Beneficial Gut Effect of a Symbiotic-Probiotic Regimen in Healthy Stressed Individuals: Effectiveness on Permeability, Microbiota and Detoxification Parameters
Received: 02-Mar-2017 / Accepted Date: 02-Apr-2017 / Published Date: 09-Apr-2017 DOI: 10.4172/2161-069X.1000560
Abstract
The aim of the present study was to test either a previously studied symbiotic, a novel probiotic mixture or a sequence symbiotic-probiotic schedule in beneficially affecting gut health parameters in otherwise healthy stressed individuals. This was a multicenter randomized study with no gender selection. A total of hundred-twenty individuals aged between 38 and 62 years were recruited for this study. Patients were selected to have an overall positive attitude towards their personal life and work but reporting high-demanding job activity regarded as stressful. Three age and gender-matched groups (40 pts each) were considered: A) given a symbiotic 10 ml t.i.d. for 5 months; B) given 1 tab t.i.d of P3T/J (a probiotic mixture) for 5 months and C) given the symbiotic 10 ml t.i.d. for 1 month and then shifted to 1 tab t.i.d of probiotic mixture for 4 months. A forth group (group D) of 20 healthy, stressed individuals coming from a prior study, supplemented a marine PUFA extract served as treatment control (a sturgeon-based fish collagen, Caviarlieri, Swiss cap packaging, Switzerland).
As compared to baseline and all other groups, group C showed a significant decrease of morning salivary cortisol at awakening. When plotting these values against the STAI scores, it appeared a significant correlation (r:0.66, p<0.05). At either 2 and 5 months observation, it appeared that the area under the curve in group B and C was significantly lesser than in all other groups (p<0.05 vs. B and D). Salivary levels of CgA sampled at 1 pm were significantly higher than at baseline in all groups and showed in C group a significant dercrease at 5 months observation. At 2 months observation the zonulin level was significantly decreased by treatment B and C. At 5 months observations, both A and C groups showed a significant further lowering (C>B). Values of serum zonulin showed a wide scattering in all groups and by clustering those individuals, who were engaged in regular moderateintense physical activity, the baseline concentration of zonulin was higher as compared to the rest of the population and it occurred a remarkably lower level of zonulin with C schedule. Fecal parameters in B and C group showed a statistically significant reduction of β-glucuronidase at 2 months (p<0.05) while only group A and C maintained such status also at 5 months observation. As for fecal level of nitroreductase, this was beneficially reduced by B treatment during all the study period. Besides treatment A, also regimen C at 5 months showed a significant reduction of this parameter. Unlike group D, all the other three groups showed a significantly lower level of p-cresol at either 2 or 5 months observation with group C yielding the absolute lowest urinary concentration but only at 2 months. The subgroup in each treatment schedule group showed a significant increase of the Bacteriodetes/Firmicutes ratio which was normalized by B and C treatment. Either A, B and C regimes brought about a significant eubiosis modification of gut flora.
Keywords: Symbiotic; Probiotic; Saliva; Stress; Cavarlieri
Introduction
While acute stress conditions are likely to cause self-limited abnormalities in gut physiology [1,2], the modern multi-factorial chronic psychosocial and dietary style stressors [3,4], may generate a constant threat and detrimental trigger to microbiota ecology [5]. These phenomena may be associated with morpho-functional gut abnormalities, such as dysregulation of the immune system [6,7] with low-grade inflammation [8,9], hypercortisolemia [10], long-term permeability of epithelial barriers [11], endotoxemia, [12], metabolic and neurodegenerative disorders etc. [5,13-15]. Indeed, the intestinal paracellular “leak” allows antigens to directly translocate from the intestinal intraluminal space and this promotes the immune system response and subsequent inflammatory mechanism and redox unbalance [16,17]. On the other hand, following the birectional gutbrain interrelationship, several experimental research have envisaged how emotional changes (stress, anxiety, depression) may modify gut microbiota [18,19] while also other contributions have proven that by modulating gut micro-ecology, brain metabolomics and neurotransmitter could be influenced as well [10,20-22]. A number of studies have shown that cortisol may be a reliable biomarker stress either in of psychological disorders and physiological conditions [23]. A further marker of interest is represented by chromogranin-A, an acidic glycoprotein present in most neuroendocrine cell types and released along with catecholamines from the adrenal medulla and the sympathetic nerve endings, is highly related to stress response. From the work of Nakane et al. [24], it appears that, when psychosomatic stress is applied, unlike the delayed cortisol detection in the saliva, a brisk increase in salivary CgA con centration occurs. Moreover, Nomura et al. [25] showed that this biomarker has a sensitive and quick dynamics by decreasing during the recovering phase in intermittent psychological stress test. Intensive physical exercise, with also its invariably associated psychological factors, has been reported to affect gut barrier. In particular, abnormally elevated serum levels of zonulin have been reported in such situations [26], besides several autoimmune diseases, type 2 diabetes, obesity and overall metabolic syndrome [27]. Within an healthy gut concept one has also to consider the remarkable detoxification processes taking place intraluminally and affecting the whole body. As a matter of fact, during dysbiosis it can be hypothised that some bacterial species can indirectly impair liver detoxification by deglucuronidation of hormones and xenobiotics [28,29]. β-glucuronidase hydrolyzes several glucuronides and thus releasing into the intestinal lumen a number of potentially mutagenic aglycones, mainly coming from enzymatic activity of β-glucuronidase from Bacteriodes [30,31]. A further interesting marker of a proper gut detoxification function is the urinary level of p-cresol, (4-methylphenol), a 108.1 Da volatile low-molecularweight compound forming as byproduct of proteolytic degradation of aromatic amino acids (phenylalanine, tyrosine, and tryptophan) by the anaerobic flora of the left colon. P-cresol is absorbed from the intestinal luminal content to the bloodstream by colonocytes, metabolized in the liver and mostly excreted by the kidneys. A lower p-cresol excretion has been shown to be associated to a stricter adherence to a Mediterranean diet [32] while higher values have been reported in cardiovascular disease [33], besides being noxious to colonocytes per sè [34].
In prior experimental and clinical work, our group had showed that a symbiotic mixture (SCM-III, based on L. acidophilus strain, L. helveticus, Bifidobacterium spp. in a phytoextracts-enriched medium. Microflorana-F, Named Co, Lesmo, Italy) had significant prokinetic, cytoprotective and antimutagenic properties [32-38] while preliminary in-house experimental work have suggested that P3T/J (a probiotic mixture composed of Bifidobacterium animals ssp. lactis Bi1, Bifidobacterium breve Bbr8, Lactobacillus acidophilus LA1, Lactobacillus paracasei 101/37, LD Proactiv 50, Named Co, Lesmo, Italy) could exert adaptogenic and eubiosis properties in stress-induced and antibiotic-induced dysbiosis. The aim of the present study was to test the symbiotic, the probiotic or a sequence symbiotic-probiotic schedule in beneficially affecting gut health parameters in otherwise healthy stressed individuals.
