Angiotensin Converting Enzyme Gene Polymorphism in an Urban Worksite Cohort from Mumbai, Western India
Received: 08-Feb-2019 / Accepted Date: 18-Feb-2019 / Published Date: 25-Feb-2019 DOI: 10.4172/2161-0681.1000362
Abstract
Objective: An insertion/deletion polymorphism in the Angiotensin Converting Enzyme (ACE) gene has been implicated in the pathogenesis of heart disease. The present cross sectional study evaluated the ACE gene I/D polymorphism in an urban worksite cohort.
Method: Fasting blood samples from 132 subjects from a worksite in Mumbai, Western India was analysed for ACE I/D polymorphism by polymerase chain reaction along with biochemical and lipid parameters. Circulating levels of ACE activity was also determined.
Results: Among the study cohort, 33 subjects (25%) had metabolic disorders. The remaining 99 subjects without metabolic disorders could be considered healthy. These subjects had a frequency of 0.36, 0.38 and 0.26 for the I/I, I/D and D/D genotypes, respectively. The genotypic frequency of subjects with metabolic disorders was not different from that of the healthy subjects. Homozygous carriers of the ‘D’ allele exhibited two-fold higher serum ACE activity compared to homozygous carriers of the ‘I’ allele. Serum ACE activity was significantly correlated to serum total cholesterol (r=0.501, p=0.006) and triglyceride (r=0.469, p=0.012) levels in D/D carriers. Contrary to this serum ACE activity was significantly related to fasting plasma glucose levels (r=0.39, p=0.03) in the I/I homozygotes.
Conclusion: In this worksite urban Indian cohort, I/D polymorphism in the ACE gene is associated with established risk factors namely fasting serum cholesterol, triglycerides and plasma glucose levels. While the ACE activity of D/D homozygotes was predictive of total cholesterol and triglyceride levels, the ACE activity of I/I homozygotes was predictive of plasma glucose levels.
Keywords: Angiotensin converting enzyme; Enzyme activity; Polymorphism; Worksite; Serum cholesterol; Plasma glucose; Metabolic disorders
Introduction
The angiotensin converting enzyme (ACE) is an important component of the Renin- Angiotensin system (RAS) involved in the regulation of blood pressure, sodium and water homeostasis. In humans, ACE converts angiotensin I to angiotensin II, a potent vasoconstrictor and degrades bradykinin, a vasodilator [1]. These activities contribute to the regulation of blood pressure. ACE inhibitors constitute an effective therapeutic option in the management of essential hypertension.
Since the discovery of a polymorphism involving insertion/deletion of a 287 bp fragment in the intron 16 of ACE gene [2], numerous studies have assessed the role of this polymorphism in hypertension [3,4], heart disease [5], stroke [6] and diabetic nephropathy [7]. The phenotypic effects of the polymorphism on ACE enzyme activity have been reported in few studies [4,8]. However, results from these studies have failed to establish a consistent role of this polymorphism as a genetic marker of cardiovascular disease.
Indian population is highly susceptible to coronary heart disease, ischemic heart disease and stroke [9,10]. Conventional risk factors such as smoking, obesity, type-2 diabetes and dyslipidaemia have been implicated in the pathogenesis among Indians similar to Caucasians. Since there is a high familial tendency to develop cardiovascular diseases in the Indian population, studies on the role of genetic factors are important in our population.
A large number of case control studies have been carried out in different parts of India. Although India is one country, there are differences in lifestyle, environment, dietary, religious and cultural habits of subjects from different regions. South Indian subjects have high incidence of type-2 diabetes and coronary artery disease, CAD while East and North Indian subjects have high incidence of stroke [9]. Further, hospital-based studies introduce selection bias and may not represent true population sample.
Cohort studies offer a unique opportunity to identify subjects in an unbiased manner. Further work related stress could also be a risk factor among Indians similar to Caucasians [11]. Worksite represents a common environment for a major part of productive life. Hence implementing preventive measures and follow up is easy. This would offer substantial benefits to the society besides improving healthcare of the workforce.
The objective of the present study is to evaluate the I/D polymorphism in the ACE gene and its metabolic implications, in an urban worksite cohort from Mumbai, Western India.
