Analysis of SHIP2 Expression and its Correlation with VEGF and HER-2 Expression in Breast Cancer
Received: 11-Jun-2017 / Accepted Date: 13-Jul-2017 / Published Date: 17-Jul-2017 DOI: 10.4172/2161-0681.1000314
Abstract
Purpose: To investigate SHIP2 expression in breast cancer tissues, and its correlation with clinicopathological features and with the expressions of VEGF and HER-2 in cancer tissues.
Methods: Immunohistochemical staining for SHIP2, HER-2 and VEGF was performed on paraffin-embedded tissue specimens, including 80 specimens of breast cancer and 53 adjacent tissue samples. Two pathologists were invited to interpret the immunohistochemistry results using double-blind method. Expression profiles of SHIP2, HER-2 and VEGF were evaluated and scored according to percentage of positive cells and positive signal intensity. The correlation between SHIP2 and clinicopathological features was analyzed by chi-square test and fourfold table exact test. The correlation among SHIP2, HER-2 and VEGF expression were analyzed using Pearson contingency coefficient analysis for evaluating prognostic relevance to breast cancer.
Results: The positive expression rates of SHIP2, HER-2 and VEGF were significantly higher than that of adjacent normal tissues (p<0.05). SHIP2 expression was significantly increased, in the case of the four risk factors (the poor histological grade, the later stages, lymph node metastasis, and higher BMI) (p<0.05). Positive rate of both SHIP2 and VEGF protein expression indicated a significant correlation between the two proteins (r=0.30, p<0.05). In addition, in the HER-2 protein positive group, SHIP2 protein expression also showed a positive correlation (r=0.71, p<0.05) between these two proteins. Expression of all three proteins were highly related to tumor histopathologic grade, clinical terms, and lymphatic metastasis (p<0.05). Our data indicates that SHIP2 expression in tumor tissue is significantly correlated with HER-2 and VEGF expression.
Conclusion: Our study provides a proof of concept that SHIP2, correlated with HER-2 and VEGF, plays important roles in tumorigenesis, infiltration, and metastasis of breast cancer, and are highly associated with prognosis.
Keywords: Breast cancer; HER-2; Immunohistochemistry; SHIP2; VEGF
315244Introduction
The increasing incidence of breast cancer has seriously endangered women’s health world widely. Currently, a large body of studies points out that the aberrant activation of PI3K/AKT signal pathway plays a critical role in breast cancer development. PI3K/AKT is an important intracellular signaling pathway, and the high frequency alterations of elements in this pathway can cause cell transformation and promote the proliferation and survival of tumor cells. Studies have shown that, PI3K-AKT signaling pathway activation may be a prerequisite for oncogene activated in breast tissue, thereby causes malignant transformation of cells [1]. PI3K-AKT signaling pathway is activated in many human malignancies, and the activation of this pathway in breast cancer is up to 70% [2]. In a normal physiological situation, this pathway is regulated by multiple factors, and SH2-containing-5'- inositol phosphatase-2 (SHIP2) and phosphatase and tensin homolog (PTEN) mainly comprise the negative feedback regulation. However, in an abnormal situation such as tumorigenesis, unlike PTEN, SHIP2 does not negatively modulate PI3K/AKT pathway. Instead, highly expressed SHIP2 is correlated with breast cancer and other malignancies, and becomes one of indicators in breast cancer [3]. Human epidermal growth factor receptor-2 (HER-2) is one of upstream factors in PI3K/AKT pathway. Overexpression of HER-2 can activate PI3K/AKT kinase pathway, thus promote tumor cell survival [4,5]. Further, overexpression of HER-2 is also associated with the prognosis of breast cancer according to recent studies [6]. Vascular Endothelial growth factor (VEGF) has been reported, in various studies, to be a key mediator of angiogenesis in cancer, and VEGF-mediated signaling occurs in tumor cells and this signaling contributes to tumorigenesis. A few studies also find that increased PI3K-AKT signaling pathway activation including HER-2 results in upregulation of VEGF mRNA as well as expression of VEGF. As a positive feedback, the increased VEGF binds to its receptor and stimulates PI3K/AKT pathway [7]. Positive VEGF has been reported highly associated with breast cancer despite cancer forms [8]. However, the influence of VEGF on breast cancer outcome is not well clarified [8,9].
