Antibacterial Activity of Crude Leaf Extracts from Selected MedicinalPlants against Shigella Flexineri
Received: 18-Feb-2022 / Manuscript No. science-22-54872 / Editor assigned: 21-Feb-2022 / PreQC No. science-22-54872 (PQ) / Reviewed: 07-Mar-2022 / QC No. science-22-54872 / Revised: 14-Mar-2022 / Manuscript No. science-22-54872 (R) / Accepted Date: 14-Mar-2022 / Published Date: 21-Mar-2022 DOI: 10.4172/science.1000117
Abstract
This study aimed at determining the effects of the selected medicinal plants (Tagetes minuta, Aloe secundiflora, Vernonia lasiopus, and Bulbine frutescens) extracts against Clinical isolate of Shigella flexineri. Shigella flexineri is a gram-negative bacterium that is associated with gastrointestinal disturbances leading to diarrhoea in human beings. The antibacterial activity of the medicinal plant extracts against Shigella flexineri was determined using the Kirby Bauer method. The extracts showed antimicrobial activity against Shigella flexineri with Bulbine frutescens extract (minimum inhibitory concentration 3.2 μg/ml; maximum bactericidal concentration 6.2 μg/ml) being the most active when compared to the others. Tagetes minuta (minimum inhibitory concentration 7.4 μg/ml; maximum bactericidal concentration 12.6 μg/ml) extract was less active when compared to the other extracts. Bulbine frutescens had the largest average zone of inhibition 19.50 ± 1.05 mm while Vernonia lasiopus and Aloe secundiflora had the least zone of inhibition of 18.17 ± 1.47mm both. Ciprofloxacin (5μg/ml) was used as a positive control producing an average zone of inhibition of 22 ± 1.84mm while negative controls (water and dimethyl sulphoxide) showed no zone of inhibition. The preliminary qualitative screening for phytochemical showed the presence; of saponins, tannins, alkaloids, and flavonoids. The study provides insight into the antibacterial activity of the medicinal plant extracts and if they can be used in the treatment of infections caused by Shigella flexineri as an antibacterial agent.
Keywords: Shigella flexineri; Phytochemicals; Kirby Bauer; Medicinal plants; Zone of inhibition
Keywords
Shigella flexineri; Phytochemicals; Kirby Bauer; Medicinal plants; Zone of inhibition
Introduction
Medicinal plants are used by almost 80% of the world’s population for their basic health care because of their low cost and ease of availability. From the dawn of civilization, people have developed a great interest in plant-based drugs and pharmaceutical products [1]. In the last few decades, many bacterial organisms have continued to show increasing resistance against current antimicrobial agents [2]. Herbal drugs made from medicinal plants have been used from ancient times to treat various diseases and their antimicrobial properties make them a rich source of many potent drugs [3]. The use of herbal medicinal plants has always played a positive role in the control or prevention of diseases such as diabetes, heart disorders, and various cancers [4]. Some medicinal plants have been used in the production of various drugs singly or combination and even as principal raw material for the production of other conventional medicines [5].
The genus Tagetes belongs to the Asteraceae family which presently comprises 56 species, 27 biennials, and 29 perennials. Tagetes species are grown all over the world as multipurpose plants. The most common species are Tagetes minuta, Tagetes patula, Tagetes erecta, and Tagetes tenuifolia [6]. Tagetes species and chemotypes from its genus have been largely examined for biologically active metabolites that can be used in industry and medicine [7]. Compounds that have antimicrobial activity in the Tagetes minuta plant are said to be accumulated in the organs of the plant and their essential oils have not only antimicrobial effects but also insecticidal properties [8]. Plant parts such as flowers and leaves have been known to contain flavonoids that are scavengers for free radicals which enhances the antimicrobial activity of the Tagetes minuta extracts [9]. Some of the Tagetes minuta phytochemicals from the plant such as carotenoids have also been used in pharmacological preparations and they have been found to contain anti-aging and anticancer effects [10]. The plant extracts have been used in treating intestinal and stomach problems [11-12]. Tagetes minuta extracts such as its volatile oil and other components have been used in the flavoring of food products and as perfumes. The plant has also shown inhibitory activity against some pathogens and insects. Studies carried out have shown that leaf extracts from most of the Tagetes species including Tagetes minuta contain flavonoids that have shown antimicrobial potential against both Gram-positive and Gram-negative bacteria. Extracts from Tagetes minuta leaf flowers and stem extracted using methanol have been shown to contain secondary metabolites including terpenes which are thought to be responsible for antibacterial activities. Aloes are perennial succulent xerophytes that develop water storage tissues in leaves to survive in areas with low or erratic rainfall [13]. The plant is mainly found in cultivation, having no naturally occurring population although closely related Aloes do grow in northern parts of Africa [14]. The plant is an almost sessile perennial herb that has leaves 30-50 centimetres long and 10 centimetres broad at the base, bright yellow tubular flowers 25-35centimetres in length arranged in a slender loose spike [15]. The genus Aloe is common in Kenya; with about 60 taxa recognized [16]. Aloe secundiflora has been used in treating ailments including; chest problems, polio, malaria, and stomach ache by herbalists in the Lake Victoria region [17]. Aloe secundiflora leaf components have been credited for antibacterial, antifungal antiviral, and anthelminthic medicinal properties [18]. Aloe