Materials And Methods
Subjects
A total of 132 unrelated individuals undergoing Annual Medical Check-up from June 2012 to July 2012 at the institute participated in the study. Blood was collected between 9 am and 10 am after 12hr overnight fasting. Informed written consent was obtained from the study subjects. Body weight was measured in kilograms. Height was measured in centimetres with the subject in the standing position without shoes. Waist circumference was measured as the smallest length around the umbilicus. Blood pressure was measured twice while resting using a mercury sphygmomanometer. Medical history and clinical data were collected using a detailed questionnaire. The project was approved by the Human Ethics committee of the Haffkine Institute.
Blood samples
About 10-12ml of venous blood was collected. This included 2ml with fluoride, 5ml in plain vaccutainers and another 5ml in the presence of ethylenediamine tetraacetic acid (EDTA). Blood collected in fluoride was immediately centrifuged at 3000 rpm for 10 minutes and plasma glucose levels were determined within 1h. Blood collected in plain vaccutainers was allowed to clot for an hour at room temperature and then centrifuged at 3000 rpm for 10 minutes. Serum was separated and dispensed into three micro centrifuge tubes. One aliquot of serum was used for biochemical analysis. The other two aliquots were stored at -800°C.
Biochemical analysis
Plasma glucose, serum total cholesterol, triglycerides, urea and creatinine levels were determined by enzymatic methods using kits from Erba Mannheim (Transasia Bio Medical Ltd., India). Activities of serum alanine transaminase (ALT) and aspartate transaminase (AST) were measured by spectrophotometry using kits from Erba Mannheim. The absorbance was measured using Erba Chem-5 semi-autoanalyser (Transasia Bio Medical Ltd., India).
Enzyme and hormone assays
Serum ACE activity was measured by spectrophotometric method using a kit (Buhlmann Laboratories AG, Schönenbuch, Switzerland) which contained a synthetic substrate, furan-acryloyl-Lphenylalanylglycylglycine. The absorbance was measured using Synergy HT multimode reader (Biotek Instruments, Vermont, US). The intra-assay precision was 2.7% and the inter-assay precision was 8.1%. Serum renin levels were assayed using renin ELISA kit (Demeditec Diagnostics Gmbh, Kiel, Germany). The intra-assay coefficient of variation (CV) was 3.8% and inter-assay CV was 5.1%. Serum insulin was assayed using an ELISA kit (Mercodia AB, Uppsala, Sweden). The intra-assay coefficient of variation (CV) was 2.8% and inter-assay CV was also 2.8%. Serum C-peptide was assayed by ELISA using a kit (Mercodia AB, Uppsala, Sweden). The intra-assay coefficient of variation (CV) was 2.9% and inter-assay CV was 0.6%.
DNA isolation & genotyping
Genomic DNA was isolated from EDTA-treated blood samples using QIAamp DNA Blood Mini Kit (Qiagen, California, US) according to manufacturer’s instructions. The DNA was genotyped by a modified PCR method [12]. Primers used were ACE-Forward primer: 5′-GCCCTGCAGGTGTCTGCAGCATGT-3′and ACE-Reverse primer: 5′-GGATGTGGCTCTCCCCGCCTTGTCTC-3′ (Eurofins, MWG Operon, Alabama, USA). The PCR consisted of an initial denaturation at 94°C for 3 min followed by 35 cycles of denaturation at 94°C for 30s, annealing at 56°C for 45s, and elongation at 72°C for 2 min. The final elongation was at 72°C for 7 min. The PCR products were separated on 2% agarose gel containing ethidium bromide.
Statistical analysis
Body mass index (BMI), the ratio of body weight in kilograms to height in meters square, was determined and expressed in Kg/m2 units. Insulin resistance and β cell function were determined using the HOMA method [13]. Data are presented as mean ± standard error of mean (SEM). For variables showing skewed distribution, median values are presented. Group means were compared using unpaired t-test. Allele frequency and genotype frequencies were compared using Chi-square test. All statistical analyses were performed using Statistical Package for Social Sciences (SPSS) software for Windows v 21.0. Minimum level of significance was taken at p<0.05.
