Anti-Psychotic Medication and the Pattern of Cardiovascular Risk Factors: A Population Based Study (The Ayrshire Diabetes Follow-Up Cohort (ADOC) Study)
Received: 07-Jul-2017 / Accepted Date: 19-Sep-2017 / Published Date: 26-Nov-2017 DOI: 10.4172/2155-6105.1000343
Abstract
Background: Life expectancy in people with severe mental illness is significantly reduced: in part due to increased cardiac risk. Aim: The aims of this study were to investigate the prevalence of cardiovascular risk factors and to determine the prevalence of regular screening in these patients. Method: Data was extracted from 48 General Practices in NHS Ayrshire and Arran (n=320,613) in April 2015. Results: There were 3857 patients on anti-psychotic medication (prevalence 1.2%). Female patients and those on first generation medication were older (p<0.001). Monitoring rates ranged from 75% for BP and smoking down to under 50% for lipids. Only 10% of patients monitored were free of a cardiovascular risk factor. Conclusion: Treatment with anti-psychotic medication was associated with clustering of cardiovascular risk factors. Screening varied in relation to age and sex of the patient, the anti-psychotic agent prescribed and cardiovascular risk factor.
Keywords: Anti-psychotic medication; Obesity; Lifestyle; Hyperlipidaemia; Type 2 diabetes
Introduction
Adults with severe mental health illness, such as schizophrenia and bipolar disorder die prematurely largely from cardiovascular disease [1]. Life expectancy in people with schizophrenia is reduced by 10-25 years [2] with 60% of the excess mortality due to physical illness [3]. Furthermore, schizophrenia itself may be a risk factor for diabetes, and there is also increasing concern that antipsychotic drugs, particularly second-generation antipsychotics, have metabolic consequences that contribute to the risk [4]. Antipsychotic medications can cause significant weight gain and this may be one of the mechanisms by which they increase the incidence of diabetes [5,6]. Weight gain alone does not explain the increase in risk of diabetes mellitus, as some people who receive antipsychotic medications develop diabetes mellitus without baseline obesity and without weight gain [7]. Other lifestyle factors, such as smoking, sedentary lifestyle and poor diet are common in patients with severe mental health illness [8]. Various mechanisms beyond lifestyle and demographic characteristics have been proposed to explain the link. These include shared susceptibility genes between severe mental illness and type 2 diabetes [9], geneenvironment interactions [10], foetal development [11] and co-morbid problems such as low vitamin D [12,13].
Through Delivering for Mental Health [14] and Improving the Physical Health and Well Being of those experiencing Mental Illness [15], the Scottish Government have committed to provide regular physical health check for people with severe and enduring mental illness. The aims of this study were to investigate the prevalence of hypertension, hyperlipidaemia, obesity, smoking status and type 2 diabetes in patients on anti-psychotic medication and to investigate the prevalence of regular screening in these patients.
Methods
48 of the 55 General Practices in NHS Ayrshire and Arran (population 320613) contributed data from their practice computer systems. There were 3857 patients on anti-psychotic medication making a prevalence of 1.2%. Ninety-two per cent were prescribed a single drug (Table 1). Most of the patients were on antipsychotics for a psychotic illness but a number of patients were on antipsychotics for a bipolar disorder, sometimes depressive illness and low dose off label use in anxiety disorders, personality disorders and Post-traumatic Stress Disorder. The data collected for each patient included body mass index (BMI), blood pressure (BP), total cholesterol, triglycerides, glucose and smoking status. The data was extracted in April 2015. The audit was registered with the Clinical Governance Department, NHS Ayrshire and Arran and Caldicott Guardian approval was obtained.