extracts have been used for many centuries for their curative and therapeutic properties. Aloe products have also been used in pharmaceuticals, cosmetic, and food industries [19]. Bulbine is a genus of plants in the family Xanthorrhoeaceae and subfamily asphodeloideae and its members are well known for their medicinal value [20]. Bulbine frutescens wild and Bulbine natalensis baker is the most common species known [21]. The Bulbine plant has been used for medicinal purposes in the early stages of the 18th century by Dutch and British settlers of South Africa in treating various ailments [22]. Many species have bulb-shaped tubers. It’s chiefly found in South Africa with a few species extending to the tropics of Africa and Australia [23]. They are succulent plants with most of the species having yellow flowers whereas some of them have white, orange, or pink flowers. Bulbine frutescens is mostly grown as an ornamental plant in the flower garden at homes in South Africa [24]. The leaves of the plant have been used in the treatment of wounds thought to be infected with bacterial pathogens and it has shown antibacterial properties [25]. Some of the species of the plant found in South Africa have been used for blood cleansing, treatment of ringworms, and gravel rush by some local communities such as the Xhosa [26]. A decoction of bulbs and roots of some of the species has been used in the treatment of some of the venereal diseases in women and stomach upsets [28]. Vernonieae is a tribe that has about 1300 species and is in the family Asteraceae (Compositae) which mostly contains herbaceous plants [29]. Vernonia shrubs grow in tropical Africa and have a height of about 2-5 metres, elliptical leaves of up to 20 centimetres, and a rough bark [31]. The plants in this genus usually have a bitter taste and in English, they are called bitter leaf [32]. Some of the common African names of plants in this genus are Olusia (Luo), Mululuza (Luganda), Onugu (Igbo), Grawa (Amharic), and Chusar-Doki (Hausa) [33]. Vernonia lasiopus decoctions from the stems and leaves have been traditionally been used by herbalists in East Africa to treat, malaria, worms, and gastrointestinal problems [34]. In the Kikuyu community, it’s traditionally known as Mucatha and it has been used in treating diarrhoea problems [35]. Studies carried out have shown some of the phytochemical components found in its extracts have antimicrobial capability [36]. Its extracts have also been used in treating some of the sexually transmitted diseases in southern parts of Africa [37]. In North America, some of the species of the genus Vernonia such as Vernonia altissima, Vernonia fasciculata, and Vernonia flaccidifolia have been found to contain effective properties for them to be used as blood purifiers, uterus toner, and also contain sesquiterpene lactone which can also help in preventing atherosclerosis. In Brazil, Vernonia condesata commonly known to locals as necroton or figatil has been used in traditional medicine to treat analgesic, antithermal, anti-anemic, anti-inflammatory and as an antibacterial agent. Shigella is a genus of Gram-negative, rod-shaped facultative bacteria responsible for shigellosis. Only a few cells of the bacteria can cause infections and its clinical manifestations include exothermic reactions, diarrhea, abdominal pain and sometimes vomiting. Shigellosis epidemic tends to occur in developing countries due to poor sanitation and the transmission rate from one person to another is more frequent especially when it’s due to water or food contamination. Shigellosis is responsible for most of the diarrhea episodes and causes approximately over one million deaths annually [38]. Over time the increase in the use of conventionally produced antimicrobials against Shigella flexineri has led to the development of resistance. The increase in resistance has led to the need for an alternative to the antimicrobials produced leading to the need to extensively study extracts from medicinal plants with antimicrobial activities [39].
Materials and Methods
Plant material collection
The fresh plant leaves of Aloe secundiflora, Bulbine frutescens, Vernonia lasiopus, and Tagetes minuta were collected at Kenyatta University Arboretum. Voucher specimens were prepared and deposited in the university herbarium in Plant Sciences Department for future reference. The plant’s leaves were brought to the laboratory and thoroughly washed in running water to remove debris and dust particles and then rinsed using distilled water and finally air-dried.
Preparation of plant extract
The air-dried plants leaves were ground into powder soaked in methanol for 72 hours and placed in a Gallenkamp shaker at 65 revolutions per minute. The contents were homogenized and filtered using Whatman filter paper no. 1. The filtrate was poured into a round bottom flask concentrated using a vacuum evaporator and stored in a labeled amber glass bottle at room temperature away from light and heat before being used for an antibacterial efficacy test.
Preparation of Media
The media used was Muller Hinton agar and it was prepared according to commercially given instructions.
Preparation of Muller-Hinton agar
38 milligram of Muller-Hinton agar powder was added into one liter of distilled water in a flat-bottomed conical flask. The mixture was heated with frequent agitation and boiled for one minute to completely dissolve the media. The flask was then tightly closed using cotton wool and further covered with aluminum foil. The mixture was autoclaved for 15 minutes at 121 ºC after which it was left to cool down to room temperature. The media was poured into the Petri dishes in a laminar flow to give a uniform depth of 3-4 millimeters. The Petri dishes containing the media were then placed in a sterile plastic bag and stored at a temperature of 2-8 ºC before use.