Results
Characteristics of the study subjects
The study included a total of 132 subjects. Table 1 presents the anthropometric and metabolic characteristics. Among the subjects, 64% were men. The study cohort was comparatively young with the mean age of 35 years and had a body weight of 65.5 ± 1.1 kg (mean ± SEM). While men were non-obese, women had abdominal obesity since the waist circumference was more than 80 cm (Table 1). Both systolic and diastolic blood pressures were within the normal limits. The levels of alanine transaminase and aspartate transaminase activities were within upper limit of the normal expected range.
| Characteristics | Value* | % |
|---|---|---|
| Number | 132 | |
| Men | 84 | 64 |
| Women | 48 | 36 |
| Age (years) | 35.2 ± 0.84 | |
| Body Weight (Kg) | 65.5 ± 1.1 | |
| Body Mass Index (Kg/m2) | ||
| Men | 23.9 ± 0.40 | |
| Women | 26.0 ± 0.80 | |
| Waist circumference (cm) | ||
| Men | 89.2 ± 1.19 | |
| Women | 89.8 ± 1.32 | |
| Systolic Blood Pressure (mm Hg) | 123.4 ± 1.13 | |
| Diastolic Blood Pressure (mm Hg) | 78.6 ± 0.75 | |
| Metabolic Parameters Glucose (mg/dL) | 92.9 ± 1.6 | |
| Triglycerides (mg/dL) | 123.7 ± 7.5 | |
| Cholesterol (mg/dL) | 184.5 ± 3.4 | |
| Alanine Transaminase (IU/L) | 29.4 ± 1.5 | |
| Aspartate Transaminase (IU/L) | 26.1 ± 1.1 |
Table 1: Characteristics of the Study Subjects (*Data are expressed as (mean ± SEM) units).
The study cohort exhibited strong family history of cardiovascular and metabolic disorders. Family history of hypertension was reported by 43 subjects (32.6%), 21 (15.9%) had a family history of Type 2 diabetes and 4 (3%) had family history of heart disease. In this cohort, there were 15 (11.4%) obese subjects, 10 (7.6%) had hypertension, 7 (5.3%) had type 2 diabetes and 1 (0.7%) had cancer. Patients with hypertension or diabetes were on oral drugs. Thus, there were 33 subjects (25%) with metabolic disorders. Excluding these subjects there were 99 subjects that can be considered healthy.
ACE genotype in the study subjects
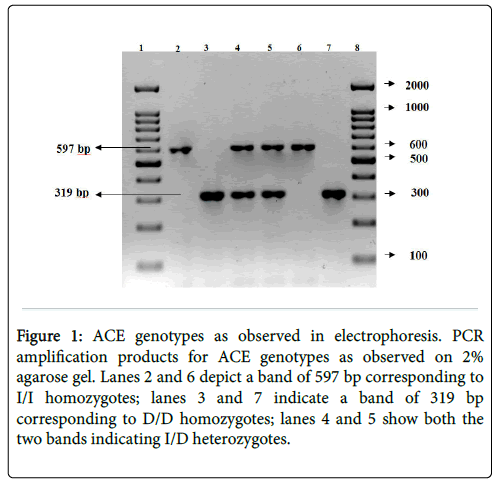
The PCR products from the I/I homozygote is seen as a single band at 597 bp (Figure 1). The D/D homozygote is visualized as a single band at 319 bp. The I/D heterozygote is identified by the presence of both the bands. Lanes 2 and 6 identify I/I homozygotes; lanes 3 and 7 identify D/D homozygotes; lanes 4 and 5 bands identify I/D heterozygotes.
Figure 1: ACE genotypes as observed in electrophoresis. PCR amplification products for ACE genotypes as observed on 2% agarose gel. Lanes 2 and 6 depict a band of 597 bp corresponding to I/I homozygotes; lanes 3 and 7 indicate a band of 319 bp corresponding to D/D homozygotes; lanes 4 and 5 show both the two bands indicating I/D heterozygotes.
In subjects without metabolic disorders (n=99) the frequency of the ‘I’ allele was 0.55 and that of the ‘D’ allele was 0.45 (Table 2). The allele frequency of subjects with metabolic disorders was not significantly different from those without metabolic disorder (χ2=0.820, P=0.423).
| Allele Frequency | Genotypic Frequency | ||||||
|---|---|---|---|---|---|---|---|
| I | D | I/I | I/D | D/D | χ2 | p | |
| Subjects without metabolic disorders (99) | 0.55 | 0.45 | 0.36 | 0.38 | 0.26 | 2.59 | 0.27 |
| Subjects with metabolic disorders (33) | 0.45 | 0.55 | 0.21 | 0.46 | 0.33 | ||
Table 2: Allele and Genotypic frequency of ACE gene in subjects with or without metabolic disorders.
Frequencies of I/I, I/D and D/D genotypes for subjects with metabolic disorders was not different from the frequencies observed in healthy subjects.