Here, we hypothesize that the activation of upstream regulators such as HER-2 and VEGF in PI3K/AKT signaling pathway may lead to increased expression of downstream proteins including SHIP2, and together they play a synergistic role in the development and progression of breast cancer. Analyzing the correlation of SHIP2, HER-2 and VEGF can provide important information for earlier detection, early treatment and prognosis assessment of breast cancer. Currently, the correlation among the three proteins in breast cancer has not been reported clearly. So we propose to investigate the expression of SHIP2, HER-2 and VEGF in breast cancer using immunehistochemical method, and explore the relevance of these proteins and their possible roles in the process of invasion and metastasis for breast cancer. Our study is a preliminary report of the correlation among these three elements in PI3K/AKT pathway in breast cancer.
Material and Methods
Specimen collection
Samples were collected from Department of Pathology of Liaocheng People's Hospital during the period of 2004-2008, including 80 cases of invasive breast ductal carcinoma and 53 cases of adjacent tissues. All samples were collected from females, aged 26-72 years old, mean age 45 years old, during their first surgeries. These patients did not undergo preoperative chemotherapy and endocrine therapy. International clinical stage of disease classification (TNM staging) for malignancies was based on the American Joint Committee on Cancer (American Joint Committee on Cancer, AJCC) criteria [10]. Among these cases, 28 cases were at stage I, 38 cases at stage II, and 14 cases at stage III. In addition, Nottingham histological grading standards were used for histological grading of breast cancer. As a result 15 cases were of grade I, 32 cases of grade II, and 33 cases of grade III. This study was approved by the Ethics Committee, Liaocheng People's Hospital. The study neither had impact on clinical diagnosis and treatment, nor had influence on final diagnosis and treatment recommendations. Therefore, informed consents can be exempted from the patients’ and family members’ signature, which was approved by the Ethics Committee consent.
Reagents
Rabbit polyclonal anti-human SHIP2 antibody (C-term) was purchased from American ABGENT companies. Rabbit polyclonal anti-human HER-2 antibody, polyclonal rabbit anti-human VEGF antibody, SP universal kit and DAB chromogenic kit were purchased from Beijing Zhongshan Biotechnology Co., Ltd.
Immunohistochemical analysis
Specimens were fixed in 10% neutral formalin, and then were embedded in paraffin. Tissue was sectioned at 4 μm thick, 6 slides for each sample were selected randomly. A routine H & E staining was performed on 2 slides for pathologists to validate the diagnosis. Three slides were used for immunostaining using SHIP2 (diluted at 1:100), HER-2 (dilated at 1:100) and VEGF (diluted at 1:100) antibodies respectively. Signal was detected using SP kit following the manufacturer’s instruction. One slice was used as negative control in the absence of primary antibody.
Evaluation Criteria for Immunohistochemical Results
Two experienced pathologists were invited to evaluate the results of immunohistochemistry using double-blind method. SHIP2 protein expression is detected in cytoplasm, as well as on cell membrane. Both HER-2 and VEGF are detected in cytoplasm. All three proteins are stained brown as positive signal. Expressions of SHIP2 and VEGF are evaluated and scored according to percentage of positive cells and signal intensity based on literature [11]. The evaluation criteria are as follows: positive cells are quantified by using 9-point scoring system: <10% scored as 1 ≥ 10% -50% scored as 2>50% of the total counted scored as 3. Staining intensity criteria is as followed: no coloring or uniform yellow background is defined as 0 point, light brownish yellow as 1 point, brown as 2 points and tan as 3 points. Finally, the combination of the two values are used for assessment criteria ( ≥ 3 positive expression, <3 divided negative). HER-2 protein was quantified by the results of a positive signal intensity determination: 0 (no staining or membrane staining in less than 10%), + (>10% of cells carrying incomplete membrane staining), + + (>10% cell carrying weaker but complete membrane staining), + + + (>10% of cells stained with a strong and complete signal on membrane). As the result, for HER-2, 0 to + is considered as negative, + + to + + + is considered as positive.