Test bacterial organism
The microorganism used was a clinical isolate of Shigella flexineri obtained from Kenyatta University Health Centre Laboratory, Nairobi. The isolate was tested against methanolic leaf extracts from Tagetes minuta, Aloe secundiflora, Bulbine frutescens, and Vernonia lasiopus.
Antimicrobial susceptibility testing
The microorganism used was a clinical isolate of Shigella flexineri obtained from Kenyatta University Health Centre Laboratory, Nairobi. Shigella flexineri was tested against methanol extracts of Tagetes minuta, Aloe secundiflora, Bulbine frutescens, and Vernonia lasiopus. Shigella flexineri inoculum was concentrated by comparing it with a 0.5 McFarland standard. Discs of 6 milliliters were prepared from Whatman no.1 filter paper. The discs were sterilized by autoclaving. After sterilization, the moisture discs were dried on a hot air oven at 50 ºC [40]. The various solvent extracts discs prepared were impregnated with the extracts from the highest concentration of 1000 μg/ml to the lowest concentration of 1 μg/ml. The antimicrobial efficacy test was carried out using the Kirby Bauer method [41]. Muller Hinton agar was used in the spread plate technique where Shigella flexineri was spread using a sterilized cotton wool swab and exposed to extracts impregnated discs in milligrams per microliter from Aloe secundiflora, Tagetes minuta, Vernonia lasiopus, and Bulbine frutescens. The discs were placed with equal distance between them on agar plates inoculated with Shigella flexineri. Positive control discs contained ciprofloxacin while negative control discs were impregnated with dimethyl sulphoxide and distilled water. The Petri dishes were incubated at 37ºC for 24 hr. Zones of inhibition formed were measured in milli meters and their average determined. The experiment was carried out in duplicates and the diameter of zones of inhibition formed was measured.
Minimal inhibitory concentration (MIC) and Minimum bactericidal concentration (MBC) determination
Minimal inhibitory concentration (MIC) was determined using the broth tube method, [43]. 100μl of 250 μg/ml of methanol extract was added to 100μl of sterile bacteriological peptone in the first well of the 96 well microplates and mixed well with a micropipette. 100μl of this dilution was transferred subsequently to wells two folding each dilution of the original extract. This was done to the extracts of Aloe secundiflora, Bulbine frutescens, Vernonia lasiopus, and Tagetes minuta. An inoculum of 100μl (0.5 McFarland standard) of an overnight clinical culture of Shigella flexineri was added in each of the wells. Triplicates of each microplate were made and the procedure was repeated. The plates were then incubated at 37ºC for 24 hrs. After incubation 40μl of 0.2 mg/μl of INT were added in each of the wells and the plates were examined after an additional 60 minutes of incubation. Growth was indicated by a red colour (conversion of INT to formazan). The lowest concentration at which the colour was invisible as compared to the next dilution was taken as the minimum inhibitory concentration [44]. Minimum bactericidal concentration (MBC) was determined by taking 100μl of suspension from micro plate wells that demonstrated no growth and inoculated on agar plates. The plates were incubated at 37ºC for 24 hrs. In the case where there was no bacterial growth and value not greater than the minimum inhibitory concentration, the concentration was used as the maximum bacterial concentration.
Phytochemical analysis
The presence of saponins, tannins, flavonoids, and alkaloids in the crude extract was determined.
Tannins: Each of the extracts were weighed to 0.5mg and dissolved in 1 ml of distilled water. Filtration was carried out after 2ml of FeCl3 was added. If there was the presence of a blue or black precipitate then it indicated the presence of tannins.
Flavonoids: Each of the extracts was weighed to 0.5mg dissolved in 1 ml of ethanol and filtered. 2ml of 1% HCL and magnesium ribbon was added to the filtrate. If there was the formation of a pink or red colour it indicated the presence of flavonoids.
Alkaloids: Each of the extracts was weighed to 0.5mg dissolved in 1ml of methanol and filtered. 1% HCL was added to the filtrate and the solution heated. Mayor`s reagent was added drop wise and if there was the formation of any colored precipitate it indicated the presence of alkaloids.
Saponins: Each of the extracts was weighed to 0.5mg dissolved in 1 ml of methanol and filtered. Distilled water was added and shaking was done for a few minutes. If there was persistence frothing then it indicated the presence of saponins.
Data Analysis
The data were expressed as means and standard deviations. SAS version 19.0 package was utilized in conducting ANOVA test to determine significant differences in antimicrobial activity of selected plant extracts against Shigella flexineri. Two-way ANOVA was carried out to determine if there was any interaction between the plant extracts and Shigella flexineri. P ≤ 0.05 is considered significant. Turkey’s posthoc was utilized to assess the difference within individual means of the zones of inhibition. A P-value ≤ 0.05 was considered statistically significant.