ACE genotype and metabolic characteristics
The metabolic characteristics of the healthy subjects (n=99) stratified according to ACE genotype are presented in Table 3. The mean age was similar among the three genotypic groups. Blood pressure levels did not vary among the different genotypes. Biochemical parameters of fasting plasma glucose, serum triglyceride and total cholesterol levels tended to be higher in D/D homozygotes compared to the levels found in I/I homozygotes. The kidney and liver function test parameters were not significantly different among the subjects with different genotypes.
| Characteristics | I/I (36) | I/D (37) | D/D (26) |
|---|---|---|---|
| Age (years) | 31.4 ± 7.3 | 34.2 ± 8.8 | 34.5 ± 9.8 |
| Systolic Blood Pressure (mm Hg) | 124.2 ± 2.1 | 119.5 ± 1.6 | 121.9 ± 2.2 |
| Diastolic Blood Pressure (mm Hg) | 78.3 ± 0.3 | 76.0 ± 0.4 | 77.3 ± 0.5 |
| Glucose (mg/dL) | 86.5 ± 1.4 | 89.9 ± 2.1 | 91.1 ± 3.5 |
| Triglycerides (mg/dL) | 102.0 ± 10.4 | 107.2 ± 9.3 | 119.7 ± 18.2 |
| Cholesterol (mg/dL) | 177.4 ± 5.8 | 175.1 ± 5.6 | 188.5 ± 7.2 |
| Insulin (μU/ml)* | 11.3(9.1-14.9) | 8.8(6.9-13.8) | 9.4(5.3-15.7) |
| C-peptide (pmol/L)* | 574(502-680) | 617(453-697) | 618(443-817) |
| HOMA IR* | 2.4(1.8-3.0) | 1.9(1.5-3.1) | 2.2(1.2-3.8) |
Table 3: Characteristics of healthy subjects distributed according to ACE genotype (Data are expressed as (mean ± SEM) units. *Median (95% CI)).
Median serum insulin levels of subjects homozygous for the I allele (I/I) tended to be higher than insulin levels of subjects with other genotypes (Table 3). Serum C-peptide levels and HOMA-IR values did not differ significantly among the genotypes.
Association of ACE genotype with phenotype
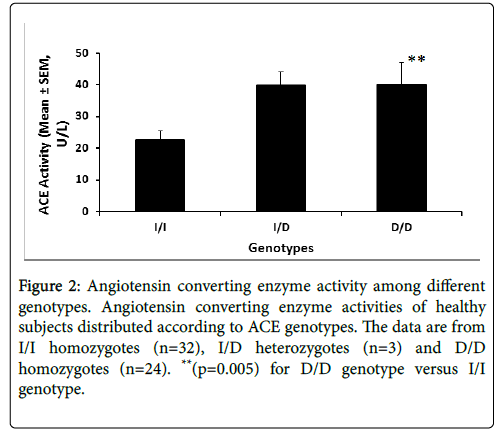
Figure 2 illustrates the serum ACE enzyme activity stratified by the genotype. ACE activity data are available for 59 of the 99 subjects.
Figure 2: Angiotensin converting enzyme activity among different genotypes. Angiotensin converting enzyme activities of healthy subjects distributed according to ACE genotypes. The data are from I/I homozygotes (n=32), I/D heterozygotes (n=3) and D/D homozygotes (n=24). **(p=0.005) for D/D genotype versus I/I genotype.
Subjects homozygous for D/D genotype exhibited 2-fold higher enzyme activity (p<0.005) compared to the I/I homozygotes (Fig 2).