Materials And Methods
Study design
This was a multicenter randomized study with no gender selection. Subjects were randomized using a table of random numbers derived from a random number generating program. All subjects provided written informed consent prior to joining in this study. This investigation was approved by an independent Ethical Committee for non-pharmacological research (ReGenera Research Group for Aging Intervention, protocol PROBIO-DeStress 23/2017). Each subject recruited from outpatient clinics for the study was fully informed and treated in compliance with the guidelines of the Declaration of Helsinki for Research on Human Subjects 1989. A total of hundredtwenty individuals aged between 38 and 62 years were recruited for this study. Patients were selected to have an overall positive attitude towards their personal life and work but reporting high-demanding job activity regarded as stressful and was eligible for the study. If they reported being sedentary, this was noted by taking into account the definition as not being engaged in an exercise regimen exceeding 20 min per day, 3 day per week over the previous six months. Subjects underwent an anthropometric and clinical examination including questions on family history, socio-economy, lifestyle factors, and medical history. Participants with a major depression/anxiety diagnosis were excluded as well as if they were excessive drinkers (>10 drinks/week) and recreational drug users, smokers (>5 cigarettes/wk) or presenting with a prior or present history of cancer, heart disease or autoimmune disorders. Participants who were obese (BMI>29,99), suffering from sleep disorders or sleep-apnea or attending mind-body relaxation programs were also excluded from the study. Similarly, cases suffering at present or in the past from severe burnout (high score for emotional exhaustion or depersonalization) as assessed by an altered Maslach Burnout Inventory [39] were also excluded. Whenever stress assessment took place, it was made sure that women participating in the study were either on hormonal contraceptives or in the first half (follicular phase) of their cycle when considering the influence of gonadal steroids on HPA axis stress responsiveness [40]. Healthy, age/gender-matched group fulfilling the above exclusion criteria and reporting no psychological pressure of any kind, were considered our non-stressed control group.
Subjects were instructed to maintain their current dietary regimen while under study, to abstain from alcohol, green tea, caffeine use and physical activity for 24 hour before testing. Moreover, no food or snack was allowed for at least min before salivary collection, as suggested by Toda et al. [41]. Due to the lack of any pilot data, the target sample size of 40 per group was selected based on the size of similar studies in the literature and what would identify a clinically meaningful group difference.
Three age, BMI and gender-matched groups (40 pts each) were considered.
• Given SCM-III 10 ml t.i.d. (Composition for 100 ml: L acidophilus strain 145 1.25 × 106, L helveticus ATC 15009 1.3 × 109, Bifidobacterium spp. 420 4.95 × 109 in a phytoextractsenriched medium. This consists of: Urtica dioica, Ribes nigrum, Vaccinium myrtillus, Taraxacum officinalis leaves and roots, Daucus carota, Equinacea purpurea leaves and roots in the amount of 82 g/100 ml. Microflorana-F, Named spa, Lesmo, Italy) for 5 months.
• Given 1 tab t.i.d of P3T/J (a probiotic mixture composed as follows: Bifidobacterium animalis sp. lactis Bi1, Bifidobacterium breve Bbr8, Lactobacillus acidophilus LA1, Lactobacillus paracasei 101/37, LD Proactiv 50, Named Co, Lesmo, Italy) for 5 months.
• Given SCM-III 10 ml t.i.d. for 1 month and then, stop and follow through with 1 tab t.i.d of P3T/J × 4 months.
A forth group (group D) of 20 healthy, stressed individuals (as for specific questionnaire and interview assessment) and ageand gender matched, coming retrospectively from a prior study, supplemented a generic marine PUFA extract previously shown to exert some biochemical and symptomatic benefit on stress parameters, served as treatment control [42]. Fecal, salivary and blood samples stored at -80°C were used. Compliance was tested by asking the subjects to return the used and unused compounds containers.
Methods
Stress assessment: Pre-selection on the basis of validated stress questionnaire Psychological stress was measured by the State Trait Anxiety Inventory (STAI), which is widely used for assessing state or acute anxiety [43]. STAI was completed by all participants at the entry and at the completion of the study. The STAI asks the subject to describe how he/she feels ‘right now’ by responding to 20 questions with a 4-point response format from ‘not at all’ (score 1) to ‘extremely’ (score 4) anxious. The answers are reported on a Licker scale. Total scores range from 20 to 80, with higher scores indicating greater anxiety. Only subjects regarded as having “mild-moderate” stress (STAI score: 2-3) were included into the study. The questionnaire was administered at the entry and then during the scheduled 1 and 5 month visit.
At the entry, 2 months and 5 months afterwards the following tests were done. Each time, salivary tests were done twice, at awakening, after entering their work premise about 30-50 min after awakening (8.30-9.00 am) and at 1.00 pm by properly instructing the patients to handle the collection by themselves.
Biochemical tests: Blood samples were processed for routine analyses, including metabolic parameters: total and high-density lipoprotein (HDL) cholesterol and glycaemia were measured using Siemens enzymatic methods (Siemens Health Diagnostics, Deerfield, USA).
Salivary markers: Saliva was collected by previously instructed patients at awakening, around 8.30-9.00 am (before having breakfast at working place) and at 1 pm (before lunch). The patients were informed that they should not eat or drink anything 90 minutes beforehand and to rinse their mouth with clear water immediately before each collection. Five ml un-stimulated saliva was collected in 5 minutes by using a sterilized Falcon tube in a quiet condition while sitting on a chair with head put a bit forward and samples put each time in ice-boxes and collected by a dedicated nurse within 30 min to be then quickly stored at -80°C until assayed. Before assay, the saliva samples were centrifuged for 3 minutes in an Eppendorf Microfuge to remove particulate material and salivary cortisol (nmol/L) activity was determined using a fluorescence immunoassay, as previously described [44], with intra and inter-assay variability of less than 10% and 12%, respectively. Salivary CgA levels also determined by an enzyme-linked immunosorbent assay kit (Chromogranin A, L-Span Bio-sciences, Co, USA). The CgA concentration was corrected by the total protein salivary concentration.
Fecal pH and biomarkers: The pH of fecal samples (homogenizing 1 g soft part of feces with 10 mL distilled water) was measured by using an Orion pH probe (Fisher Scientific, USA) connected to an Accumet Model 25 pH meter (Fisher Scientific).
Raw stool samples were immediately refrigerated after collection and then they were mixed with a storage solution (pH 7.2) consisting of 1 g yeast extract, 1 g KH2PO4, 0.15 g K2HPO4, 0.15 g NHCl, 1 g NaCl, 0.6 g MgCl26H2O, 0.1 g KCl, and 0.5 g cysteine-HCl, (quantities per 1 L) and stored at -80°C within 10 hour after the sampling. Before the laboratory analysis, stool samples were thawed, homogenized eluted by using a proper fecal sample preparation kit (Roche Diagnostics, Germany). Briefly, 100 mg of stool sample was suspended in 5 mL of vortexed extraction buffer and centrifuged (5 min at 2000 g). Stool sample eluates were used immediately after preparation for ELISA analysis.
Fecal and serum zonulin determination: Overall the zonulin analysis is based on a competition between the free antigen in the samples or standards and the antigen coated on the wells of the microplate. Standards, aliquots of the treated preparations and the polyclonal primary anti-zonulin antibody were transferred into the pre-coated microplate wells. The antigen competes with the antigen immobilized on the wells for the binding sites of the antizonulin antibody. ELISA assays were used to measure serum and fecal zonulin (ImmundiagnostikAG, Bensheim,Germany). The unbound components were washed out. During a second incubation step, a streptavidin-labeled-peroxidase antibody, which binds to the biotinylated zonulin tracer, was added into each microtiter well. After a washing step to remove the unbound components, a peroxidaseconjugated antibody is used for detection and tetramethylbenzidine as a peroxidase substrate. The enzymatic reaction is terminated by acidic stop solution. The sensitivity of the assay was less than 0.01 ng/ mL. Mean intra-andinterassay coefficients of variance were 5% and 8.9%, respectively. The quantification is based on the optical density at 450 nm. Data are expressed in ng/mL.