Results
The extracts from Tagetes minuta, Aloe secundiflora, Bulbine frutescens, and Vernonia lasiopus all showed significant antimicrobial activity when tested against the clinical isolate of Shigella flexineri. The extract from Bulbine frutescens was more active against Shigella flexineri as compared to others producing the highest average zone of inhibition (19.50 ± 1.05 mm). Aloe secundiflora and Vernonia lasiopus extract were the least active both producing an average zone of inhibition of 18.17 ± 1.47mm. The extract from Tagetes minuta also showed a considerable antimicrobial activity producing a zone of inhibition of 19.00 ± 1.41, Ciprofloxacin antibiotic discs used as positive control produced a zone of inhibition of 22.00 ± 1.84 mm. The negative controls of dimethyl sulphoxide (DMSO) did not produce any zones of inhibition (0.0 ± 0.00). The results showed that Bulbine frutescens produced the highest zone of inhibition hence it was a more potent antibacterial agent when compared to the other plant extracts (Table 1).
| Plant extracts | Zone of Inhibition (mm) |
|---|---|
| Tagetes minuta | 19.00±1.41bc |
| Aloe secundiflora | 18.17±1.47c |
| Bulbine frutescens | 19.50±1.05bc |
| Vernonia lasiopus | 18.17±1.47c |
| Controls | Zone of inhibition (mm) |
| Ciprofloxacin | 22±1.84b |
| Methanol | 31.67±2.88a |
| 4% (DMSO) | 0.0±0.00d |
| P value | 0.0001 |
Table 1: Efficacy test of the plant's leaf extracts against Shigella flexineri.
The value of average zones of inhibition ± standard error after one-way ANOVA followed by Tukey`s HSD test. A value followed by the same superscript within the same column is not significantly different (P˃0.05).
Key: DMSO - Dimethyl sulphoxide, Plant extracts concentration (1000μg/ml) Antibiotic standard discs of; Ciprofloxacin (5μg/ml) (+ve control).
The analysis of the interaction between the plant extracts and shigella flexineri showed that the average zone of inhibition formed by the plant’s extracts when used against shigella flexineri was significantly different from those formed by antibiotic, methanol, and DMSO (P<0.05; (Table 2). Moreover, zones formed by Bulbine frutescens extract against shigella flexineri was significantly different from those formed by the other plant extracts (17.19 ± 0.42 mm) (P<0.05) Table 2. The interaction between shigella flexineri and the plant extracts was significant in Table 2. The value of average zones of inhibition ± standard error of the mean (SEM) after two-way ANOVA was followed by Tukey`s HSD test. A value followed by the same superscript within the same column is not significantly different (P˃0.05).
| Extract | Zone of inhibition±SEM |
|---|---|
| Aloe secundiflora | 16.69±0.40cd |
| Bulbine frutescens | 17.19±0.42c |
| Tagetes minuta | 16.06±0.56d |
| Vernonia lasiopus | 15.81±0.61d |
| Controls | Zone of inhibition±SEM |
| Antibiotic | 23.5±0.43b |
| Methanol | 31.17±0.34a |
| DMSO | 0.00±0.00e |
| Test microorganism | Zone of inhibition±SEM |
| Shigella flexineri | 18.69±1.45a |
| P values of the main factors and their interactions | |
| Extract | <0.001 |
| Test microorganism | <0.001 |
| Extract*Test microorganism | <0.001 |
Table 2: Interactions between the plants extract and Shigella flexineri.
Key: Antibiotic (ciprofloxacin), DMSO - dimethyl sulphoxide
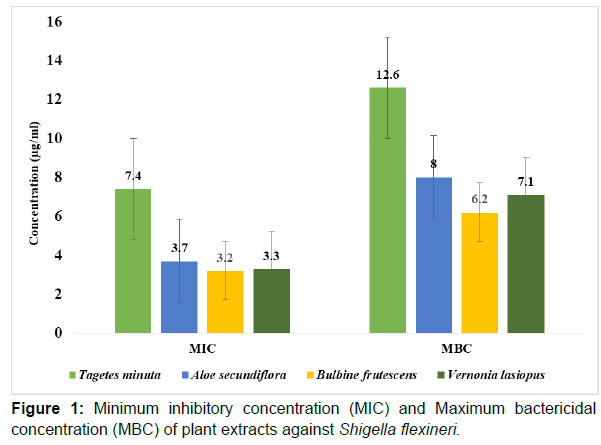
When the plant extracts were used in low concentra¬tions against Shigella flexineri, Bulbine frutescens was the most active with a minimum inhibitory concentration (MIC) of 3.2μg/ml and maximum bactericidal concentration (MBC) of 6.2μg/ml. Tagetes minuta extract was the less active; MIC of 7.4μg/ml and MBC of 12.6μg/ml in comparison to all other plant extracts. The other two extracts, Vernonia lasiopus, and Aloe secundiflora also had a pronounced antimicrobial activity against Shigella flexineri with; MIC of 3.3μg/ml and 3.7μg/ml; MBC of 7.1μg/ml and 8.0μg/ml respectively (Figure 1).