NICE CG178 (2014) recommends screening every 12 months. We chose to review screening over 15 months to allow slippage. Most of the risk factor screening took place in General Practice along the intervention and treatment.
| Antipsychotic drug category | Medication | Generation | No of patients prescribed | % |
|---|---|---|---|---|
| Quetiapine | Second | 1379 | 35.80% | |
| Olanzapine | Second | 894 | 23.20% | |
| Risperidone | Second | 634 | 16.40% | |
| Haloperidol | First | 311 | 8.10% | |
| Chlorpromazine | First | 277 | 7.20% | |
| Aripiprazole | Second | 234 | 6.10% | |
| Amisulpride | Second | 162 | 4.20% | |
| Other first generation (n=226) | Sulpiride | First | 79 | 5.90% |
| Levomepromazine | First | 35 | ||
| Trifluoperazine | First | 31 | ||
| Zuclopenthixol | First | 30 | ||
| Flupentixol | First | 28 | ||
| Promazine | First | 9 | ||
| Pericyazine | First | 8 | ||
| Pimozide | First | 3 | ||
| Benperidol | First | 3 | ||
| Clozapine | Second | 29 | 0.80% | |
| Depot (n=57) | Haloperidol decanoate | Depot | 15 | 1.50% |
| Flupentixol decanoate | Depot | 16 | ||
| Zuclopenthixol decanoate | Depot | 8 | ||
| Pipotiazine Palmitate | Depot | 5 | ||
| Risperidone depot | Depot | 5 | ||
| Paliperidone depot | Depot | 4 | ||
| Fluphenazine decanoate | Depot | 4 |
Table 1: Prevalence of drug prescriptions.
Risk factors were defined for patients:
If their BMI was over 30 kg/m2
If either their systolic >140 mm Hg or diastolic BP >90 mm Hg or if they were on anti-hypertensive medication,
If either total cholesterol >5 mmol/L or triglyceride >1.7 mmol/L or if they were on lipid lowering treatment,
If they were a current smoker
If their glucose >6.1 mmol/L (or HbA1c>6% [42 mmol/mol]).
Statistics
An overall risk score based on the five risk factors was calculated for each patient based on their last monitored level. Where a patient was missing one or two measures the scores were adjusted but if a patient was missing more than 2 measures a score was not calculated. Risk scores were calculated for 3443 (89%) patients and ranged from 0 to 5.
Interval level data was analysed using t-tests, ANOVA or ANCOVA; categorical data was analysed using chi-square tests. Analysis was carried out using SPSS V21.
Results
There were 3857 patients on anti-psychotic medication with slightly more females (51.2%) than males (48.8%). The mean age of female patients was significantly higher than male patients (56.5 ± 18.5 vs. 50.0 ± 17.7, t (3855)=11.2, p<0.001). Seventy-eight per cent of the patients had been prescribed second generation anti-psychotic agents with quetapine (35.8%) and olanzapine (23.2%) being the two most commonly used. 18% had been prescribed first generation drugs and 1% depot drugs. 3% of patients had been prescribed a combination of first/second and depot drugs. Those on first generation medication were significantly older than those on second generation drugs with a mean difference of 10 years (t (3691)=13.2, p<0.001; 95% CI 8.6,11.5). There were also slightly more females (chi-square (1)=4.2, p=0.04;OR 1.2) on first generation drugs. The age difference between first and second generation was similar for both males and females (9.3 years female; 10.3 years male).
Monitoring rates ranged from 75% in the last 18 months for BP and smoking down to under 50% for lipids. For almost a third of patients there was no monitoring data for lipids. In the previous 15 months, 88.5% had been monitored for at least one measure and 36.2% for all six while 34.6% were monitored for less than half the measures.
For those patients on only a single drug (3557, 92% of the sample) a breakdown by drug prescribed showed that screening was not equitable across medication. Those on Clozapine (n=21), a second generation drug, had 4.1 ± 1.5 (mean ± SD) parameters monitored in the previous 15 months. By comparison, patients on Quetiapine (n=1224), had 3.2 ± 2.2 (mean ± SD) parameters monitored in the previous 18 months.