Foecal assay of β-glucuronidase and nitroreductase: The fecal samples were collected in four replicates from the same stool by each participant at the end of the run-in period (Baseline, week 0) and treatment period (2 months and 5 months), put in 0.2 mL of 1:10 v/v dilution of the caecal digesta in 100 mM phosphate buffer at pH 7.0, centrifuged at 10000 xg for 15 min at 4ºC to be then used for quantitative enzyme assay of β-glucuronidase (β-Glucuronidase -EC 3.2.1.31; substrate phenolphthalin mono β-D-glucuronic acid; Sigma, St. Louis, MO) and nitroreductase. The β-glucuronidase enzymatic activity was assessed in triplicate by spectrophotometry, by measuring the amount of phenolphthalein (p-nitrofenol) released following the hydrolysis of 10 mM phenolphthalein-β-D-glucopyranoside (20 mM, Sigma Aldrich, USA, p-nitrophenyl-β-D-glucuronide). One gram of feces was diluted 10-fold in 0.1 M potassium phosphate buffer. The specimens were homogenized to be then sonicated for 15 min, and centrifuged at 12,000 rpm for 10 min at 4°C. Then the enzymerich collected supernatant was transferred to test tubes and 1 ml of 0.02 M phosphate buffer pH=7, and 0.1 ml of phenolphthalein-β-Dglucopyranoside (p-nitrophenol-β-D-glucopyranoside) was added to it. The samples were incubated in a water bath for 15 min at 37°C (for 60 min at 37°C), after adding glycine buffer the reaction was stopped. The bacterial enzyme activities were measured by using commercial assay kits (human ELISA kits for β-glucuronidase and nitroreductase (carried out in an anaerobic chamber); R&D Systems, USA). One unit of β-glucuronidase and nitroreductase was defined as the amount that released 1 mmol of β-nitrophenyl, p-nitrophenyl, and p-aminobenzoic acid per minute, respectively. Results were expressed as units per gram (U/g) of wet feces. The tubes were mixed thoroughly and the absorbance level was read at wavelength λ=540 nm using the methods detailed by Goldin & Gorbach [45]. The total concentration of protein in bacterial cells was determined by the Lowry method.
Determination of urinary p-cresol content: A specific care was taken as to ascertain that partecipants would not substantially vary their protein intake. This is because; an increase of protein intake being degraded by the microbiota proteases and peptidases to release free amino acids fueling bacterial formation of p-cresol [46] would raise its urinary concentrations [47]. The p-cresol was measured by gas chromatography (GC) mass spectrometry (MS) technology. Urine samples with a volume of 950 μl were taken, and the pH of the samples was adjusted to pH 1 with concentrated H2SO4. The conjugated phenolic content of the solution was deproteinized and hydrolyzed by heating at 90°C for 30 min. After cooling down to room temperature, 50 μl of 2,6-dimethylphenol (20 mg/100 ml, Sigma-Aldrich Chem, USA) were added as an internal standard. P-cresol was extracted with 1 ml of ethyl acetate and after its drying 0.5 μl of this solution was transferred to GCMS. Helium GC grade was used as a carrier gas with a constant flow of 1.3 ml/min. The oven was run at 75°C for 5 min with 10°C/min increase up to 160°C and at with 20°C/min steps up to 280°C. MS detection was carried out in full-scan mode from m/z 59 to 590 at 2 scans/s. Results were expressed as mM/mM creatinine p-cresol excreted in urine.
Gut microbiota assessment: DNA extraction and gene sequencing: Stool specimens were collected at home and transported to the laboratory within 3 hours in ice, fixed in Transwab tubes (Sigma, Dorset, UK and immediately frozen and stored at -80°C until assay when about 200 mg of stool was homogenized. Briefy, total bacterial DNA was extracted from 250 mg of fecal sample using QIAamp DNA Stool Mini Kit (Qiagen, California, USA) by applying a slightly modified protocol [48] in a MagNA Pure LC2.0 device (Roche Diagnostics, Mannheim, Germany). The DNAs samples were resuspended in 100 μl of TE buffer and treated with 2 μl of 10 mg/ml DNase-free RNase at 37˚C for 15 min. After protein removal, DNA was purified by QIAamp Mini Spin columns (QIAGEN). Final DNA concentration was quantified by using NanoDrop ND-1000 (NanoDrop Technologies, Wilmington, DE). The V2-V3 region of the 16S rRNA gene was amplified by using the universal primer set HDA1-GC/HDA2 eubacteria. The amplification reactions were conducted in Thermal Cycler T Gradient, with AmpliTaq Gold DNA Polymerase (Applied Biosystem) as DNA polymerase thermostable. The composition of 50 μL of the reaction mixture was as follows: 0.5 μM of each primer, dNTPs 200 μM, 1X PCR Buffer, MgCl2 2 mM, 1.25 U of DNA polymerase, and 4 μl of genomic DNA template 20 ng/μl. Nextera adapter sequence (Illumina, California, USA) was added to the 5’-end of the primer set for library preparation. PCR using 50~150 ng DNA was performed with 1 cycle of 98°C/30 sec, 30 cycles of 98°C/10 sec, 60°C/30 sec, 72°C/30 sec, and 72°C/5 min. Libraries were purified and successively pooled at equimolar concentrations (4 nM), denatured and diluted to 6 pmol/L to be then quantified using Nanophotometer (G-Implen, München, Germany). The purified libraries were checked by 2% agarose gel electrophoresis, Qubit (Thermo Fisher Scientific, Massachusetts, USA) and qPCR methods. Finally, libraries were normalized to the same concentration and sequenced by Illumina Miseq sequencer.
Statistical Analysis
SPSS for Windows software, version 19.0 was used for statistical analyses. Data are presented as mean ± SD. The Shapiro-Wilk test was used to determine normal distribution. Baseline characteristics, and clinical chemistry data, were compared by unpaired Student’s t-test and factors: treatment was analyzed using a univariate, three-factorial, repeated measures ANOVA. Significant interactions and main effects were analyzed by using Bonferroni correction.
Results
State Anxiety assessment (STAI-Y1) Data from the state anxiety inventory revealed that at 5 month observation, group A and C showed a trend reduction of the score.