The plants extract from Tagetes minuta, Aloe secundiflora, Bulbine frutescens, and Vernonia lasiopus, when qualitatively analyzed for the presence of phytochemicals showed the presence of saponins, alkaloid, tannins, and flavonoids (Table 3).
| Name of test | Plants leaf extracts | |||
|---|---|---|---|---|
| T.minuta | A.secundiflora | B.frutescens | V.lasiopus | |
| Saponins test | + | + | + | + |
| Tannin’s test | + | + | + | + |
| Alkaloid’s test | + | + | + | + |
| Flavonoid’s test | + | + | + | + |
Table 3: Phytochemical tests on the plant extracts.
Key: (+) present
Discussion
The increase of antimicrobial resistance has led to the need for the invention of new antimicrobial agents. The use of plant extracts to test for antimicrobial activity has been brought forward as one of the ways of achieving this goal. The plants used in the study have been said to be of medicinal value. This study evaluated the use of the plant’s leaf extracts in treating Shigella flexineri and tested if they are effective or not. Furthermore, qualitative analysis to test for the phytochemical’s presence was also done. This is because phytochemicals have been said to be responsible for some of the antimicrobial activity by extracts from plants with medicinal value. From the findings, the leaf extracts from the plants were found to have antimicrobial activity when used against Shigella flexineri.
Antimicrobial activity
Medicinal plant extracts from various studies have shown that plants from similar genera with Aloe secundiflora have been shown to have antimicrobial activity. In this study, the antimicrobial activity of leaf extracts from medicinal plants was tested against Shigella flexineri. It was interesting to note that the leaf extract from Aloe secundiflora showed antimicrobial activity against Shigella flexineri. Findings from the study were similar to those obtained in Nigeria [45] from activities against medicinal plants. The leaf extract from Aloe secundiflora had antimicrobial activity against Shigella flexineri and other bacterial pathogens such as; Escherichia coli, Salmonella typhi, Staphylococcus aureus, and Enterococcus faecalis. The findings were similar to those obtained in a study carried out on Gram-negative and Gram-positive bacteria in India and England. These findings were also similar to those obtained in a previously carried out study in Kenya [46] which found, methanol extracts of Aloe secundiflora along the lake region in Kenya to be effective against Shigella flexineri and other bacterial pathogens such as Escherichia coli, Salmonella typhi, and Staphylococcus aureus among others. The leaf extract from Tagetes minuta showed antimicrobial activity against Shigella flexineri. The findings were similar to a previously carried out study in Pakistan who found out that extracts from Tagetes minuta had antimicrobial activity against not only Shigella flexineri but also other bacterial organisms such as Salmonella typhi, Escherichia coli, and Staphylococcus aureus. Furthermore, the minimum inhibitory concentration against these test microorganisms ranged from 4 to 100μg/ml, the average zones of inhibition formed were ≥ 17.00mm and the standard antibiotic used (Ciprofloxacin) produced zones of inhibition ≥ 20.00mm. This means that if the concentration of Tagetes minuta could be standardized, it could be used as an alternative therapy for Ciprofloxacin, against Shigella flexineri. Bulbine frutescens extract showed antimicrobial activity against Shigella flexineri. Its antimicrobial activity was significantly higher when compared to the other plant extracts. This finding was contrary to the one in South Africa [47] who found out that, extract from Bulbine frutescens had no antimicrobial activity against gram-negative bacterial pathogens. These differences could be due to the different geographical and environmental conditions during the growth of the plant, the method of extraction used, and the age of the plant. The extract from Vernonia lasiopus showed antimicrobial activity against Shigella flexineri. The findings concurred with those previously obtained in Kenya who found out that leaf extracts from Vernonia lasiopus had antimicrobial activity against gram-negative bacterial pathogens.