A greater percentage of those on first generation drugs were monitored more recently that for those on second generation drugs. While the numbers were small, fewer of those on depot drugs and more of those on a combination of drugs were monitored in the previous 15 months (Table 2). However the difference between first and second generation monitoring can be explained by age as those on first generation agents were older. This was confirmed by an analysis of the number of monitored measures by drug generation looking only at those on first and second generation drugs. There was a significant difference in the unadjusted mean number of measures monitored (t (3691)=2.6, p=0.009). However, when adjustment was made for age, there was no significant difference in the mean number of measures monitored between first and second generation drugs (ANCOVA, F (1,3690)=0.17, p=0.68).
| Last monitored | |||
|---|---|---|---|
| Measure | In the last 15 months | More than 15 months ago | No data available |
| BMI | 2274 (59%) | 1057 (27.4%) | 526 (13.6%) |
| BP | 2899 (75.2%) | 718 (18.6%) | 240 (6.2%) |
| Triglyceride | 1847 (47.9%) | 727 (18.8%) | 1283 (33.3%) |
| Cholesterol | 1895 (49.1%) | 786 (20.4%) | 1176 (30.5%) |
| Glucose* | 2200 (57%) | 817 (21.2%) | 840 (21.8%) |
| Smoking status | 2911 (75.5%) | 845 (21.9%) | 101 (2.6%) |
Table 2: Last monitoring date by measure, * this includes both glucose and HbA1c monitoring.
There was an increasing trend with older patients being monitored on more measures (F (1,3850)=223.1, p<0.01; eta=0.25). The mean number of measures monitored in the previous 18 months was 3.64 ± 2.21. Females were monitored for more measures than males (3.76 ± 2.12 vs. 3.50 ± 2.29, t (3855)=3.71, p<0.001; difference (95% CI) 0.26 (0.13,0.42)). However once allowance was made for age (women patients tended to be older) there was no significant gender effect (ANCOVA, F (1,3854)=1.35, p=0.25).
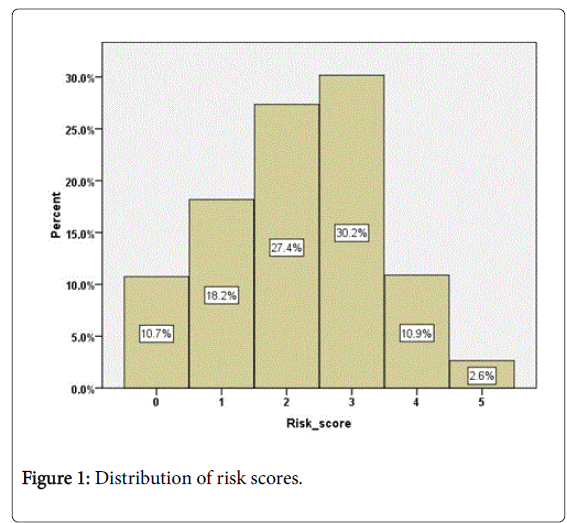
The overall risk score (0-5) was calculated for each patient based on their last monitored level. Where a patient was missing one or two measures the scores were adjusted but if a patient was missing more than 2 measures a score was not calculated. Risk scores were calculated for 3443 (89%) patients. The mean risk score was 2.2 ± 1.2 (range 0 to 5). The distribution of scores is shown in Figure 1 with 56% having a score of 0 to 2 and 44% of patients having a score of 3 to 5. 13.5% of patients had a score of 4 or 5. There was no significant difference in mean risk score by gender once allowance was made for age (ANCOVA, F (1,3440)=0.68, p=0.80).
However, although mean age differed significantly by risk the effect size was small (F (5,3437)=12.7, p<0.01, eta=0.13). Figure 2 shows the mean age (and 95% CI) for patients at each risk score. Those with both zero and the maximum risk score were younger with a mean under 50 years while those with risk score between 1 and 4 were older with mean ages around the mid-fifties (Figure 2).
The mean risk score of patients by the number of measures monitored in the previous 18 months is shown in Figure 2. Those patients who have been monitored for all 5 measures had a significantly higher mean risk score than the others so there was evidence that monitoring had been focused on those most at risk (ANOVA, F (6,3436)=24.9, p<0.001; eta=0.20). This remained true even when age was taken into account (ANCOVA, F (6,3435)=22.6, p<0.001; eta 0.19). However, among those patients with high risk scores of 4 or 5 (466,13.5%), only 58% (270/466) had been monitored for all measures in the previous 18 months while 7% (31/466) had no monitoring. There was no significant difference in the risk profile of patients who had been monitored for less than 5 measures.
