Case Report Open Access
Administration of Alpha-1 Antitrypsin in Haemodialysis
| Martin-Gomez MA*, Martinez de la Plata E, Hidalgo Rico MA, Palacios Gomez ME and Garcia-Marcos S | |
| Units Nephrology and Neumologia, Public Enterprise Hospital de Poniente, Almeria, Spain | |
| *Corresponding Author : | Martin-Gomez MA Units Nephrology and Neumologia Public Enterprise Hospital de Poniente Almeria, Spain Tel: 677081239 E-mail: doritamg@gmail.com |
| Received September 14, 2015; Accepted December 15, 2015; Published December 18, 2015 | |
| Citation:Martin-Gomez MA, Martinez de la Plata E, Hidalgo Rico MA, Palacios Gomez ME, Garcia-Marcos S (2015) Administration of Alpha-1 Antitrypsin in Haemodialysis. Kidney Disord Clin Pract 1:102. doi:10.4172/kdc.1000102 | |
| Copyright: © 2015 Martin-Gomez MA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at
Abstract
The deficit of alpha-1-antitrypsin (AATD) is the most common potentially fatal hereditary disease in adulthood, causing the onset of emphysema, various liver diseases and favouring the development and progression of tumours and systemic vasculitis. Treatment is replacement rate. The dosing schedule alpha1-antitrypsin (α-1AT) has been modified over time by infraestrutura centers dedicated to it. In the beginning, a weekly administration agreed, but given the saturation of the hospitals and the deterioration of the quality of life of patients having to go to hospital every week, it was decided to increase the interval to every 15-21 days.
| Keywords |
| Alpha-1 Antitrypsin; Human serum protease; Perfusion; Nephelometry |
| Introduction |
| The α-1AT, 52KD glycoprotein, is the most abundant inhibitory human serum proteases [1] has significant anti-inflammatory properties (blocked cytotoxicity conducted by neutrophils and stimulating síntetis IL-8, IL-6, IL1β, TNF α- and other cytokines), increasing their activity in inflammatory, tumoral or infectious processes [2]. Its deficit (plasma levels below 35% of normal) is transmitted with a recessive autosomal pattern and leads to the development of emphysema and chronic liver (cholestasis, cirrhosis), and predisposes to the onset and progression of tumours and systemic vasculitis [3,4], therefore it is considered life threatening. |
| The only treatment currently available, in addition to the usual support of any bronchial condition, is symptomatic substitute, not “curable” and consists of intravenous (IV) of purified human α-1AT, which maintains its enzyme activity in plasma and in broncho alveolar lavage. Lung activity correlates directly with their plasma concentration, allowing monitor treatment [1]. |
| The administration schedule α-1AT has been changing over time as the infraestrutura centers dedicated to it. Thus, in the eighties, with the emergence of drug, administration of 60 mg/kg/week agreed, given the half-life of 5-6 days after infusion [1,5]. Due to the supersaturation of hospitals and deterioration of the quality of life of the patients because of going to hospital every week, it was decided to increase the interval between doses every 15-21 days, since the average life of the new molecules permitted [2,6,7]. |
| To date there is no literature on administration in hemodialysis patients, case presented. |
| Clinical Case |
| 44 patients with a family history of AAT (ZZ phenotype) with hepatic impairment (centrilobular cholestasis with portal hypertension) and pulmonary (panlobular widespread emphysema/panacinar, bullosa dystrophy and bronchiectasis colonized by Pseudomonas aeruginosa conditioning recurrent respiratory infections). Moderate OSA, rejecting treatment with nasal CPAP intolerance. In 2006 it presents criteria for chronic airflow obstruction (FVE1 26-28%) and dyspnea on minimal exertion, requiring home oxygen. α-1AT replacement therapy with purified human (Prolastina®, Talecris Bio therapeutics) from diagnosis in 1998, in addition to tiotropium, salmeterol-fluticasone, acetylcysteine and nebulized colistin since April 2011. From the point of view nephrology, has advanced chronic kidney disease secondary not biopsied chronic glomerulonephritis (nephrotic syndrome), on hemodialysis since December 2007. Guideline dialysis: 4 hours/session, 3 sessions/week, through a FAV humerobasilica reaching a Qb 450 ml/ min and dialysis polyacrylonitrile 1.65 m2, CUF 50 ml/h/mmHg). |