Phytochemicals
The extract from Aloe secundiflora showed that the plant leaf contained pharmacologically active components. The extract contained flavonoids, saponins, alkaloids, and tannins which may be responsible for the antimicrobial activity. Similar studies previously carried out have shown that some of the pharmacologically active components have antimicrobial activity. These findings concurred with a previously carried out study in Kenya who carried out qualitative analysis of phytochemical components of Aloe secundiflora extract used against bacterial and fungal pathogenic microbes from a plant collected along the Kenya lake region found it too contained tannins, saponins, flavonoids, and alkaloids. Furthermore, similar findings were obtained in a study carried out in India who confirmed the presence of flavonoids, saponins, tannins, and alkaloids in Aloe extract. Qualitative analysis for the presence of phytochemicals in Tagetes minuta extract showed the presence of flavonoids, saponins, alkaloids, and tannins. This pharmacologically active component might be responsible for Tagetes minuta extract antimicrobial activity against Shigella flexineri. The study findings were similar to those obtained in a study carried out in Pakistan in Argentina who also confirmed the presence of phytochemicals flavonoids, saponins, tannins, and alkaloids in Tagetes minuta extract used against Gram-positive and Gram-negative bacteria. The extract from Bulbine frutescens contained pharmacologically active compounds namely saponins, tannins, alkaloids, and saponins which could be responsible for antimicrobial activity. This study concurred with those of a previously done study in South Africa by [48] who found out the presence of alkaloids, saponins, tannins, and flavonoids in plant extract from Bulbine frutescens. However, the findings were contrary to those obtained in a previously carried out study in South Africa by who found out that, the extract from Bulbine frutescens did not contain any of the four phytochemicals. This could be due to diverse plant metabolites associated with a geographical and ecological difference from where the plant was obtained and also the age of the plant used. The extract from Vernonia lasiopus had active pharmacological compounds; flavonoids, saponins, tannins, and alkaloids which could be responsible for the antimicrobial activity. These findings were similar to those of a previously carried out study by who found out that, extract from Vernonia lasiopus contained alkaloids, flavonoids, saponins, and tannins when used against Grampositive and Gram-negative bacteria. In Nigeria also found out that extract from the plant from the family Vernonia contained flavonoids, alkaloids, tannins, and saponins. However, the findings were contrary to those obtained in a study carried out in the Southwestern region in Nigeria by who found out the extract from Vernonia lasiopus did not contain alkaloids. This difference may be associated with the geographical and environmental factors of the area from which the plant was collected.
Conclusion
All the plant’s leaf extracts showed significant antibacterial action against Shigella flexineri. This demonstrates that the extracts from the plants have the potential to be employed as an antibacterial agent against Shigella flexineri and related bacterial pathogens of the nature (Gram-negative). There is also a requirement to purify the plant extracts further to identify the key bioactive components of phytochemicals that are responsible for this antibacterial action. This will help in the supply of a natural source for treating illness caused by a bacterial pathogen (gram-negative) and others of its kind which have been gradually gaining resistance against commercially available antibiotics.
Acknowledgments
Special appreciation to Professor L.E. Newton for the help and guidance offered in the process of identification of the plants. I also acknowledge the support and assistance from Mr. Daniel Ng`ang`a Ms. Mary Njeri, Mr. Roy Mulanda, and the entire Microbiology Laboratory staff. May Almighty God shower you with his everlasting blessings?
Ethics approval
The ethical approval for the use of clinical isolates of Shigella flexineri obtained from Kenyatta University Health Centre Laboratory was obtained from the university research and ethical approval committee.
Declaration
I declare that this manuscript submitted for publication is from my original research work. All the sections in this manuscript, the concept, and the argument developed by other authors have been referenced to show the material has been used to support my manuscript.
Consent of publication
I hereby give consent for the publication of this manuscript because it’s my original work and it does not contain any specific individual data.
Availability of data and Material
The data and material can only be shared on request by the author but it depends on the reasons for such request being clearly articulated pending a decision of acceptance or rejection from the respondent author.
Competing interests
The authors declare there are no competing interests.
Funding
Self-funded project.
Author’s contributions
Opinde H.R. – Research Student.
Nyamache A.K. – Research Supervisor and Advisor.
Gatheri G.W. – Research Supervisor and Advisor.
Authors’ information
Not applicable
References
- Acocks JPH, (1988) Veld types of South Africa, Memoirs of the Botanical Survey of South Africa. 57:100-146.
- Agarry OO, Olaleye MT (2005)Comparative Antimicrobial Activities of Aloe Vera Gel and Leaf. Afr J Biotechnol 4:1410-1413.
- Akinyele BO, Odiyi AC (2007) Comparative Study of the Vegetative Morphology and The ExistingTaxonomic Status of Aloe Vera L. J Plant Sci 2:558-563.
- Arunkumar S, MuthuselvamM (2009) Analysis of Phytochemical Constituents and Antimicrobial Activities of Aloe Vera L. against clinical pathogens. World JAgric Res 5:572-576.
- Ayoola GA, Coker HA, Adesegun SA, Adepoju-Bello AA, Obaweya K, et al. (2008) Phytochemical Screening and Antioxidant Activities of Some Selected Medicinal Plants used for Malaria Therapy in Southwestern Nigeria. TropJPharmRes 7:1019-1024.
- Bennish ML, SalamMA, Hossain MA, Myaux J, Khan EH, et al. (1992) Antimicrobial Resistance of Shigella Isolates in Bangladesh, 1983 - 1990: Increasing Frequency of Strains Multiply Resistant to Ampicillin, Trimethoprim-Sulfamethoxazole, and Nalidixic Acid. Arch Clin Infect Dis 14:1055-1060.
- Broussalis AM, Ferraro GE, MartinoVS, Pinzón R, Coussio JD, et al. (1999)Argentine Plants as A Potential Source of Insecticidal Compounds. J Ethnopharmacol 67:219-223.
- Coopoosamy RM (2016) Traditional Information and Antibacterial Activity of Four Bulbine Species (Wolf). Afr J Biotechnol 10:118-220.
- Coopoosamy RM, Magwa ML, Mayekiso B (2000) Proceedings: Science and Society University of Fort Hare, Bhisho, Eastern Cape, South Africa.