11.9% (457/3857) of patients on anti-psychotic medication were diagnosed with type 2 diabetes compared with a prevalence of diabetes of 5.5% in of Ayrshire & Arran in 2014 (16) (z=16.9, p<0.001; OR 2.29 (2.08,2.53). 20.2% (777/3857) of patients on anti-psychotic medication were diagnosed with hypertension compared with a prevalence of 16.0% in Ayrshire & Arran in 2014 (z=6.9, p<0.001; OR 1.32 (1.22,1.43) [16]. 42.2% of the patients on anti-psychotic medication smoked.
Approximately a third of patients on anti-psychotic medication did not have any lipid measures. However, 42.4% of those measured had a raised total-cholesterol or were on lipid lowering treatment and 39.8% had raised triglyceride levels. It is likely that the missing data is not random. It is therefore not possible to calculate a true measure of hyperlipidemia and our figures probably reflect a conservative estimate of cardiovascular risk in patients on anti-psychotic medication.
Discussion
This study confirmed that in patients on anti-psychotic medication there is a clustering of cardiovascular risk factors with hypertension, smoking, type 2 diabetes and probably hyperlipidaemia [2-7]. Type 2 diabetes occurs in mental health illness, particularly patients with schizophrenia, at higher rates than in the general population [15]. The reasons for this increased risk are poorly understood. In addition to general diabetes risk factors, such as age, hypertension, and dyslipidemia, diabetes appears to be promoted by anti-psychotic agents such as olanzapine and mid-potency mid-potency first-generation antipsychotics [17]. In this study the prevalence of hypertension was significantly higher in the patients on anti-psychotic medication compared with the rest of the population and the prevalence of type 2 diabetes nearly double.
In our study only 10% of patients on anti-psychotic medication were free of a cardiovascular risk factor. Furthermore, although the majority of patients in our study were on antipsychotic medication for a psychotic illness, a number of patients were on antipsychotic medications for other reasons, such as bipolar disorder, depressive illness and some of off label usage. This study therefore probably underestimates the risk associated with psychotic illness, antipsychotic medication, and cardiovascular risk factors.
Screening for cardiovascular risk factors varied significantly in relation to age and sex of the patient, the anti-psychotic agent prescribed and cardiovascular risk factor. In this study, blood pressure and smoking status were screened most often and lipid status least often. As one would expect, older patients tended to have more risk factors. Furthermore, older patients were monitored more frequently than younger patients, suggesting that they were more compliant and willing to adhere to a screening process. Alternatively, it is possible that younger patients had more risk factors with a significantly higher early mortality rate.
This cross sectional studies has allowed us to determine prevalence but does not permit distinction between cause and effect. This research, however, is part of a much larger diabetes follow-up study: the Ayrshire Diabetes Follow-up Cohort (ADOC) study. We have been able to flag up these patients on anti-psychotic medication and will be able to monitor their progress over the next 5 to 10 years. A further advantage of our study is the accuracy of the data collected. The data were collected directly from the GPs database (EMIS) and a significant part of the data has been used for Quality Outcome Framework payment [18].
This study also confirmed that the screening for cardiovascular risk factors in patients on antipsychotic medication is unstructured and poorly undertaken [19]. Some of the possible barriers to screening in practice include uncertainty as to whether such physical health screening was the responsibility of the psychiatric team rather than a primary care clinician [20], a lack of confidence in the interpretation of abnormal screening results [21] and limited access to basic infrastructure such as IT equipment [22]. Other possible explanations for the relatively low level of screening is that clinicians may target patients for the assessment of metabolic side effects rather than routinely screen all patients prescribed antipsychotic medication. The quality of guidelines for screening and monitoring of cardiometabolic risk in people with schizophrenia and other mental health illnesses are often not of sufficient quality to fully inform clinicians in screening and monitoring practices [19]. It has also been suggested that patients with psychiatric diagnoses often seem to receive inferior quality of care [19] and that physical co-morbidity is often unrecognized and inadequately treated in those with mental health illnesses [23]. A lack of knowledge about the additive burden of cardiometabolic complications in individuals with mental health problems is another possible explanation for poor monitoring practices. However, this does not seem to be supported by any evidence. For example, in 2005 Buckley et al. found that US psychiatrists rated metabolic monitoring in patients on anti-psychotic medications as a very serious (36%) or serious (61%) concern [24].