Statistical analysis
All statistical analyses are carried out using SPSS 19.0 statistical software. Data are represented as mean ± SD. Enumeration data are analyzed by chi-square test and fourfold table exact test to determine the association between SHIP2 and the clinical and pathological features, including patient's ages, tumor grades, clinical stages, and lymph node. The correlation among SHIP2, HER-2 and VEGF expression is analyzed using Pearson contingency coefficient analysis to evaluate prognostic relevance to breast cancer. Moreover, we can speculate the correlation between the expression of SHIP2 and the prognosis of breast cancer. Results with P<0.05 are considered statistically significant.
Results
Expression of SHIP2, VEGF-C and HER-2 protein in breast cancer tissues
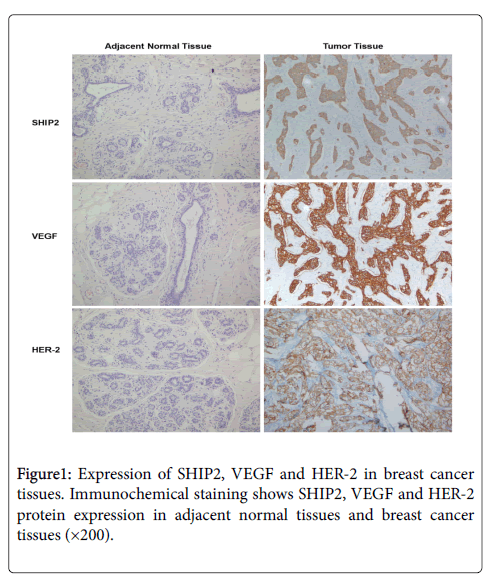
SHIP2 protein in breast cancer cells is mainly localized in the cytoplasm (Figure 1), and positive expression is characterized by brown color staining in cytoplasm in a patchy distribution fashion. VEGF protein is mainly localized in the cytoplasm of tumor cells and some nuclei (Figure 1). HER-2 protein is mainly located on cancer cell membranes (Figure 1). In 80 cases studied here, the positive expression rates for SHIP2, HER-2 and VEGF are 42.5%, 50.0% and 71.2% respectively in breast cancer tissues, significantly higher than the positive rate in 53 adjacent normal tissues which has 9.4% for SHIP2, 11.3% for VEGF and 7.55% for HER-2 (p<0.05) (Table 1).
| cases | SHIP2 | VEGF | HER-2 | ||||
|---|---|---|---|---|---|---|---|
| +(R %) | P | +(R %) | P | +(R %) | P | ||
| Breast cancer | 80 | 34 (42.5%) | X2=16.819 p=0.000 | 57 (71.2%) | X2=45.926 | 40(50.0%) | X2=25.953 |
| p=0.000 | p=0.000 | ||||||
| Normal tissues | 53 | 5 (9.4%) | 6 (11.3%) | 4(7.55%) | |||
| R: expression rate | |||||||
Table 1: Expression of SHIP2, VEGF and HER-2 in breast cancer tissues and adjacent normal tissues.