Salivary tests
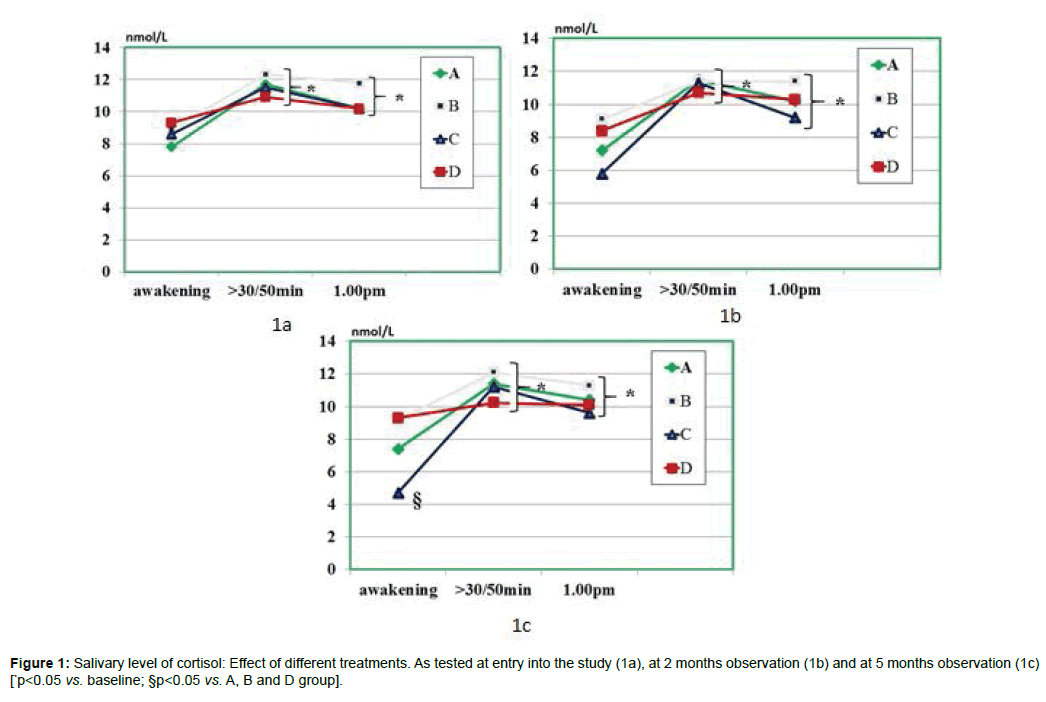
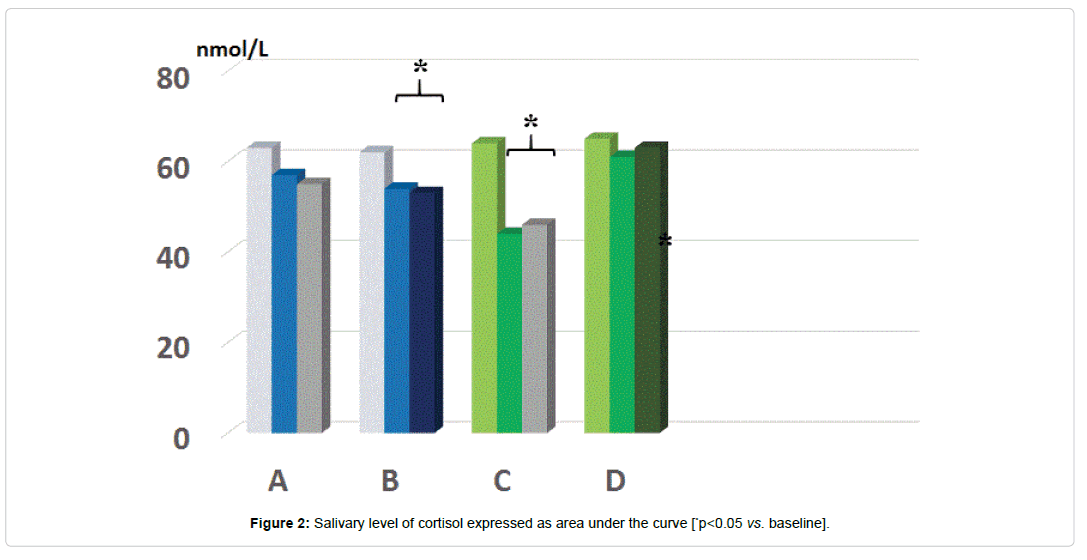
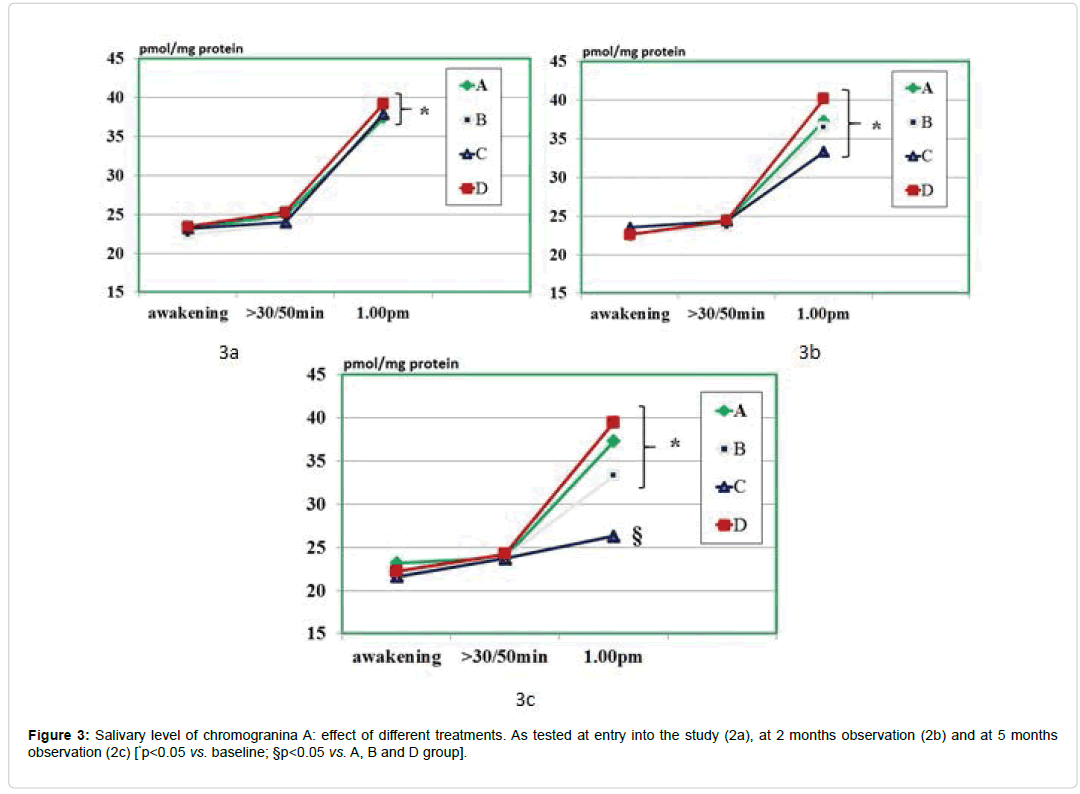
As compared to baseline, group A, B and D, group C showed a statistically significant decrease of morning salivary cortisol at awakening throughout the study period (p<0.05) but not at 8.30-9.00 am. When plotting these values against the STAI scores, it appeared a significant correlation (r: 0.66, p<0.05) either at baseline and at 5 month observation (but not at 2 months observation due to scattered values) (Figures 1 and 2). While absolute values of salivary cortisol were not significantly affected at 1 pm testing by any of the treatments, at either 2 and 5 months observation, it appeared that by calculating the area under the curve out of the three samplings in either group A and C, this was significantly lesser than in all other groups (p<0.05 vs. A and D). At awakening and 8.30-9.00 am, salivary levels of CgA were not affected by any of the treatments employed. However, 1 pm assessment showed that this parameter was significantly higher than at baseline in all groups (Figure 3a) prior to treatment (retrospective group D included as well) as compared to awakening value (p<0.05). This parameter showed in C group a trend decrease at 2 months (Figure 3b) and reached a statistically significance at 5 months observation (Figure 3c, <0.05 vs. groups A and B).