In addition, the cardiovascular risk factors appear at a younger age in patients on anti-psychotic medication. In the US Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) study, more than a quarter of men with schizophrenia aged 20-29 years had the metabolic syndrome at baseline, compared with fewer than 10% in the general US population [25]. This would suggest that healthcare professionals need to pay attention to cardiovascular risk factor management in people with severe mental illness from the point of diagnosis. Recent studies have also reported that obesity rates are approximately doubled in people with schizophrenia or bipolar illness. Body composition is also altered in people with severe mental illness: higher waist-to-hip ratios and increased visceral fat have been found even at first presentation of psychosis [26]. Although no antipsychotic can be viewed as weight neutral, the risk of weight gain differs between antipsychotics. Interestingly, the best predictor of long-term weight gain is weight change in the first few weeks of treatment, emphasising the need for regular weight measurement during the early phase of treatment [26].
Conclusion
Health improvement should be considered in a broader and more holistic sense with both physical and mental health improvement built into everyday care and support [27]. Patients with mental health issues should be encouraged to participate in health improvement activities including weight management, physical activity, smoking cessation [28] and health care needs to be tailored to the individual [28]. A combination of motivational and behaviour change interventions, alongside appropriate pharmacological treatments, appear to provide the best results in terms of both health gain and adherence to health improvement activities. In order to gain maximum benefit, individuals need support not only to participate but to sustain their involvement in health improvement activities [29]. All patients on antipsychotic medication should have their cardiovascular risk factors checked on at an annual basis and treated appropriately. This requires a structured screening process and good lines of communication between Mental Health Teams, Primary Care and Physicians with an interest in cardiovascular risk.
References
- Vinogradova Y, Coupland C, Hippisley-Cox J, Whyte S, Penny C (2010) Effects of severe mental illness on survival of people with diabetes. Br J Psychiatry 197: 272-277.
- Laursen TM, Munk-Olsen T, Vestergaard M (2012) Life expectancy and cardiovascular mortality in persons with schizophrenia. Curr Opin Psychiatry 25: 83-88.
- De Hert M, Correll CU, Bobes J, Cetkovich-Bakmas M, Cohen D, et al. (2011) Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psychiatry 10: 52-57.
- Leucht S, Burkard T, Henderson J, Maj M, Sartorius N (2007) Physical illness and schizophrenia: A review of the literature. Acta Psychiatr Scand 116: 317-333.
- De Hert M, Detraux J, Van Winkel R, Yu W,Correll CU (2012) Metabolic and cardiovascular adverse effects associated with antipsychotic drugs. Nat Rev Endocrin 8: 14-126.
- Rojo LE, Gaspar PA, Silva H (2015) Metabolic syndrome and obesity among users of second generation antipsychotics: A global challenge for modern psychopharmacology. Pharmacol Res 101: 74-85.
- Holt RI, Peveler RC (2006) Antipsychotic drugs and diabetes-An application of the Austin Bradford Hill criteria. Diabetologia 49: 1467-1476.
- Mitchell AJ, Vancampfort D, Sweers K (2013) Prevalence of metabolic syndrome and metabolic abnormalities in schizophrenia and related disorders-A systematic review and meta-analysis. Schizophr Bull 39: 306-318.
- Schizophrenia working group of the psychiatric genomics consortium (2014) Biological insights from 108 schizophrenia-associated genetic loci. Nature 511: 421-427.
- Kao AC, Muller DJ (2013) Genetics of antipsychotic-induced weight gain: update and current perspectives. Pharmacogenomics 14: 2067-2083.