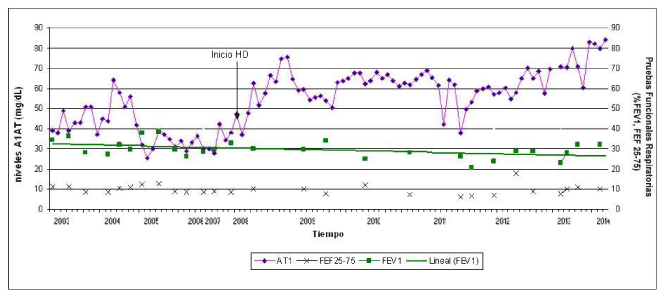
| Prior to the start of hemodialysis Prolactina® administration took place in “day hospital” the pattern of 400 ml (10 grams) to spend 2 hours every 21 days, with blood drug levels far below what is considered therapeutic or protective (Figure 1). |
| Since the start of renal replacement treatment in our unit, the intradialysis administration was decided by drug infusion pump postfilter a maximum rate of 4 ml/min. To do this, we ensure that Prolastina® molecule (52 kDa) not spread through the dialyzer: plasma samples pre and post-dialysis were taken and in the effluent of ultrafiltration (Table 1). |
| In January 2008, we start with the usual pattern of pulmonology at 180 mg/kg/21 days, but to persist infraterapeuticos levels frequency to fortnightly (April 2008: 120 mg/kg/15 d) is increased and last week (July 2008: 60 mg/kg/week), reaching levels in rank order. |
| The sample for measurement Prolactina® predialysis levels taken before the infusion (Cmin trough levels), considering the normal range by nephelometry between 90-200 mg/dl. The drug preparation is performed in hospital pharmacy, by reconstituting the powder solution perfusion solution. Each vial contains 1000 mg human α-1AT and each milliliter of reconstituted solution of 25 mg protein. This solution should be administered within three hours of its preparation at a rate not exceeding 0.08 ml/kg/min. |
| The evolution to 7 years since initiating treatment intradialysis has been very satisfactory. The patient is with marked improvement, without dyspnea or need for home oxygen since 2012 and performing daily activities without difficulty. He has presented few bronchial exacerbations (an average of two per year) without requiring hospitalization or increased baseline bronchodilator treatment. Imaging techniques have not been shown progression centrilobular emphysema or bronchiectasis. Pulmonary function tests show no worsening (Figure 1). The only adverse event related to treatment was the presence on a single occasion of grade I dyspnea associated with infusion faster than usual, giving the endentecer up. |
| We present a patient with homozygous AAT that, to clarify the situation of renal replacement therapy with hemodialysis, he increased the frequency of administration of the α-1AT (intradialysis once weekly administration as we confirmed molecule was not dialyzed), thereby entering levels drug in the therapeutic range, while allowing a slowing in the progression of the disease. |
| Discussion |
| AAT deficiency is the second most important cause of lung disease of genetic nature. The only specific treatment that is currently available replacement with the purified protein from human plasma, indicated in homozygous patients with airflow obstruction on spirometry, obtaining maximum benefit in those with forced expiratory volume (FEV1) between 30-65% [1-6]. It is not curative but their periodical administration has been associated with clinical improvement, especially in moderate stages [8,9]. Although there is some discrepancy in clinical trials for improving lung function, its efficacy has been demonstrated in numerous experimental and observational studies, especially by reducing the incidence of chest infections and exacerbations [2,10-12]. Its mechanism of action is based on its neutralizing neutrophil elastase activity, inhibiting tissue destruction in tissue bronchioloalveolar [2]. It does not happen just in liver tissue, which does not confer on protection [2,3]. In addition, he is credited ability to inhibit replication and infectivity of viruses and bacteria [1]. |
| Its rate of infusion makes a hospital stay of at least two hours. This was in the 90s a saturation of day centers in which the product was administered together with a deterioration of the quality of life of patients who had to go to hospital every week, which created different patterns treatment described above, which spreaded the frequency of its administration up to 30 days [1,2,10-12], although sheet will continue to recommend the weekly, to be the best documented [1,13,14] and which allows better pharmacokinetic levels. Therefore, using the patient should go to their scheduled dialysis three days a week and since no therapeutic levels were achieved with monthly administration of α-1AT sessions, we decided to administer in our unit shortening intertherapy first fortnightly and then weekly period, obtaining very good clinical, functional and analytical results. |