Permeability test
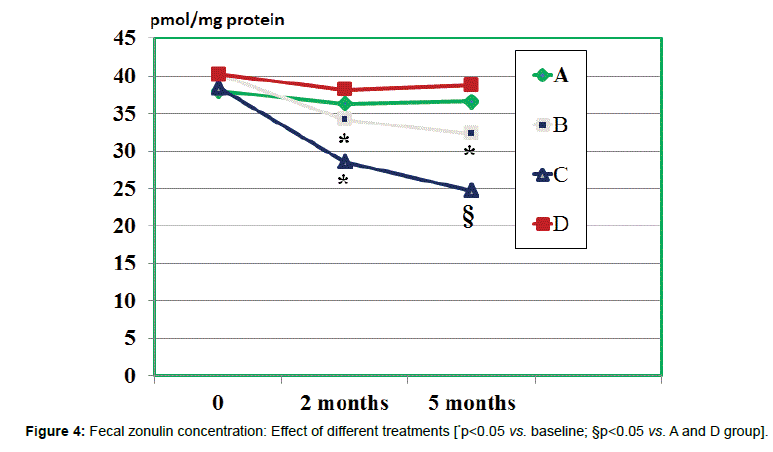
Fecal zonulin concentration: All groups showed a comparable fecal level of zonulin at baseline ranging from 38.7 to 40.2 ng/ml (Figure 4). At 2 months observation the zonulin level was significantly decreased by treatment B and C (p<0.05 vs. baseline and vs. other groups). There was an albeit not significant trend showing lower values of the C group ss compared to B group (n.s.). However, when evaluating the values measured at 5 months observations, both A and C groups showed a significant further lowering (p<0.05 vs. baseline and vs. other groups) but values of C groups were significantly lower than in B group (p<0.05).
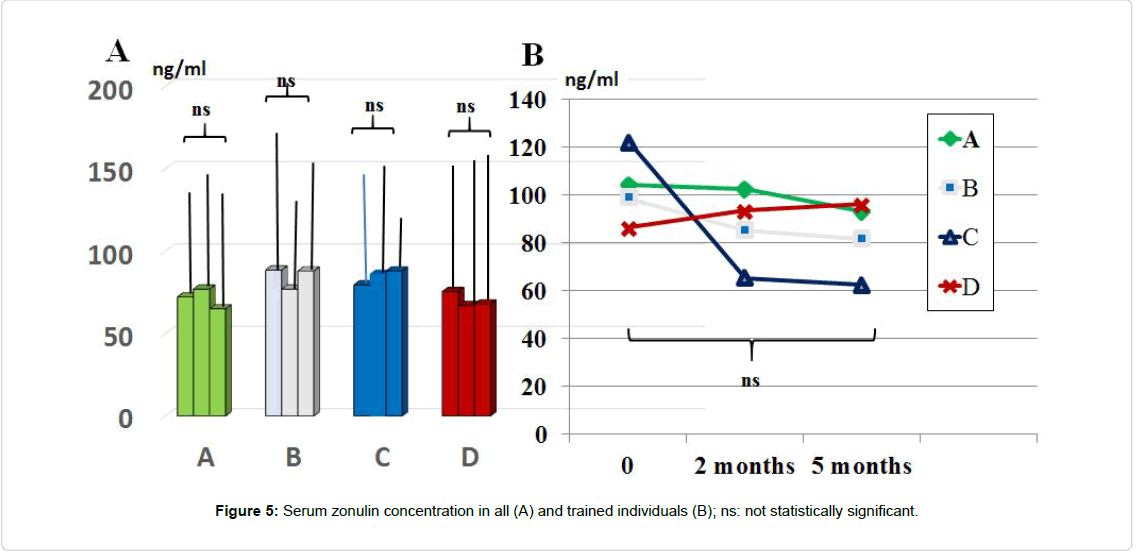
Serum level of zonulin: Serum zonulin concentration throughout the study period was not significantly affected by any of the treatment although there was a not significant trend decrease in group C and A treatment (Figure 5). Values showed a wide scattering in all groups (range 37.5 ng/ml to 163.4 ng/ml). By clustering those individuals who were engaged in regular moderate-intense physical activity (total 29 individuals: 6 in A group, 9 in B, 8 in C and 6 in D group), the baseline values of zonulin were higher as compared to the rest of the population (range 89.5 ng/ml to 163.4 ng/ml). Interestingly, this group yielded a remarkable lower level of zonulin with C schedule. However, the limited number of subjects did not allow carrying out a statistical analysis.
Detoxification tests
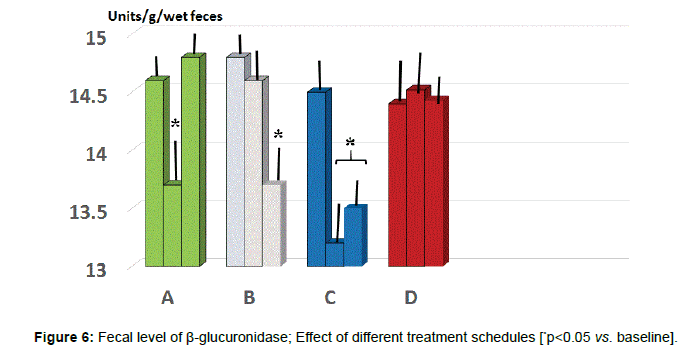
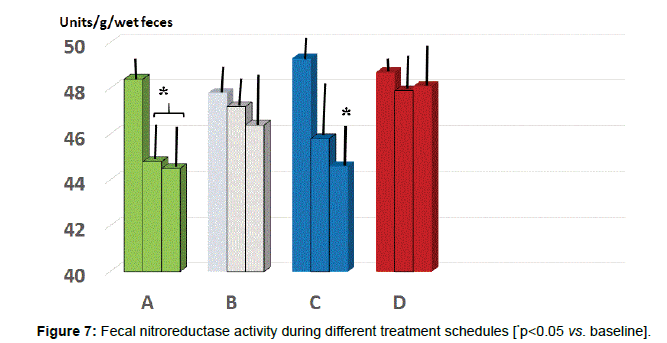
Fecal parameters: Either A and C group showed a statistically significant reduction of β-glucuronidase at 2 months (p<0.05 vs. baseline, group A and D, Figure 6) while only group B and C maintained such significant difference also at 5 months observation (p<0.05 vs. B and D). As for fecal level of nitroreductase, this was beneficially reduced by B treatment starting from 2 months observation (Figure 7, p<0.05 vs. baseline and all other groups). Besides treatment B, also regimen C at 5 months testing showed a significant reduction of this parameter (p<0.05, vs. baseline, group A and D).