Association between ACE phenotype and metabolic risk factors
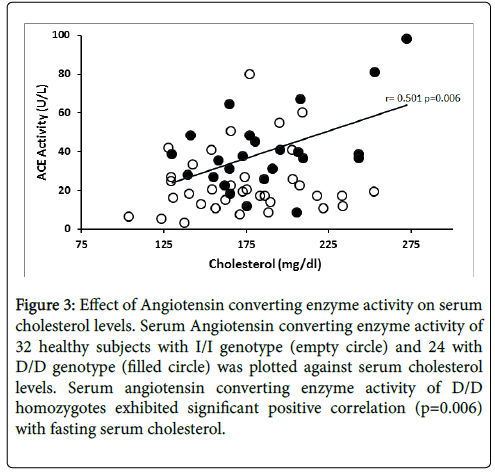
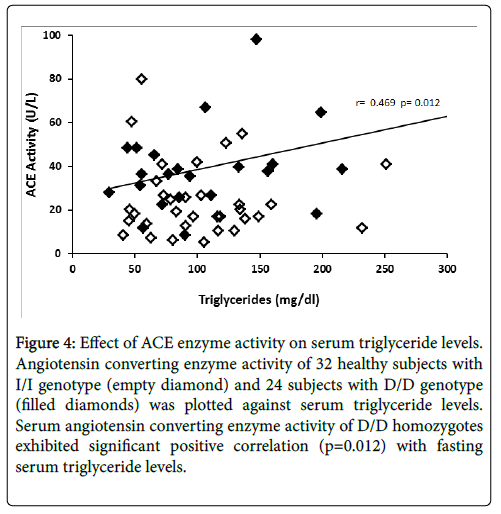
The relationship between ACE enzyme activity and fasting serum total cholesterol levels is presented in Figure 3. Linear regression analysis showed that circulating ACE activity was significantly related to total cholesterol levels among the D/D homozygotes (r=0.501, p=0.006) while ACE activity of I/I homozygotes did not show significant correlation (r=0.156, p=0.193). Figure 4 illustrates the relationship between ACE activity and fasting serum triglyceride levels in healthy subjects. Serum ACE activity was also significantly (p=0.012) correlated to triglyceride levels in homozygous carriers of the D allele (r=0.469).
Figure 3: Effect of Angiotensin converting enzyme activity on serum cholesterol levels. Serum Angiotensin converting enzyme activity of 32 healthy subjects with I/I genotype (empty circle) and 24 with D/D genotype (filled circle) was plotted against serum cholesterol levels. Serum angiotensin converting enzyme activity of D/D homozygotes exhibited significant positive correlation (p=0.006) with fasting serum cholesterol.
Figure 4: Effect of ACE enzyme activity on serum triglyceride levels. Angiotensin converting enzyme activity of 32 healthy subjects with I/I genotype (empty diamond) and 24 subjects with D/D genotype (filled diamonds) was plotted against serum triglyceride levels. Serum angiotensin converting enzyme activity of D/D homozygotes exhibited significant positive correlation (p=0.012) with fasting serum triglyceride levels.
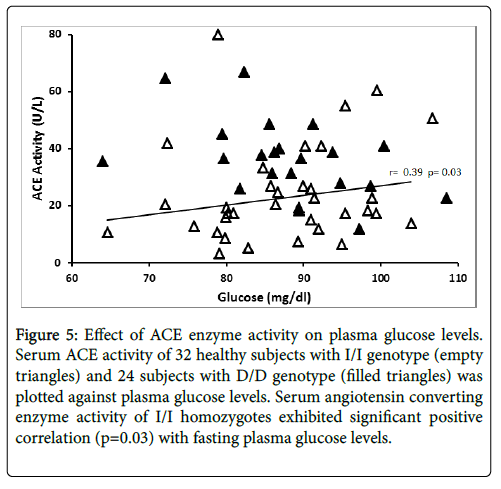
Serum ACE activity was significantly related to fasting plasma glucose levels (r=0.39, p=0.03) in subjects with I/I genotype, while the D/D homozygotes did not exhibit this relationship (Figure 5). ACE activity was not related to BMI, blood pressure, insulin, C-peptide and HOMA-IR in this cohort.
Figure 5: Effect of ACE enzyme activity on serum triglyceride levels. Angiotensin converting enzyme activity of 32 healthy subjects with I/I genotype (empty diamond) and 24 subjects with D/D genotype (filled diamonds) was plotted against serum triglyceride levels. Serum angiotensin converting enzyme activity of D/D homozygotes exhibited significant positive correlation (p=0.012) with fasting serum triglyceride levels.
Serum renin levels are available for the 59 healthy subjects who were also analysed for ACE activity. The mean renin level of these 59 healthy subjects was 55.6 ± 4.7 pg/mL. Renin levels did not differ in relation to the genotype.