- Colman I, Ataullahjan A, Naicker K, Van Lieshout RJ (2012) Birth weight, stress, and symptoms of depression in adolescence: Evidence of fetal programming in a national Canadian cohort. Can J Psychiatry 57: 422-428.
- Peet M (2004) Diet, diabetes and schizophrenia: Review and hypothesis. Br J Psychiatry Suppl 47: S102-105.
- Mitri J, Muraru MD, Pittas AG (2011) Vitamin D and type 2 diabetes: A systematic review. Eur J Clin Nutr 65: 1005-1015.
- Mental health in Scotland: Improving the physical health of those experiencing mental illness.
- Lean ME, Pajonk FG (2003) Patients on atypical antipsychotic drugs: Another high-risk group for type 2 diabetes. Diabetes Care 26: 1597-1605.
- Nialsen J, Skadhede S, Correll CU (2010) Antipsychotics Associated with the Development of Type 2 diabetes in antipsychotic-naïve schizophrenia patients. Neuropsychopharmacology 35: 1997-2004.
- Information Services Division Scotland (2014) Quality and outcomes framework.
- De Hert M, Bobes J, Cetkovich-Bakmas M, Cohen D, Leucht S, et al. (2011) Physical illness in patients with severe mental disorders. II. Barriers to care, monitoring and treatment guidelines, and recommendations at the system and individual level. World Psychiatry 10: 138-151.
- Mitchell AJ, Delaon V, Vancampfort D, Correll CU, De Hert M (2012) Guideline concordant monitoring of metabolic risk in people treated with antipsychotic medication: Systematic review and meta-analysis of screening practices. Psychol Med 42: 125-147.
- Organ B, Nicholson E, Castle D (2010) Implementing a physical health strategy in a mental health service. Australas Psychiatry 18: 456-459.
- Cochrane L, Olson C, Murray S, Dupuis M, Tooman T, et al. (2007) Gaps between knowing and doing, understanding and assessing the barriers to optimal health care. J Contin Educ Health Prof 27: 94-102.
- Morrato EH, Newcomer JW, Kamat S, Baser O, Harnett J, et al. (2009) Metabolic screening after the American diabetes association’s consensus statement on antipsychotic drugs and diabetes. Diab Care 32: 1037-1042.
- Buckley P, Miller D, Singer B, Arena J, Stirewalt E (2005) Clinicians’ recognition of the metabolic adverse effects of antipsychotic medications. Schizophr Res 79: 281-288.
- Nasrallah H, Meyer J, Go D, McEvoy J, Davis S, et al. (2006) Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: Data from the CATIE schizophrenia trial sample at baseline. Schizophr Res 86: 15-22.
- Alvarez-Jimenez M, González-Blanch C, Crespo-Facorro B, Hetrick S, RodrÃguez-et al. (2008) Antipsychotic-induced weight gain in chronic and first-episode psychotic disorders: A systematic critical reappraisal. CNS Drugs 22: 547-562.
- Weiss A, Henderson D, Weilburg J, Go D, Meigs J, et al. (2006) Treatment of cardiac risk factors among patients with schizophrenia and diabetes. Psychiatric Services 57: 1145-1152.
- Green CA, Yarborough BJ, Leo MC, Yarborough MT, Stumbo SP, et al. (2015) The STRIDE weight loss and lifestyle intervention for individuals taking antipsychotic medications: A randomized trial. Am J Psychiatry 172: 71-81.
- Chwastiak L, Tek C (2014) Management of obesity in the psychiatrist's office. World Psychiatry 13: 193-195.
Citation: Collier A, Kessavalou K, Sit LE, Hair M, Cameron L, et al. (2017) Anti-Psychotic Medication and the Pattern of Cardiovascular Risk Factors: A Population Based Study (The Ayrshire Diabetes Follow-Up Cohort (ADOC) Study). J Addict Res Ther 8: 343 DOI: 10.4172/2155-6105.1000343
Copyright: ©2017 Collier A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 5058
- [From(publication date): 0-2017 - Dec 22, 2025]
- Breakdown by view type
- HTML page views: 4081
- PDF downloads: 977