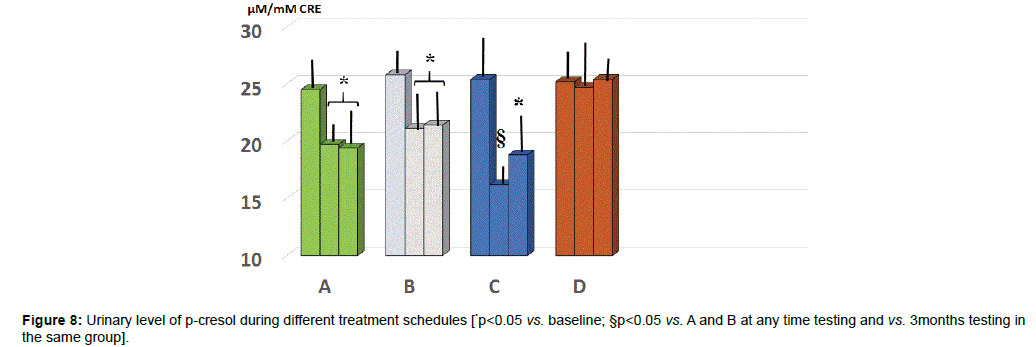
Urinary parameters: At baseline the values of p-cresol showed a wide scattering of the data. Unlike group D, all the other three groups showed a significantly lower level at either 2 or 5 months observation (Figure 8, p<0.05) with group C yielding the absolute lowest urinary concentration but only at 2 months (p<0.05 vs. group A and B).
Main gut microbiota assessment
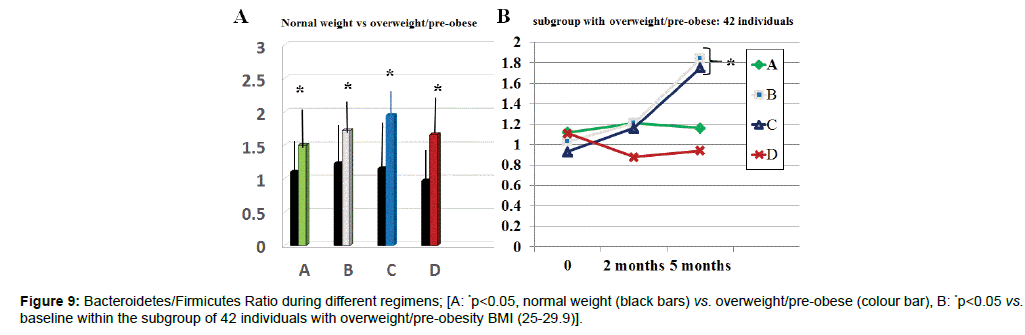
There was a wide scattering of the Bacteroidetes/Firmicutes ratio throughout all groups allowing a statistically significant discrimination among groups (data not shown). However, by selecting in each groups individuals with BMI over BMI 25 (A10, B12, C11, D9), these showed a statistically significant lower ratio as compared to the rest of the group (Figure 9 p<005). Only regimes B and C brought about a normalization at 5 months observation (p<0.05 vs. baseline and vs. A and D group at any time). Moreover, group B and C showed a statistically significant increase of lactobacilli population (p<0.01, Table 1). Same significant increase was observed in A, B and C group as far as Bifidobacteria were concerned.
| Bacterial Population | Group A | Group B | Group C | Group D |
|---|---|---|---|---|
| Lactobacillus (cfu/g) | ||||
| Entry | 6.1 ± 0.5 | 6.1 ± 0.2 | 6.0 ± 0.4 | 5.9 ± 0.4 |
| 2 months | 6.3 ± 0.2 | 6.9 ± 0.3 | 7.5± 0.2* | 6.2 ± 0.3 |
| 5 months | 6.6 ± 0.6 | 7.6 ± 0.2* | 8.1 ± 0.4* | 6.0 ± 0.3 |
| Bifidobacterium (cfu/g) | ||||
| Entry | 6.9 ± 0.3 | 7.1 ± 0.4 | 6.9 ± 0.1 | 7.1 ± 0.5 |
| 2 months | 8.1 ± 0.2* | 9.1 ± 0.2* | 9.4 ± 0.3* | 6.9 ± 0.2 |
| 5 months | 8.0 ± 0.4* | 9.6 ± 0.2* | 10.1 ± 0.2* | 6.8 ± 0.4 |
Table 1: Stool concentration of Lactobacilla and Bifidobacteria during different treatment regimens [*p<0.01 vs. baseline and other groups at the same point testing].
Conclusion
While acute stress responses elicits adaptation and survival by positively acting on neural, cardiovascular, immune and metabolic pathways, chronic stress deregulates those systems by switching their function to a pathophysiological mode. Indeed, it is likely that acute stressful events may affect microbiota only at minor extent owing to the relative stability of the gut ecology over time [49], However, when persistent stressors cross some sort of breaking point, this brings about a substantial and, at times, self-maintaining eubiosis imbalance. Glucocorticoids and catecholamines are the two landmark hormones of stress response and, we found that otherwise healthy individuals psychologically “adapted” to their demanding working environment, would develop a transient significant increase of salivary cortisol. None of the treatment employes brought about a significant difference at each single testing point but the probiotic (B) and symbiotic-probiotic regimes enabled a significant lower area under the curve of salivary cortisol output. Interestingly, at the peak of working activity, the more fast-acting stress parameter, i.e. chromogranin-A [50-52] yielded a comparably significant increase and only symbiotic-probiotics (C) treatment normalized it. B and C treatment were also the one beneficially affecting fecal zonulin and this may be ascribed to the eubiotic action exerted, whereas in the serum there was a similar trend in those individual undergoing intensive physical activity. There is an ever increasing data on the importance of microbiota modulation of stress response [53-56] and albeit limited report on the beneficial gut ecology interventions [57-59]. In this regard, our present results further corroborate such rationale.
As for the detoxification parameters (beta-glucuronidase and nitroreductase) the symbiotic (A) seems to play a more relevant tole and this could be indirectly in agreement with the antimutagenic property our group showed in the past [38]. Indeed, these two colonic enzymes are synthesized by a set of detrimental bacteria [60] antagonizing hepatic metabolism by hydrolyzing the biliary conjugates such and also being potentially carcinogenetic. We do not have a solid explanation why the studied population had a slightly but significantly raised fecal concentration of beta-glucuronidase and nitroreductase. From a dietary analysis it appeared that most of the recruited individuals were on a reduced carbohydrate intake (either for dieting or personal dietary style). When there is limited carbohydrate availability, proteins more easily undergo fermentative metabolism by proteolytic bacteria bringing to potential formation of potentially toxic metabolites (ammonia, amines, thiols, phenols and indoles). As a matter of fact, p-Cresol (4-methylphenol), a low-molecular-weight compound, is a metabolite of protein breakdown and an increase of the nutritional protein load in healthy individual’s results in enhanced generation and urinary excretion [47]. This dietary finding is worth attention, given some popular trends of protein-rich dietary regimens. Under normal intake, about 6-18 g of proteins and peptides, mainly coming from diet, but also from endogenous sources, travel through the large intestine daily [61]. From our data it looked like the “prebiotic” component of regimen A played an important role in this regard and it is not a surprise that by using the same symbiotic we pioneered over 10 years ago a clinical study showing a significantly decrease ammonia-related benzodiazepine-like neurotoxicant in patients with advanced liver disease [12]. Such gut ecology modification in otherwise healthy subjects meets even more an interesting overall health strategy [9,62] after the data of Amar et al. [63] showing that some degree of endotoxemia ensues after calorie-rich meal also in healthy subjects and this may be a subtle abnormality in those cases prone to metabolic disease too [5,64].