Subjects with metabolic disorder
The cohort of 33 subjects with metabolic disorders (Table 4) was older (41.0 ± 1.6 years) than the subjects without metabolic disorders (33.3 ± 0.8 years n=99). This group had more women compared to the healthy subjects. The subjects were overweight. The systolic blood pressure was slightly higher in D/D homozygotes (134.1 ± 5.1 mm Hg, n=8) compared to subjects with I/I genotype (123.4 ± 5.1 mm Hg, n=6). Similarly, the diastolic blood pressure was slightly more in the D/D homozygotes than in I/I homozygotes.
| Variables | I/I | I/D | D/D |
|---|---|---|---|
| Number (%) | 7 | 15 | 11 |
| Men: Women ratio | 2:5 | 7:8 | 6:5 |
| Age (years) | 38.8 ± 3.8 | 42.4 ± 2.8 | 40.5 ± 3.3 |
| Body weight (Kg) | 63.5 ± 5.8 | 73.0 ± 3.7 | 71.0 ± 3.3 |
| Body Mass Index (Kg/m2) | 27.0 ± 2.6 | 29.2 ± 1.2 | 30.4 ± 1.5 |
| Waist circumference (cm) | 92.1 ± 5.6 | 96.0 ± 2.9 | 94.2 ± 1.7 |
| Systolic Blood Pressure (mm Hg) | 123.4 ± 5.1 | 125.7 ± 3.8 | 134.1 ± 5.1 |
| Diastolic Blood Pressure (mm Hg) | 78.0 ± 2.6 | 81.6 ± 2.2 | 87.0 ± 3.2 |
| Glucose (mg/dL) | 107.4 ± 8.7 | 98.4 ± 5.2 | 111.0 ± 10.6 |
| Triglycerides (mg/dL) | 158.9 ± 40.1 | 133.8 ± 26.3 | 189.9 ± 43.0 |
| Cholesterol (mg/dL) | 177.4 ± 8.4 | 195.6 ± 9.2 | 218.9 ± 19.6 |
| Renin (pg/mL) | 46.0 ± 12.6 (n=6) | 29.7 (n=1) | 36.6 ± 7.2 (n=8) |
Table 4: Characteristics of subjects with metabolic disorder distributed according to ACE genotype (Data are expressed as (mean ± SEM) units)).
Discussion
The present cross sectional study reveals that:
1. In this small cohort of 132 young working subjects, 25% suffer from various metabolic disorders;
2. Among the subjects without metabolic disorders, carriers of D/D genotype exhibited two fold higher circulating ACE enzyme activity compared to carriers of I/I genotype;
3. Serum ACE activity was significantly related to fasting serum total cholesterol, triglycerides and plasma glucose levels;
4. While carriers of D/D genotype exhibited significant correlation of ACE activity to serum cholesterol and triglyceride levels the I/I homozygotes exhibited correlation of ACE activity to fasting plasma glucose levels.
Characteristics of our total study cohort revealed a high family history of CVD risk factors of 50%. Further, 25% of the subjects have been currently suffering from various metabolic disorders even at a relatively young age of around 35 years. These results are similar to the findings on subjects from Rajasthan and North India [9,14]. Our data on the prevalence of obesity (11.4%), hypertension (7.6%) and diabetes (5.3%) are different from a study on urban Indians from Delhi of similar age wherein a higher prevalence was reported [15].
Mumbai is a big metropolitan city with large population and reported to have the highest cardiovascular mortality [16] in India. There is lacunae on the role of risk factors (genetic/environmental) responsible for the increased propensity of Mumbai urban Indians to heart diseases. The present study could be considered as a first effort in this regard.
Allele frequencies of the ‘I’ and ‘D’ alleles observed in our healthy cohort is comparable to other studies reported from India [17,18]. Increase in ACE activity of carriers of the ‘D’ allele compared to the carriers of the ‘I’ allele seen in our study is similar to the previous reports [19-21] in Caucasian subjects. It is known that the I/D polymorphism accounts for nearly 50% of the variation in ACE enzyme activity in various population. Enzyme activity of ACE has not been studied extensively among Indian subjects. One study involving patients suffering from myocardial infarction from South India [5] did not find significant changes in activity, among the different genotypes although elevated levels were reported in patients compared to controls.
We found significant positive association between ACE enzyme activity and established cardiovascular risk factors in this cohort. ACE activity was correlated to fasting cholesterol levels only in carriers of D/D genotype but not in the carriers of the I/I genotype. Further, ACE activity was also significantly related to serum triglycerides in the D/D homozygotes. This is a novel finding in our study.
