable and followed a general trend. The slight elevation in body weight after t he 1 4-day t reatment c an be a ttributed t o normal g rowth o f the animals over the period [18]. None of the experimental groups suffered loss in weight or gained overweight which suggested that the plant extracts did not induce significant changes in the appetite and did not exert any deleterious effects on the general health status and metabolic growth of the rats. The animals in the control group showed a negative change in body weight towards the end of the observations. This change could be merely as a result of environmental f actors as the control group only received water and food. The pattern of body weight was altered after day 8 during the phase 1 experiment. However, this change was also observed with the control group and is therefore not caused by the administration of methanol extract. This suggested that the plant extracts did not induce any deleterious effects on growth and development of the rats [13, 19, 20]. A positive change in body weight of animals has also been reported by Adewoye et al., (2010) after oral administration of non-toxic plant extracts).
Conclusion
From the data generated from the current study, it could be concluded that a single intraperitoneal dose of 10 mg/kg of methanol extract of Chrysophyllum albidum (leaves) was not able to induce mortality or toxic effects in rats, while a single intraperitoneal dose of 100 mg/kg was able to induce clinical symptoms of toxicity with no mortality and a single intraperitoneal dose of 600 mg/kg, 1000 mg/kg, 1600 mg/kg and 2900 mg/kg of methanol extract of Chrysophyllum albidum (leaves) were able to induce mortality or toxic effects in rats. This shows that this extract is very toxic at higher doses but safe at very low doses when administered through the intraperitoneal route. While the oral administration of methanol leaf extract of Chrysophyllum albidum did not show any toxicity within the test doses. The plant material can be classified as safe when taken via oral route.
Acknowledgment
We wish to express our gratitude to Rev. Chinekeokwu and Mr. Tony Nani, both of Biochemistry department, FUTO for their assistance during the laboratory work.
References
- Mensah LK, Komlaga G, Forkuo GA, Firempong C, Anning AK, et al. (2019) Toxicity and safety implications of Herbal medicines used in Africa. Intechopen 63-86.
- Grandjean P (2015) Toxicology research for precautionary decision making and the role of human and experimental toxicology. Hum Exp Toxicol 34: 1231-1237
- Boukandou MM, Ludovic MS and Aboughe A (2015) Toxicity studies of medicinal plants used in sub-Saharan Africa. J Ethnopharmacol 174: 618-627.
- Ene AC, Atawodi SE, and Fatihu MY (2014) Acute Toxicity of Chloroform Extract of Artemisia macivera Linn in swiss albino mice. B J Pharm Res 4(15): 1900-1908
- Olaniyann JM, Muhammad HL, Makun HA, Busari MB, and Abdullah AS (2015) Acute and sub-acute toxicity studies of aqueous and methanol extracts of Nelsonnia campestris in rats. J Acute Dis. 5: 62-70
- Sharma OP (2011). Clinical Biochemistry of Hepatotoxicity. J ClinToxicol and J Ethnopharm 121: 140-147.
- Langlois-Klassen D, Kipp W, Jhangri GS, and Rubaale T (2007) Use of traditional herbal medicine by AIDS patients in Kabarole district, Western Uganda. Am J Trop Med Hyg. 77: 757-763.
- Yuan X, Chapman RL, and Wu Z (2011) Analytical methods for heavy metals in herbal medicines. Phytochem Anal 22:189–198
- Adebayo AH, Abolaji AO, Kela R, Ayepola OO, Olorunfemi TB, et al. (2011) Antioxidant activities of the leaves of Chrysophyllum albidum. Pak J Pharm Sci 24: 545-551.
- Adisa SS, Garba SA, Iyagbo OA, and Iyamo AO (2000) Vitamin C, protein and mineral contents of African star apple (Chrysopyllum albidum). 18th Annual conference of Nigerian Institute of Science Laboratory Technology, Ibadan.
- Emudainowoho JO, Erhirhie EO, Moke EG, and Edje KE (2015) A comprehensive review of Ethno-medicine, phytochemistry and Ethnopharmacology of Chrysophyllum albidum. J Adv Med Pharm Sci 3(4): 147-154.
- Maheshwari DG, and Shaikh NK (2016).  An  overview  on  toxicity  testing method. Int J Pharm Technol 8(2): 3834– 3849.
- Adewoye EO, Salami AT, Lawal TO, and Adeniyi BA (2011) The antimicrobial and kill kinetics of Chrysophyllum albidum stem bark extract. Eur J Sci Res 56: 434-444.
- Bada SO (1997). Preliminary information on the ecology of Chrysophyllum albidum done in the West and Central Africa; In proceedings of a National Workshop on the potentials of Star Apple in Nigeria.
- Ekene EM, Odigie OM (2019) Hematological changes in administration of Chrysophyllum albidum stem extract to wistar rats. Int J Res Rep Hematol 2: 1-8.
- Ezeokeke EE, Ene AC, Igwe CU (2017) Sub-Acute Toxicity Studies of Alchornea cordifolia Leaf Extract in Swiss Albino Rats. J Anal Bioanal Tech 8: 2
- Marcus C, Karin L, Jain G, Matthias L, Jorns F, et al. (2003) Captive roe deer (Capreolus capreolus) select for low amount of tannic acid but not quebracho: Flunctuation of preference and potential benefit. Biochem Mol Biol 136: 369-382.
- Medu EO, Idowu TO, Oyedele AO, Adesanya SA (2016) Antimicrobrial activity of eleagnine isolated from Chrysophyllum albidum. Nig J Nat Prod and Med. 20: 27-34.
- Shobo AA, Daniyan MA, Olayiwola G, Idowu TO, Ogundiani AO (2019) Toxicity evaluation of the extract and fraction of Chrysophyllum albidum seed cotyledons in rats. SOJ pharm pharm sci 6: 1-12
- WHO (1986) Epidemiology and control of African trypanosomiasis. World Health Organ Tech Rep Ser 739:1-127
Citation: Chinedu EA, Obianuju NR, Blessing ON, Chibueze OV, Onuoha EE, et al. (2021) Acute Toxicity Studies of Methanol Leaf Extract of Chrysophyllum albidum in Swiss Albino Rats . J Anal Bioanal Tech. 12: 001 DOI: 10.4172/2155-9872.1000001
Copyright: © 2021 Chinedu EA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 3658
- [From(publication date): 0-2021 - Dec 20, 2025]
- Breakdown by view type
- HTML page views: 2798
- PDF downloads: 860