- Crellin JK, Philpott J (1990) A Reference Guide to Medicinal Plants: Herbal Medicine Past and Present. J R Anthropol Inst 4:95
- Da Silva JB, Temponi VDS, Gasparetto CM, Fabri RL, Aragão DMDO, et al. (2013)Vernonia condensata Baker (Asteraceae): A promising source of antioxidants. Oxid Med Cell Longev 2013:698018.
- Eilers B, MayerScholl A, WalkerT , Tang C, WeinrauchY, et al. (2010) Neutrophil Antimicrobial Proteins Enhance Shigella Flexineri Adhesion and Invasion. Cell Microbiol 12:1134-1143.
- Eshun K, He Q (2004) Aloe Vera: A Valuable Ingredient for the Food, Pharmaceutical and Cosmetic Industries - a Review. Crit Re Food Sci Nutr 44:91-96.
- Green MM, Singer JM, Sutherland DJ, Hibben CR (1991) Larvicidal Activity of Tagetes Minuta (Marigold) Toward Aedes Aegypti. JA m MosqControl Assoc 7:282-286.
- Hendricks L, Wright N (1979) Diagnosis of Cutaneous Leishmaniasis by In Vitro Cultivation of Saline Aspirates in Schneider's Drosophila Medium. Am JTrop MedHyg 28:962-964.
- Ibrahim HA, Imam IA, Bello AM, Umar U, Muhammad S, et al. (2012) The Potential of Nigerian Medicinal Plants as an Antimalarial Agent: A Review. Int jscitechnol 2:600-605.
- Ijeh II, Ejike CE (2011) Current perspectives on the medicinal potential of Vernonia amygdalina Del. J Med Plant Res 5:1051 - 1061.
- Kaingu F, Kibor A , Waihenya R, Shivairo R, Mungai L (2013) Efficacy of Aloe Secundiflora Crude Extracts on Ascaridia Galli In Vitro. Int J Sustain Agric Res 2: 45-49.
- Kaithwas G, Kumar A, Pandey H, Acharya AK, Singh M, et al. (2008)Investigation of Comparative Antimicrobial Activity of Aloe Vera Gel and Juice. Pharmacologyonline 1:239-243.
- Kambizi L, Afolayan AJ (2001) An ethnobotanical study of plants used for the treatment of sexually transmitted diseases (njovhera) in Guruve District, Zimbabwe. J Ethnopharmacol 77:5-9.
- Kareru PG, Gachanja AN, Keriko JM, Kenji GM (2007) Antimicrobial Activity of Some Medicinal Plants used by Herbalists in the Eastern Province, Kenya. Afr J Tradit Complement Altern Med 5:51-55.
- Keeley SC, Forsman ZH, Chan R (2007) A phylogeny of the “evil tribe” (Vernonieae: Compositae) reveals Old/New World long-distance dispersal: Support from separate and combined congruent data sets (trnL-F, ndhF, ITS). Mol Phylogenet Evol M 44:89-103.
- Kelmanson JE, Jäger AK, Van Staden J (2000) Zulu Medicinal Plants with Antibacterial Activity. J.Ethnopharmacol 69: 241-246.
- Kigondu EV, Rukunga GM, Keriko JM, Tonui WK, Gathirwa JW, et al. (2009) Antiparasitic Activity and Cytotoxicity of Selected Medicinal Plants from Kenya. J Ethnopharmacol 123:504-509.
- Kokwaro JO (2009) Medicinal plants of East Africa. University of Nairobi Press.
- Kotloff KL, Winickoff JP, IvanoffB, Clemens JD, Swerdlow DL, et al. (1999)Global Burden of Shigella Infections: Implications for Vaccine Development and Implementation of Control Strategies. Bull World Health Organ 77:651-666.
- Koul JL, Koul S, Singh C, Taneja SC, Shanmugavel M, et al. (2003) In Vitro Cytotoxic Elemanolides from Vernonia Lasiopus. Planta Med 69:164-166.
- López ML, Bonzani NE, Zygadlo JA (2008)Allelopathic Potential of Tagetes Minuta Terpenes by A Chemical, Anatomical and Phytotoxic Approach. Biochem Syst Ecol BIO 36:882-890.
- Mariita RM, Orodho JA, Okemo PO, Kirimuhuzya C, Otieno JN, et al. (2011) Methanolic Extracts of Aloe Secundiflora Engl. Inhibits in Vitro Growth of Tuberculosis and Diarrhea-Causing Bacteria. Pharmacogn Res 3: 90-95.
- Mohanta B, Chakraborty A, Sudarshan M, Dutta R, Baruah M (2003)Elemental Profile in some Common Medicinal Plants of India. It’s Correlation with Traditional Therapeutic usage. J Radioanal Nucl Chem 258: 175-179.
- Nascimento GG, Locatelli J, Freitas PC, Silva GL (2000) Antibacterial Activity of Plant Extracts and Phytochemicals on Antibiotic-Resistant Bacteria. Braz J Microbiol 31: 247-256.