Many investigators have studied ACE polymorphism in CAD as a potential causal link to serum cholesterol and other risk factors. Our results are somewhat similar to the reports of Borzyszkowska and coworkers who found association of ACE polymorphism with severity in atherosclerosis [5] in CAD patients from Poland. They reported that severity of CAD, defined by Gencini score, was the highest among male D/D carriers who also have high cholesterol. Studies from India have not reported association of ACE polymorphism with cholesterol or any other metabolic variables either in controls or patients with metabolic syndrome (Mittal et al. 2011) or diabetes [17]. A study from South India found association of ACE polymorphism with heart disease among diabetic patients only in subjects with polymorphisms in CETP (Cholesterol Ester Transfer Protein) gene, involved in the cholesterol metabolism [18]. However, these authors did not evaluate the relationship between ACE polymorphism and cholesterol or triglyceride levels. Our finding, if confirmed in larger population, is of clinical significance. Genotyping of subjects with high cholesterol could help in the early identification of a subset with D/D genotype, which could be targeted for preventive or therapeutic measures aggressively.
Contrary to the trend seen in cholesterol and triglyceride levels, plasma glucose was significantly related to ACE activity in carriers of ‘I’ allele. It is not clear whether this is related to their lower plasma glucose levels (Table 3) compared to D/D carriers.
The mechanism behind the association of ACE activity to serum cholesterol or triglyceride in D/D homozygotes is not clear. The I/D polymorphism is in the intron 16 of the ACE gene and hence, a direct effect on ACE activity is unlikely. It has been reported that this I/D polymorphic region is in linkage disequilibrium with another major gene controlling the enzyme activity [22,23]. Since ACE is also expressed in vascular endothelium, one possibility is that the D/D homozygotes, with additional vascular enzyme activity could be prone to atherosclerosis through angiotensin II mediated vasoconstriction, in the presence of high serum cholesterol.
The limitation of our study is the small size. Because of this we could not find the difference in genotype distribution between subjects with (n=33) and without metabolic disorders in this cohort. These findings need to be confirmed and validated in another cohort involving larger population size.
In conclusion, the present cross sectional study suggests that I/D polymorphism in ACE gene is associated with established CVD risk factors namely fasting serum cholesterol, triglycerides and plasma glucose levels. In this cohort, homozygosity for the ‘D’ allele was predictive of serum cholesterol and triglycerides levels while homozygosity in the ‘I’ allele was indicative of fasting plasma glucose levels. Future studies involving large number of subjects are needed to validate current findings.
Acknowledgment
The authors thank Dr. S. Vaidya for co-ordination of blood sampling and Dr. R. D. Lele for encouragement. Financial support was from the Haffkine Institute.
Conflict of interest
All the authors have no conflict of interest to decline.
Data Availability
The original data could be made available if requested
References
- Carluccio M, Soccio M and De Caterina R (2001) Aspects of gene polymorphisms in cardiovascular disease: the renin-angiotensin system. Eur J Clin Invest 31: 476-488.
- Rigat B, Hubert C, AlhencGelas F, Cambien F, Corvol P, et al. (1990) An insertion/deletion polymorphism in the angiotensin I-converting enzyme gene accounting for half the variance of serum enzyme levels. J Clin Invest 86: 1343-1346.
- Chung CM, Wang RY, Fann CS, Chen JW, Jong YS, et al. (2013) Fine-mapping angiotensin-converting enzyme gene: separate QTLs identified for hypertension and for ACE activity. Plos one 8: e56119.
- He Q, Fan C, Yu M, Waller G, Zhang ZF, et al. (2013) Associations of ACE gene insertion/deletion polymorphism, ACE activity, and ACE mRNA expression with hypertension in a Chinese population. Plos one 8: e75870.
- Borzyszkowska J, StanislawskaSachadyn A, Wirtwein M, Sobiczewski W, Ciecwierz D, et al. (2012) Angiotensin converting enzyme gene polymorphism is associated with severity of coronary artery disease in men with high total cholesterol levels. J Appl Genetics 53: 175-182.
- Yadav S, Hasan N, Marjot T, Khan MS, Prasad K, et al. (2013) Detailed analysis of gene polymorphisms associated with Ischemic stroke in South Asians. Plos one 8: e57305.
- Faerch LH, Seijing AS, Lajer M, Tarnow L, Thorsteinsson B (2013) ACE genotype, phenotype and all-cause mortality in different cohorts of patients with type 1 diabetes. J Renin Angiotensin Aldosterone Syst 16: 374-381.