Taken overall, our study suggest that otherwise healthy individuals leading an active life may potentially harbor weak links whose natural history has to be ascertained by longer and larger studies. Subtle gut ecology imbalance can now be tracked down also in clinical practice by clinically-handled novel new generation sequencing technology (Next Genomics, Prato, Italy) which avails itself of a kit allowing small sampling and remaining stable for 14 days at room temperature and with a detection accuracy of 0.0001% of the original sample and easily applicable report. This may help fostering interventional plan for health strategy leaning also on this fundamental metagenomic factor, as we recently suggested in a most cited paper [65]. Limitations of our study remain the lack of expression data and in vitro systems to explore the regulation and determinants of microbial enzymatic as well as a profound analysis on alpha-diversity of the microbial community observed throughout a longer period. Finally, previous short-term positive data obtained by using a generic surgeon-based fish collagen, have to be considered ephymeral while uneffective on gut microbiota and its detoxyfication function.
References
- Martin CR, Mayer EA (2017) Gut-brain axis and behavior. Nestle Nutr Inst Workshop Ser 88: 45-53.
- Liu RT (2017) The microbiome as a novel paradigm in studying stress and mental health. Am Psychol 72: 655-667.
- Shimakawa M, Makizaki Y, Tsunemine S, Tanaka Y, Ohno H, et al. (2017) Effect of probiotics on visceral pain threshold in an experimental model of irritable bowel syndrome. Int J Probiotics Prebiotics 12: 167-174.
- Watanabe Y, Arase S, Nagaoka N, Kawai M, Matsumoto S, et al. (2016) chronic psychological stress disrupted the composition of the murine colonic microbiota and accelerated a murine model of inflammatory bowel disease. PLoS One 11: e0150559.
- Nagpal R, Kumar M, Yadav AK, Hemalatha R, Yadav H, et al. (2016) Gut microbiota in health and disease: an overview focused on metabolic inflammation. Benef Microbes 7: 181-194.
- Kang X, Sun G, Ling N, Zhou Q, Zhang L, et al. (2012) Effects of orally administered milk fermented by streptococcus thermophilus mn-zlw-002 on murine cell-mediated immune responses. Int J Probiotics Prebiotics 7: 59-64.
- Habil N, Beal J, Foey AD (2012) Lactobacillus casei strain shirota selectively modulates macrophage subset cytokine production. Int J Probiotics Prebiotics 7: 1-12.
- Holzer P, Farzi A, Hassan AM, Zenz G, JaÄan A, et al. (2017) Visceral inflammation and immune activation stress the brain. Front Immunol 8: 1613.
- Nagpal R, Yadav H, Marotta F (2014) Gut microbiota: The next-gen frontier in preventive and therapeutic medicine? Front Med (Lausanne) 1: 15.
- Biasucci LM, La Rosa G, Pedicino D, D'Aiello A, Galli M, et al. (2017) Where does inflammation fit? Curr Cardiol Rep 19: 84.
- Takadanohara H, Catanzaro R, Chui de H, He F, Yadav H, et al. (2012) Beneficial effect of a symbiotic preparation with S. boulardii lysate in mild stress-induced gut hyper-permeability. Acta Biomed 83: 208-216.
- Lighthouse J, Naito Y, Helmy A, Hotten P, Fuji H, et al. (2004) Endotoxinemia and benzodiazepine-like substances in compensated cirrhotic patients: A randomized study comparing the effect of rifaximine alone and in association with a symbiotic preparation. Hepatol Res 28: 155-160.
- Westfall S, Lomis N, Kahouli I, Dia SY, Singh SP, et al. (2017) Microbiome, probiotics and neurodegenerative diseases: deciphering the gut brain axis. Cell Mol Life Sci 74: 3769-3787.
- Catanzaro R, Anzalone M, Calabrese F, Milazzo M, Capuana M, et al. (2015) The gut microbiota and its correlations with the central nervous system disorders. Panminerva Med 57: 127-143.
- Misra S, Mohanty D (2017) Psychobiotics: A new approach for treating mental illness? Crit Rev Food Sci Nutr 30: 1-7.
- Fasano A (2011) Zonulin and ist regulation of intestinal barrier function: The biological door to inflammation, autoimmunity, and cancer. Physiol Rev 91: 151-175.
- Groschowitz KR, Hogan SP (2009) Intestinal barrier function: Molecular regulation and disease pathogenesis. J Allergy Clin Immunol 124: 3-20.
- Sudo N, Chida Y, Aiba Y, Sonoda J, Oyama N, et al. (2004) Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J Physiol 558: 263-275.
- Farzi A, Fröhlich EE, Holzer P (2018) Gut microbiota and the neuroendocrine system. Neurotherapeutics 15: 5-22.
- Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, et al. (2011) Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci USA 108: 16050-16055.
- Diaz Heijtz R, Wang S, Anuar F, Qian Y, Bjorkholm B, et al. (2011) Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci USA 108: 3047-3052.
- Desbonnet L, Garrett L, Clarke G, Kiely B, Cryan JF, et al. (2010) Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression. Neuroscience 170: 1179-1188.
- Dickerson S, Kemeny M (2004) Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychol Bull 130: 355-391.
- Nakane H (1999) Salivary chromogranin A as an index of psychosomatic stress response. Bio-Med Res 34:17-22.
- Nomura S, Mizuno T, Nozawa A, Asano H, Ide H, et al. (2010) Characteristics of salivary chromogranin A as a short-term mental stress biomarker. Trans Jpn Soc Med Biol Eng 48: 207-212.
- Karhu E, Forsgård RA, Alanko L, Alfthan H, Pussinen P, et al. (2017) Exercise and gastrointestinal symptoms: running-induced changes in intestinal permeability and markers of gastrointestinal function in asymptomatic and symptomatic runners. Eur J Appl Physiol. 117: 2519-2526.
- Ohlsson B, Orho-Melander M, Nilsson PM (2017) higher levels of serum zonulin may rather be associated with increased risk of obesity and hyperlipidemia, than with gastrointestinal symptoms or disease manifestations. Int J Mol Sci 18. pii: E582.
- Plotnikoff GA (2014) Three measurable and modifiable enteric microbial biotransformations relevant to cancer prevention and treatment. Glob Adv Health Med 3: 33-43.
- Baker JM, Al-Nakkash L, Herbst-Kralovetz MM (2017) Estrogen-gut microbiome axis: Physiological and clinical implications. Maturitas 103: 45-53.
- Pattananandecha T, Sirilun S, Duangjitcharoen Y, Sivamaruthi BS, Suwannalert P, et al. (2016) Hydrolysed inulin alleviates the azoxymethane-induced preneoplastic aberrant crypt foci by altering selected intestinal microbiota in Sprague-Dawley rats. Pharm Biol 54: 1596-1605.
- Graston SM, Juntunen KS, Poutanen KS, Gylling HK, Miettinen TA, et al. (2000) Rye bread improves bowel function and decreases the concentrations of some compounds that are putative colon cancer risk markers in middle-aged women and men. J Nutr 130: 2215-2221.