Association between SHIP2 expression and clinicopathological features in breast cancer
The correlation between SHIP2 expression and clinicopathological features of breast cancer is shown in Table 2. In total 33 of G3 grading cancer, 24 are SHIP2 positive (72.7%, p=0.000). In 52 of stage II+III cancers, 32 are SHIP2 positive (61.4%, p=0.002). In total 46 breast cancers with lymph node metastasis, 31 are SHIP2 positive (67.4%, p=0.000). In BMI greater than 28 group, 81.3% are SHIP2 positive (p=0.005). These results indicate that SHIP2 protein expression is significantly increased in breast cancers and this increase is highly associated with four risk factors: the poor histological grade, the later stages, lymph node metastasis, and higher BMI (p<0.05); whereas there is no significant association between SHIP2 expression with age, tumor size and menstruation (p>0.05).
| Pathological parameters | Cases | SHIP2 expression | χ2 value | p value | |
|---|---|---|---|---|---|
| + | - | ||||
| Ages | |||||
| <35 | 12 | 10 | 2 | 3.374 | 0.111 |
| ≥ 35 | 68 | 24 | 44 | ||
| Tumor size | |||||
| ≤ 2 cm | 32 | 13 | 19 | 0.212 | 0.818 |
| >2 cm | 48 | 22 | 26 | ||
| Histological grading | |||||
| G1+G2 | 47 | 12 | 35 | 17.448 | 0 |
| G3 | 33 | 24 | 9 | ||
| Clinical stage | |||||
| I | 28 | 7 | 21 | 9.725 | 0.002 |
| II+III | 52 | 32 | 20 | ||
| Lymph node metastasis | |||||
| Positive | 46 | 31 | 15 | 21.926 | 0 |
| Negative | 34 | 5 | 29 | ||
| BMI | |||||
| <28 | 64 | 26 | 38 | 8.455 | 0.005 |
| ≥ 28 | 16 | 13 | 3 | ||
| Menopausal status | |||||
| Before | 42 | 19 | 23 | 0.08 | 0.824 |
| After | 38 | 16 | 22 | ||
Table 2: Correlation between SHIP2 protein and clinicopathological features in breast cancer.
Correlation between SHIP 2 and VEGF or HER-2 expression in breast cancer
Expression of HER-2 and VEGF and their correlation are listed in Table 3. In total 33 of G3 grading cancers, 24 of them are HER-2 positive (72.7%, p=0.003), and 28 of them are VEGF positive (84.8%, p=0.004). In total 52 of stage II+III cancers, 37 are positive for HER-2 (71.2%, p=0.000) and 37 are positive for VEGF (71.2%, p=0.017). In total 46 cancers with lymph node metastasis, 35 are positive for HER-2 (76.1%, p=0.001) and 40 are positive for VEGF (87%, p=0.000). These results indicate that not only there is a correlation between VEGF and HER-2 protein expression in breast cancer tissue, but also this correlation is highly associated with classification, stage and lymph node metastasis.
| Pathological parameters | Cases | HER-2 | VEGF | ||
|---|---|---|---|---|---|
| + | Significance | + | Significance | ||
| Histological grading | |||||
| G1+G2 | 47 | 18 | X2=9.216 | 25 | X2=8.690 |
| G3 | 33 | 24 | p=0.003 | 28 | p=0.004 |
| Clinical stage | |||||
| I | 28 | 3 | X2=19.226 | 12 | X2=6.140 |
| II+III | 52 | 37 | p=0.000 | 37 | p=0.017 |
| Lymphnode metastasis | |||||
| Positive | 46 | 35 | X2=13.095 | 40 | X2=20.755 |
| Negative | 34 | 5 | p=0.001 | 13 | p=0.000 |
Table 3: Correlation between HER-2 and VEGF protein and clinicopathological features of breast cancer.