| Clinical markers at our disposal to monitor the effectiveness of substitution treatment are mainly [13-15] 1) FEV1, 2) TAC density, 3) frequency of respiratory infections, 4) sputum markers. All of them are considered to FEV1 as the main predictor of survival in these patients, who at 2 years is almost 100% until the FEV1 reaches 33%, and thereafter, decreases exponentially and poses 50% when FEV1 is 15% predicted [11]. The current goal in the treatment of AAT deficiency is the increase in the level of AAT in plasma and interstitial lung both above the protection threshold, whereby said plasma levels are used as a guide for replacement therapy. Minimum or trough concentration at steady state (Cmin) in the pre-administration of the next dose time is measured. It is considered a Cmin of 50 mg/dl the right to provide a similar normal non-smoker (1.3) and 70 mg/dl high therapeutic lung protection. In our case, drug levels are held weekly, functional tests semi-annually and chest scanner as pneumology demand. Scanner service Pneumology functional tests. |
| By the molecular weight of the product (52 KD), should not spend the hemodialysis membrane [16]. To ensure this premise, serial measurements in the ultrafiltration without finding significant concentrations of the molecule were performed. However, the suspension of plasma protein is not the same as in the ultra-filtrate and nephelometry is ready to blood plasma, so that it cannot safely ensure that a minimum amount of the product is not dialyzing. If so, in our case, this amount should not be significant since it achieve and maintain therapeutic levels. |
| We observed in our patient, since entering dialysis and thus the increasingly frequent administration α-1AT how the same levels increased progressively to be therapeutic (Figure 1). We objectify also a correlation between plasma levels of these improved with a decrease in the frequency of acute respiratory infections especially with stability over time of the operating parameters and a clear improvement of the general condition of the patient. The latter cannot be fully adjudicated change in the treatment regimen, and the patient began dialysis while improving their anaemia (started 9-10 g/dl), their nutritional status and general uremic syndrome, so as the treatment of bronchial infection by Pseudomonas aeruginosa. AATD mortality is high, dated in some studies 30% at 5 years, the main predictor FEV 1 below 50% [17]. |
| The adverse effects most commonly associated with replacement therapy are headache (47%), dizziness (17%), nausea (9%) and dyspnea (9%). In our case, only grade I dyspnea was observed in a related infusion faster than usual, and it disappeared when the infusion rate was slowed down. |
| Another drawback of the administration of this treatment outside the hemodialysis session is repeated puncture of peripheral veins, often considering channeling central access type reservoir to precisely reduce or avoid such punctures. Therefore, the advantage becomes use of a single vascular access during dialysis. |
| Conclusion |
| According to the recommendations of the Spanish and American Societies of Pneumology, in the absence of conclusive studies linking clinical efficacy pharmacokinetic measures, the choice of alfa1Antitripsina administration schedule should be individualized and emerge from compromise between biochemical efficacy, expectations and availability of patients and the hospital. We therefore conclude that the administration should be weekly in patients undergoing hemodialysis, since this scheme maintains higher levels of drugs in blood with good safety profile and tolerance, improves quality of life and presumably the same effect on survival patient. |
| References |
|
Tables and Figures at a glance
| Table 1 |
Figures at a glance
 |
| Figure 1 |
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 21525
- [From(publication date):
December-2015 - Aug 17, 2025] - Breakdown by view type
- HTML page views : 16947
- PDF downloads : 4578