- Newall CA, Anderson LA, Phillipson JD (1996) Herbal medicines. The Pharmaceutical Press ,London.
- Palpasa K, Pankaj B, Sarala M, Gokarna RG (2011)Antimicrobial Resistance Surveillance on Some Bacterial Pathogens in Nepal: A Technical Cooperation. J Infect Dev Ctries 5:163-168.
- Piccaglia R, Marotti M, Pesenti M, Mattarelli P, Biavati B (1997)Chemical Composition and Antimicrobial Activity of Tagetes Erecta and Tagetes Patula, in Essential oils. J basic appl resp 49 - 51.
- Qu F, Bao C, Chen S, Cui E, Guo T, et al. (2012) Genotypes and Antimicrobial Profiles of Shigella Sonnei Isolated from Diarrheal Patients Circulating in Beijing between 2002 and 2007. Diagn Microbiol Infect.Dis 74: 166-170.
- Rabe T, Mullholland D, Van Staden J(2002).Isolation and Identification of Antibacterial Compounds from Vernonia Colorata Leave. J Ethnopharmacol 80: 91-94.
- Rivas JD (1991) Reversed-Phase High-Performance Liquid Chromatographic Separation of Lutein and Lutein Fatty Acid Esters from Marigold Flower Petal Powder. J Chromatogr A 464: 442-447.
- Robson MC, Heggers JP, Hagstrom WJ (1982) Myth, magic, witchcraft, or fact? Aloe vera revisited. J Burn Care Res 3: 154-163.
- Shahzadi I, Hassan A, KhanUW, ShahMM (2010) Evaluating Biological Activities of The Seed Extracts from Tagetes Minuta L. Found in Northern Pakistan. J Med Plant Res 4: 2108-2112.
- Soule JA (1993) Tagetes minuta: A potential new herb from South America. New Crops, New York: 649-654.
- Srivastava J, Lambert J, Vietmeyer N (2005) Medicinal Plants: An Expanding Role from Western India for Potential Antimicrobial Activity. Indian J Pharmacol 37: 406 - 409.
- Tahir L, KhanN (2012) Antibacterial potential of crude leaf, fruit, and flower extracts of Tagetes Minuta L. IJHBS 1: 70-74.
- Talmadge J, Chavez J, Jacobs L, Munger C, Chinnah T, et al. (2004)Fractionation of Aloe Vera L. Inner Gel, Purification and Molecular Profiling of Activity. Int Immunopharmacol 4: 1757-1773.
- Tereschuk ML, Riera MV, Castro GR, Abdala LR (1997) Antimicrobial Activity of Flavonoids from Leaves of Tagetes Minuta. J Ethnopharmacol 56: 227-232.
- Van Staden LF, Drewes SE (1994) Knipholone from Bulbine Latifolia and Bulbine Frutescens. J Phytochem 35: 685-686.
- Van Wyk BE (2008) A Broad Review of Commercially Important Southern African Medicinal Plants. J Ethnopharmacol 119: 342-355.
- Van WykBE, Gericke N (2000) People's plants: A guide to useful plants of Southern Africa. Briza Publications.
- WHO (1999) WHO monographs on selected medicinal plants, world health organization. Geneva. 1:9241545178.
Indexed at , Google Scholar , Crossref
Indexed at , Google Scholar , Crossref
Indexed at , Google Scholar , Crossref
Indexed at , Google Scholar , Crossref
Indexed at , Google Scholar , Crossref
Indexed at , Google Scholar , Crossref
Indexed at , Google Scholar , Crossref
Indexed at , Google Scholar , Crossref
Indexed at , Google Scholar , Crossref
Indexed at , Google Scholar , Crossref
Indexed at , Google scholar , Crossref
Indexed at , Google Scholar , Crossref
Indexed at, Google scholar , Crossref
Indexed at , Google Scholar , Crossref
Indexed at , Google Scholar , Crossref
Indexed at , Google Scholar , Crossref
Indexed at , Google Scholar , Crossref
Indexed at , Google Scholar , Crossref
Indexed at , Google Scholar , Crossref
Indexed at , Google Scholar , Crossref
Indexed at , Google Scholar , Crossref
Indexed at , Google Scholar , Crossref
Indexed at , Google scholar , Crossref
Indexed at, Google Scholar , Cross Ref
Indexed at , Google Scholar , Crossref
Indexed at , Google Scholar , Crossref
Indexed at , Google Scholar , Crossref
Indexed at , Google Scholar , Crossref
Indexed at , Google Scholar , Crossref
Indexed at , Google Scholar , Crossref
Indexed at , Google Scholar , Crossref
Citation: Rachuonyo HO, Nyamache AK, Gatheri GW (2022) Antibacterial Activity of Crude Leaf Extracts from Selected Medicinal Plants against Shigella Flexineri. Arch Sci 6: 117. DOI: 10.4172/science.1000117
Copyright: © 2022 Rachuonyo HO, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 3210
- [From(publication date): 0-2022 - Dec 20, 2025]
- Breakdown by view type
- HTML page views: 2453
- PDF downloads: 757