Of the 57 VEGF positive cancers, 31 are also positive for SHIP2, which leads to a 38.8% positive rate of SHIP2 and VEGF expression in total 80 cases studied, compared to only 6.2% of SHIP2 positive but VEGF negative tissues (r=0.30, p<0.05), suggesting a positive correlation between SHIP2 and VEGF. Of the 40 HER-2 positive cancers, 31 are also positive for SHIP2, leading to a 38.8% positive rate of HER-2 and SHIP2 expression in total 80 cases studied, compared to only 3.8% of SHIP2 positive but HER-2 negative tissues (r=0.71, p<0.05) (Table 4), suggesting a positive correlation between SHIP2 and HER-2.
| Cases | SHIP2 (+) | SHIP2 (-) | Significance | |||
|---|---|---|---|---|---|---|
| Cases | % | Cases | % | |||
| VEGF expression | ||||||
| Negative | 23 | 5 | 6.20% | 18 | 22.50% | X2=7.057 |
| Positive | 57 | 31 | 38.80% | 26 | 32.50% | p=0.012 |
| R=0.297 | ||||||
| HER-2 | ||||||
| Negative(0-+) | 40 | 3 | 3.80% | 37 | 46.20% | X2=28.872 |
| Positive(++-+++) | 40 | 31 | 38.80% | 9 | 11.20% | p=0.000 |
| R=0.708 | ||||||
Table 4: Correlation among the expression of SHIP2 and VEGF and HER-2 in breast cancer.
Together, these results strongly demonstrate the correlation of SHIP2, HER-2 and VEGF in breast cancer.
Discussion
Angiogenesis is an important factor in tumor progression, which is regulated by a variety of pro-angiogenic factors such as VEGF and anti-angiogenic factors. Previous studies have shown that, PI3K/AKT signaling pathway not only takes effect in cell proliferation and apoptosis [11], but also plays an important role in angiogenesis as a regulation center of signaling pathways. Together, they decide the fate of vascular status generation [12]. Activated PI3K/AKT can increase the expression of VEGF and thus induces tumor angiogenesis and formation, and increased activation of PTEN reduces VEGF expression and suppresses tumor angiogenesis, suggesting that PTEN may be a modulator in the pathway [13-15].
VEGF is one of the most critical factors to promote tumor angiogenesis, which participates in the invasion and lymph node metastasis of breast cancer. Therefore it has been considered as an independent prognostic indicator for breast cancer [16]. Our results show that VEGF expression in breast cancer tissues is significantly higher than that in normal tissues. We also identify that higher expression of VEGF is significantly correlated with poor clinicopathological features in breast cancer, such as lymph node metastasis, a later TNM staging. This means the later the TNM stage patients are, the higher VEGF expression they have in breast cancer tissues. Our results are consistent with the findings reported by Linderholm et al. [17] who suggest that VEGF expression has prognostic significance for breast cancer patients.
Large amount of studies has shown that HER-2 overexpression enhances invasiveness of malignant cells, and ultimately leads to tumor invasion and distant metastasis-prone and also been a target for breast cancer treatment [18]. In breast cancer, HER-2 activates NF-κB through the PI3K/AKT signaling pathway and thus promotes cell malignant transformation [4]. After analyzing 506 cases of breast cancer, Yamashita et al. confirm that positive HER-2 expression is correlated with prognosis of breast cancer [6]. Yang et al. [19] report in their study that HER-2 overexpression is significantly correlated with cancer histological grade and lymph node metastasis. Our study shows that HER-2 expression in breast cancer tissues is significantly higher than the adjacent normal tissues, and this elevated expression is positively correlated with histological grade and lymph node metastasis, which suggests a poor prognosis.
The correlation between HER-2 and VEGF shown in this study synchronizes with others. A large scale of study shows that there are 72.5% of breast cancers are positive for VEGF despite the cancer forms, with high frequency in HER-2 positive cancers [8]. In cancer cells, overexpression of HER-2 elevates VEGF significantly [20], which may underline this positive correlation between HER-2 and VEGF in breast cancers.