- Pulla Reddy B, SrikanthBabu BM, VenkataKarunakar K, Yasovanthi J, Munshi A, et al. (2010) Angiotensin-converting enzyme gene variant and its levels: risk factors for myocardial infarction in a South Indian population. Singapore Med J 51: 576-81.
- Gupta R, Guptha S, Sharma KK, Gupta A and Deedwania P (2012) Regional variations in cardiovascular risk factors in India: India heart watch. World J Cardiol 4: 112-120.
- Gupta R, Joshi P, Mohan V, Reddy KS, Yusuf S (2008) Epidemiology and causation of coronary heart disease and stroke in India. Heart 94: 16-26.
- Kivimaki M, Nyberg ST, Batty GD, Fransson EI, Heikkilä K, et al. (2012) Job strain as a risk factor for coronary heart disease: a collaborative meta-analysis of individual participant data. Lancet 380 : 1491-1497.
- Kothari ST, Chheda P, Chatterjee L, Das BR (2012) Molecular analysis of genetic variation in angiotensin I-converting enzyme identifies no association with sporting ability: First report from Indian population. Indian J Hum Genet 18: 62-65.
- Matthews DR, Hosker JP, Naylor BA, Treacher DF, Turner R C (1985) Homeostasis model assessment: Insulin resistance and beta – cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28: 412- 419.
- Mittal G, Gupta V, Haque SF, Khan AS (2011) Effect of angiotensin converting enzyme gene I/D polymorphism in patients with metabolic syndrome in North Indian population. Chinese Med J 124: 45-48.
- Huffman MD, Prabhakaran D, Osmond C, Fall CHD, Tandon N, et al. (2011) Incidence of Cardiovascular Risk Factors in an Indian Urban Cohort - Results From the New Delhi Birth Cohort. J Am CollCardiol 57: 1765-1774.
- Pednekar MS, Gupta R, Gupta PC (2011) Illiteracy, low educational status, and cardiovascular mortality in India. BMC Public Health 11: 567.
- Dwivedi M, Laddha N, Imran M, Ansarullah, Bajpai P, et al. (2011) ACE gene I/D polymorphism in type 2 diabetes: the Gujarat population. Br. J of Diabetes and Vasc Dis 11: 153.
- Ganesan M, Bhaskar S, Mani R, Idris M, Khaja N, et al. (2011) The relationship of ACE and CETP gene polymorphisms with cardiovascular disease in a cohort of Asian Indian patients with and those without type 2 diabetes. J Diabetes Complications 25: 303-308.
- Danser AH, Batenburg WW, van den Meiracker AH, Danilov SM (2007) ACE phenotyping as a first step toward personalized medicine for ACE inhibitors. Why does ACE genotyping not predict the therapeutic efficacy of ACE inhibition? PharmacolTher 113: 607–618.
- Tsantes AE, Kopterides P, Bonovas S, Bagos P, Antonakos G, et al. (2013) The Effect Of Angiotensin Converting Enzyme Gene I/D Polymorphism And Its Expression On Clinical Outcome In Acute Respiratory Distress Syndrome. Minerva Anestesiol 79: 861-70.
- Camós S, Cruz MJ, Morell F, Solé E (2012) Genetic-based reference values for angiotensin-converting enzyme (ACE) according to I/D polymorphism in a Spanish population sample. ClinChem Lab Med 50: 1749-1753.
- McKenzie CA, Zhu X, Forrester TE, Luke A, Adeyemo AA, et al. (2008) A genome wide search replicates evidence of a quantitative trait locus for circulating angiotensin-I-converting enzyme (ACE) unlinked to the ACE gene. BMC Med Genomics 1:23.
- Cambien F, Poirier O, Lecerf L, Evans A, Cambou JP, et al. (1992) Deletion polymorphism in the gene for angiotensin converting enzyme is a potent risk factor for myocardial infarction. Nature 359: 641-644.
Citation: Kasarpalkar N, Pandya R, Deepak S (2019) Angiotensin Converting Enzyme Gene Polymorphism in an Urban Worksite Cohort from Mumbai, Western India. J Clin Exp Pathol 9:362. DOI: 10.4172/2161-0681.1000362
Copyright: © 2019 Kasarpalkar N, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 3530
- [From(publication date): 0-2019 - Dec 10, 2025]
- Breakdown by view type
- HTML page views: 2674
- PDF downloads: 856