- Almanza-Aguilera E, Urpi-Sarda M, Llorach R, Vázquez-Fresno R, Garcia-Aloy M, et al. (2017) Microbial metabolites are associated with a high adherence to a Mediterranean dietary pattern using a 1H-NMR-based untargeted metabolomics approach. J Nutr Biochem 4 8: 36-43.
- Poesen R, Viaene L, Verbeke K, Augustijns P, Bammens B, et al. (2014) Cardiovascular disease relates to intestinal uptake of p-cresol in patients with chronic kidney disease. BMC Nephrol 15: 87.
- Andriamihaja M, Lan A, Beaumont M, Audebert M, Wong X, et al. (2015) The deleterious metabolic and genotoxic effects of the bacterial metabolite p-cresol on colonic epithelial cells. Free Radic Biol Med 85: 219-227.
- Lamiki P, Tsuchiya J, Pathak S, Okura R, Solimene U, et al. (2010) Probiotics in diverticular disease of the colon: An open label study. J Gastrointestin Liver Dis 19: 31-36.
- Metugriachuk Y, Marotta F, Pavasuthipaisit K, Kuroi O, Tsuchiya J, et al. (2006) The aging gut motility decay: May symbiotics be acting as "implantable" biologic pace-makers? Rejuvenation Res 9: 342-345.
- Tsuchiya J, Barreto R, Okura R, Kawakita S, Fesce E, et al. (2004) Single-blind follow-up study on the effectiveness of a symbiotic preparation in irritable bowel syndrome. Chin J Dig Dis 5: 169-174.
- Marotta F, Naito Y, Minelli E, Tajiri H, Bertuccelli J, et al. (2003) Chemopreventive effect of a probiotic preparation on the development of preneoplastic and neoplastic colonic lesions: An experimental study. Hepatogastroenterology 50: 1914-1918.
- Maslach C, Jackson SE, Leiter MP (1996) Maslach Burnout Inventory (3rd edn) Consulting Psychologists Press, Palo Alto, CA.
- Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH (1999) Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosom Med 61: 154-162.
- Toda M, Ichikawa H (2012) Effect of laughter on salivary flow rates and levels of chromogranin A in young adults and elderly people. Environ Health Prev Med 17: 494-499.
- Chui DH, Marcellino M, Marotta F, Sweed H, Solimene U, et al. (2014) A double-blind, rct testing beneficial modulation of BDNF in middle-aged, life style-stressed subjects: A clue to brain protection? J Clin Diagn Res 8: MC01-MC06.
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA (1983) Manual for the State-Trait Anxiety Inventory. Consulting Psychologists Press, Palo Alto, CA.
- Dressendorfer RA, Kirschbaum C, Rohde W (1992) Synthesis of a cortisol-biotin conjugate and evaluation as a tracer in an immunoassay for salivary cortisol measurement. J Steroid Biochem Mol Biol 43: 683-692.
- Goldin R, Gorbach SL (1976) The relationship between diet and rat fecal bacterial enzymes implicated in colon cancer. J Nat Cancer Inst 57: 371-375.
- Blachier F, Mariotti F, Huneau JF, Tome D (2007) Effects of amino acid-derived luminal metabolites on the colonic epithelium and physiopathological consequences. Amino Acids 33: 547-562.
- Geypens B, Claus D, Evenepoel P (1997) Influence of dietary protein supplements on the formation of bacterial metabolites in the colon. Gut 41: 70-76.
- Biagi E, Nylund L, Candela M, Ostan R, Bucci L, et al. (2010) Through ageing, and beyond: Gut microbiota and inflammatory status in seniors and centenarians. PLoS One 5: 1-14.
- Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R (2012) Diversity, stability and resilience of the human gut microbiota. Nature 489: 220-230.
- Nakane H, Asami O, Yamada Y (1998) Salivary chromgranin A as an index of psychosomatic stress response. Biomed Res 19: 401-406.
- Takatsuji K, Sugimoto Y, Ishizaki S (2008) The effects of examination stress on salivary cortisol, immunoglobulin A, and chromogranin A in nursing students. Biomed Res 29: 221-224.
- Lee T, Shimizu T, Iijima M (2006) Evaluation of psychosomatic stress in children by measuring salivary chromogranin A. Acta Paediatr 95: 935-939.
- Allen AP, Dinan TG, Clarke G, Cryan JF (2017) A psychology of the human brain–microbiome axis. Soc Personal Psychol Compass 11: e12309
- Neufeld KA, Kang N, Bienenstock J, Foster JA (2011) Effects of intestinal microbiota on anxiety-like behavior. Commun Integr Biol 4: 492-494.
- Bailey MT (2016) Psychological stress, immunity, and the effects on indigenous microflora. Adv Exp Med Biol 874: 225-246.
- Bharwani A, Mian MF, Foster JA, Surette MG, Bienenstock J, et al. (2016) Structural & functional consequences of chronic psychosocial stress on the microbiome & host. Psychoneuroendocrinol 63: 217-227.
- Ait-Belgnaoui A, Durand H, Cartier C, Chaumaz G, Eutamene H, et al. (2012) Prevention of gut leakiness by a probiotic treatment leads to attenuated HPA response to an acute psychological stress in rats. Psychoneuroendocrinol 37: 1885-1895.
- Ait-Belgnaoui A, Ahrne S, Hagslatt ML (2011) Effect of lactobacilli on paracellular permeability in the gut. Nutrients 3: 104-117.
- Hida M, Aiba Y, Sawamura S, Suzuki N, Satoh T, et al. (1996) Inhibition of the accumulation of uremic toxins in the blood and their precursors in the feces after oral administration of Lebenin®, a lactic acid bacteria preparation, to uremic patients undergoing hemodialysis. Nephron 74: 349-355.
- Selmer T, Andrei PI (2001) p-Hydroxyphenylacetate decarboxylase from Clostridium difficile: A novel glycyl radical enzyme catalyzing the formation of p-cresol. Eur J Biochem 268: 1363-1372.
- Yao CK, Muir JG, Gibson PR (2016) Review article: Insights into colonic protein fermentation, its modulation and potential health implications. Aliment. Pharmacol Ther 43: 181-196.
- Naito Y, Marotta F, Kantah MK, Zerbinati N, Kushugulova A, et al. (2014) Gut-targeted immunonutrition boosting natural killer cell activity using Saccharomyces boulardii lysates in immuno-compromised healthy elderly subjects. Rejuvenation Res 17: 184-187.
- Amar J, Burcelin R, Ruidavets JB, Cani PD, Fauvel J, et al. (2008) Energy intake is associated with endotoxemia in apparently healthy men. Am J Clin Nutr 87: 1219-1223.
- Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, et al. (2007) Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 56: 1761-1772.
- Vaiserman AM, Koliada AK, Marotta F (2017) Gut microbiota: A player in aging and a target for anti-aging intervention. Ageing Res Rev 35: 36-45.
Citation: Kantah MK, Catanzaro R, Kumar M, Jeong WS, Marcellino M, et al. (2017) Beneficial Gut Effect of a Symbiotic-Probiotic Regimen in Healthy Stressed Individuals: Effectiveness on Permeability, Microbiota and Detoxification Parameters. J Gastroint Dig Syst 8: 560. DOI: 10.4172/2161-069X.1000560
Copyright: © 2017 Kantah MK, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 6783
- [From(publication date): 0-2018 - Dec 08, 2025]
- Breakdown by view type
- HTML page views: 5798
- PDF downloads: 985