SHIP2 is currently known as a very important negative regulator in the insulin-signaling pathway [21]. In PI3K/PIP3/AKT signaling pathway, SHIP2 as well as PTEN protein make PIP3 phosphate groups dephosphorylated at different sites, therefore execute their negative regulatory roles by inhibiting AKT in PI3K/PIP3/AKT signaling pathway [22]. Previous studies have found that SHIP2 deletion could prevent obesity caused by high-fat diet [23]. Unlike PTEN, SHIP2 plays a key role in the development and progression of malignant tumors. Clinical studies indicate that the incidence of breast cancer may increase in premenopausal women over 35 years old with a high level of body mass index (BMI) [24]. After analyzing 285 cases of primary breast cancer by using immunohistochemical assay, Prasad et al. [3] find that SHIP2 is highly expressed in invasive breast cancer, and the disease-free survival and overall survival are significantly shortened in these patients with SHIP2 over-expression. The study suggests that SHIP2 overexpression can promote breast cancer cell proliferation, thereby promoting disease progression and metastasis. So SHIP2 is considered as a very important indicator for breast cancers.
The BMI of Chinese women is significantly different from that of European or American women [25,26]. Based on population study, we refer to indicators used in Chinese population, which is different from the BMI cut off index used in previous study [26]. Our study BMI was divided into three grades: <18.5 for low body mass, ≥ 18.5 to <28 as normal, ≥ 28 for overweight.
SHIP2 protein expression in different BMI groups was analyzed by using immunohistochemistry in our study. We find that the high expression of SHIP2 is correlated with obesity, clinical stage, lymph node metastasis, estrogen and progesterone receptors. In physiological situation, like PTEN, SHIP2 plays a negative regulator in the PI3K/ PIP3/AKT signaling pathway. We speculate the possible mechanisms for SHIP2 playing a pro-oncogenic role instead of anti-oncogenic role in cancer cells: studies have reported, AKT activation is via PI3K directly, and EGF/EGFR pathway may also indirectly activates AKT [27]. SHIP2 overexpression activated AKT by blocking EGFR endocytosis degradation [28]. In addition, SHIP2 can dephosphorylated PI3P, and then generate phosphatidylinositol 3,4- bisphosphate, which is necessary for activating AKT signaling pathway [29]. SHIP2 can regulate tumor cytoskeletal reorganization and filopodia formation, thereby accelerating the migration of tumor cells and promote tumor metastasis [30]. Future study of PI3K pathway activity such as pAkt correlated with positive SHIP2, HER-2 and VEGF in breast cancer will provide information for the correlation and mechanism.
In our study, SHIP2 expression rate is 56% in the group with positive VEGF expression, indicating a significant positive correlation between SHIP2 and VEGF expression in breast cancer (p<0.05). In addition, in the group with positive HER-2, SHIP2 expression rate is 75%, indicating a significant correlation between SHIP2 and HER-2 in breast cancer (p<0.05). These results suggest that these three proteins possibly correlate synergistically to promote the development of breast cancer. We recommend that the combination of these three proteins expression can be considered as factors for poor prognosis.
In conclusion, our study finds that SHIP2, VEGF and HER-2 are highly expressed in breast cancer. SHIP2 is associated with obesity, and co-expression of three proteins may indicate a poor prognosis for breast cancer. Further study needs to be done such as expanding sample volume to and dissecting focal location of these three proteins to validate this conclusion. In addition, quantification of transcripts and proteins of SHIP2, VEGF and HER-2 combined with other elements in PI3K/AKT pathway in breast cancer tissues will help us understand the dynamic alteration of these elements, which will provide valuable information for disease prediction and treatment. However, tumor metastasis involves multiple aspects, which is an extremely complex process. In order to improve understanding for the mechanism of metastasis, we need to analyze multiple factors involved in PI3/AKT pathway, and thus provide much accurate assessment for prognosis and better guidance for clinical treatments.
Acknowledgment
The study was supported by Key Technologies R & D Program of Shandong province (Grant No. 2012GSF11845).
References
- Amundadottir LT, Leder P (1998) Signal transduction pathways activated and required for mammary carcinogenesis in response to specific oncogenes. Oncogene 16: 737-746.
- López-Knowles E, O'Toole SA, McNeil CM, Millar EK, Qiu MR, et al. (2010) PI3K pathway activation in breast cancer is associated with the basal-like phenotype and cancer-specific mortality. Int J Cancer 126: 1121-1131.
- Prasad NK, Tandon M, Handa A, Moore GE, Babbs CF, et al. (2008) High expression of obesity-linked phosphatase SHIP2 in invasive breast cancer correlates with reduced disease-free survival. Tumor Biol 29: 330-341.
- Rohan TE, Li SQ, Hartwick R, Kandel RA (2006) p53 Alterations and protein accumulation in benign breast tissue and breast cancer risk: a cohort study. Cancer Epidemiol Biomarkers Prev 15: 1316-1323.
- Li-Ning TE, Ronchetti R, Torres-Cabala C, Merino MJ (2005) Role of chromogenic in situ hybridization (CISH) in the evaluation of HER2 status in breast carcinoma: comparison with immunohistochemistry and FISH. Int J Surg Pathol 13: 343-351.
- Yamashita H, Nishio M, Toyama T, Sugiura H, Zhang Z, et al. (2004) Coexistence of HER2 over-expression and p53 protein accumulation is a strong prognostic molecular marker in breast cancer. Breast Cancer Res 6: R24-R30.
- Laughner E, Taghavi P, Chiles K, Mahon PC, Semenza GL (2001) HER2 (neu) signaling increases the rate of hypoxia-inducible factor 1alpha (HIF-1alpha) synthesis: novel mechanism for HIF-1-mediated vascular endothelial growth factor expression. Mol Cell Biol 21: 3995-4004
- Liu Y, Tamimi RM, Collins LC, Schnitt SJ, Gilmore HL, et al. (2011) The association between vascular endothelial growth factor expression in invasive breast cancer and survival varies with intrinsic subtypes and use of adjuvant systemic therapy: results from the Nurses’ Health Study. Breast Cancer Res Treat 129: 175-184.
- Jin K, Zhang Y, Zheng L, Fu K, Zhu T, et al. (2011) Vascular Endothelial Growth Factor in Breast Cancer: A Systematic Review. Journal of US-China Medical Science 8: 175-180.
- Warner CL, Cockerell CJ (2011) The new seventh edition American joint committee on cancer staging of cutaneous non-melanoma skin cancer. Am J Clin Dermatol 12: 147-154.
- Liu Y, Yu C, Qiu Y, Huang D, Zhou X, et al. (2012) Downregulation of EphA2 expression suppresses the growth and metastasis in squamous-cell carcinoma of the head and neck in vitro and in vivo. J Cancer Res Clin Oncol 138: 195-202.
- Ribatti D, Vacca A, Nico B, Quondamatteo F, Ria R, et al. (1999) Bone marrow angiogenesis and mast cell density increase simultaneously with progression of human multiple myeloma. Br J Cancer 79: 451-455.
- Steelman LS, Chappell WH, Abrams SL, Kempf RC, Long J, et al. (2011) Roles of the Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR pathways in controlling growth and sensitivity to therapy-implications for cancer and aging. Aging (Albany NY) 3: 192-222.
- Ma J, Sawai H, Ochi N, Matsuo Y, Xu D, et al. (2009) PTEN regulates angiogenesis through PI3K/Akt/VEGF signaling pathway in human pancreatic cancer cells. Mol Cell Biochem 331: 161-171.
- Tian T, Nan KJ, Wang SH, Liang X, Lu CX, et al (2010) PTEN regulates angiogenesis and VEGF expression through phosphatase-dependent and -independent mechanisms in HepG2 cells. Carcinogenesis 31: 1211-1219.
- Nicolini A, Campani D, Miccoli P, Spinelli C, Carpi A, et al. (2004) Vascular endothelial growth factor (VEGF) and other common tissue prognostic indicators in breast cancer: a case-control study. Int J Biol Markers 19: 275-281.
- Linderholm BK, Lindahl T, Holmberg L, Klaar S, Lennerstrand J, et al. (2001) The expression of vascular endothelial growth factor correlates with mutant p53 and poor prognosis in human breast cancer. Cancer Res 61: 2256-2260.
- Lian J, Li L, Sun R, Wang Q (2009) Correlation of Ki67, C-erbB-2 and VEGF expression in breast cancer and their clinical significance. Journal of Shanxi Medical University 2: 013.
- Yang YL, Fan Y, Lang RG, Gu F, Ren MJ, et al. (2012) Genetic heterogeneity of HER2 in breast cancer: impact on HER2 testing and its clinicopathologic significance. Breast Cancer Res Treat 134: 1095-1102.
- Wen XF, Yang G, Mao W, Thornton A, Liu J, et al. (2006) HER2 signaling modulates the equilibrium between pro- and antiangiogenic factors via distinct pathways: implications for HER2-targeted antibody therapy. Oncogene 25: 6986–6996.
- Bertelli DF, Ueno M, Amaral ME, Toyama MH, Carneiro EM, et al. (2003) Reversal of denervation-induced insulin resistance by SHIP2 protein synthesis blockade. Am J Physiol Endocrinol Metab 284: E679-E687.
- Gupta A, Dey CS (2009) PTEN and SHIP2 regulates PI3K/Akt pathway through focal adhesion kinase. Mol Cell Endocrinol 309: 55-62.
- Sleeman MW, Wortley KE, Lai KM, Gowen LC, Kintner J, et al. (2005) Absence of the lipid phosphatase SHIP2 confers resistance to dietary obesity. Nat Med 11: 199-205.
- Cecchini RS, Costantino JP, Cauley JA, Cronin WM, Wickerham DL, et al. (2012) Body mass index and the risk for developing invasive breast cancer among high-risk women in NSABP P-1 and STAR breast cancer prevention trials. Cancer Prev Res (Phila) 5: 583-592.
- Flegal KM, Carroll MD, Ogden CL, Curtin LR (2010) Prevalence and trends in obesity among US adults 1999-2008. JAMA 303: 235-241.
- Ko GT, Tang J, Chan JC, Sung R, Wu MM, et al. (2001) Lower BMI cut-off value to define obesity in Hong Kong Chinese: an analysis based on body fat assessment by bioelectrical impedance. Br J Nutr 85: 239-242.
- Bonaccorsi L, Carloni V, Muratori M, Formigli L, Zecchi S, et al. (2004) EGF receptor (EGFR) signaling promoting invasion is disrupted in androgen-sensitive prostate cancer cells by an interaction between EGFR and androgen receptor (AR). Int J Cancer 112: 78-86
- Wu CJ, O'Rourke DM, Feng GS, Johnson GR, Wang Q, et al. (2001) The tyrosine phosphatase SHP-2 is required for mediating phosphatidylinositol 3-kinase/Akt activation by growth factors. Oncogene 20: 6018-6025.
- Scheid MP, Huber M, Damen JE, Hughes M, Kang V, et al. (2002) Phosphatidylinositol (3,4,5)P3 is essential but not sufficient for protein kinase B (PKB) activation; phosphatidylinositol (3,4) P2 is required for PKB phosphorylation at Ser-473: studies using cells from SH2-containing inositol-5-phosphatase knockout mice. J Biol Chem 277: 9027-9035.
- Larue L, Bellacosa A (2005) Epithelial-mesenchymal transition in development and cancer: role of phosphatidylinositol 3' kinase/AKT pathways. Oncogene 24: 7443-7454.
Citation: Han H, Wang B, Zhao J, Xu G, Wang X, et al. (2017) Analysis of SHIP2 Expression and its Correlation with VEGF and HER-2 Expression in Breast Cancer. J Clin Exp Pathol 7:314. DOI: 10.4172/2161-0681.1000314
Copyright: © 2017 Han H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 4173
- [From(publication date): 0-2017 - Aug 29, 2025]
- Breakdown by view type
- HTML page views: 3278
- PDF downloads: 895