Bevacizumab (Bmab)+Dihydropyrimidine Dehydrogenase (DPD) Inhibitory Fluoropyrimidine (DIF) Combined Chemotherapy for the Treatment of Late-Stage Elderly Patients with Advanced/Recurent Colorectal Cancer (ARCC)
Received: 18-Dec-2017 / Accepted Date: 25-Jan-2018 / Published Date: 02-Feb-2018
Abstract
Purpose: In Japan, elderly people account for more than 20% of the total population. The incidence of elderly patients with advanced/recurrent colorectal cancer (ARCC) has also been increasing. However, the optimal regimen for elderly patients, especially those older than 75 years of age (late-stage elderly), has not been established. This study aims to examine the optimal chemotherapeutic regimens for late-stage elderly patients, and focuses on combined chemotherapy with Bevacizumab (Bmab)+dihydropyrimidine (DPD) dehydrogenase inhibitory fluoropyrimidine (DIF).
Methods: Between January 1996 and October 2014, 30 late-stage elderly chemotherapy-naïve patients with ARCC (male/female=16/14; average age, 79.1 years) were retrospectively reviewed. The treatment regimens were: Bmab+DIF (n=11) and other regimens (n=19).
Results: The MST was 979 days, the median PFS was 350 days and the RR was 23.3%. The grade ≥3 AEs with each of the regimens were as follows: Bmab+DIF, 1.9%, other regimens, 14.4%. Although no significant differences were observed in the OS or PFS between Bmab+DIF and the other regimens, the rate of transition to a 2nd-line chemotherapy after disease progression following first-line treatment was higher with Bmab+DIF (54.6% [6/11]) than with the other regimens (38.9% [7/18]); however, this difference did not reach statistical significance.
Conclusions: It is possible to prolong survival through chemotherapy in both late-stage elderly patients and younger patients with ARCC. Although the only appropriate specific regimen was not confirmed, given that it was associated with a 100% disease control rate, good feasibility and smooth transition to 2nd-line chemotherapy, Bmab+DIF was suggested to be a candidate treatment for late-stage elderly patients with ARCC.
Keywords: Elderly patient; Colorectal cancer; Bevacizumab; DIF
Introduction
The United Nations and World Health organization define individuals of 65-75 years of age as early-stage elderly; individuals of 75-89 years of age as late stage elderly; and individuals older than 90 years of age as hyper-stage elderly. The ratio of elderly individuals in a society is used to define its level of aging. An aging society is defined by a ratio of 7-14%, an aged society is defined by a ratio of 14-21%, and a hyper-aged society is defined by a ratio of ≥21%. According to these definitions, Japan, which had a ratio of 24.2% in 2012, is defined as a hyper-aged society. At present, the elderly population is growing. With the progressive aging of the society, the incidence of colorectal cancer in elderly patients and the associated mortality rate are increasing [1]. Since the ASCO2012 presentation of the AVEX trial, Bevacizumab (Bmab)+capecitabine has been widely used as a first-line treatment for elderly patients with advanced and recurrent colorectal cancer (ARCC) [2]. It has also been a standard chemotherapy regimen for patients who are not eligible for intensive treatment according to the 2014 guidelines for the treatment of colorectal cancer [3]. However, it is associated with a high incidence of hand-foot syndrome (>50%) and a lower quality of life (QOL) in patients with grade 3/4 colorectal cancer [2]. In Japan, UZEL/UFT (teagfur/urasil) or S-1 have been widely used for ARCC. These agents are dihydropyrimidine dehydrogenase (DPD) inhibitory fluoropyrimidines (DIFs). They consist of tegafur, which is a prodrug of 5-FU, and a DPD inhibitor which inhibits DPD activity. They maintain a high blood concentration of 5-FU and are associated with a lower incidence of digestive tract toxicities and reduced hand-foot syndrome [4,5].
We herein report the results of the treatment of elderly patients with ARCC, with a focus on Bmab+DIF combination chemotherapy.
Patients and Methods
The present study included late-stage elderly chemotherapy-naïve patients with ARCC who were treated between January 1996 and October 2014 and who received at least one course of chemotherapy. The patients’ medical records were retrospectively reviewed to compare the results of chemotherapy with Bmab+DIF and other regimens. The response evaluation criteria for solid tumors (RESIST) and the National Cancer Institute-Common Toxicity Criteria (NCI-CTC) ver.4.0 were used to evaluate the chemotherapy regimens. The median survival time (MST) and median progression free survival (mPFS) were calculated by the Kaplan-Meier method. Other factors were evaluated by Fisher’s exact test. The Stat View J 5.0 software package (Abacus Concepts, Stat View. Abacus Concepts, Inc., Berkeley, CA) was used to perform the statistical analyses. P values of <0.05 were considered to indicate a statistically significant difference.
Results
Patient characteristics
Thirty patients (male, n=16; female, n=14) satisfied the eligibility criteria. Eleven and 19 patients received Bmab+DIF and other regimens, respectively. The detailed regimens are shown in Table 1a. Six patients received S-1 and 5 patients received UZEL/UFT (L-leucovorin/ tegafur・urasil) in the course of Bmab+DIF treatment. The characteristics of the patients are shown in Table 1b. The median age of the Bmab+DIF-treated patients was older than that of the patients who received other regimens. Two patients with PS2 were treated with other regimens; no patients with PS2 received Bmab+DIF. However, there were no statistically significant differences among any of the factors.
| Bmab+DIF (11) | Other regimens (19) |
|---|---|
| Bevacizumab: 11 +S-1: 6 +UZEL/UFT: 5 |
L-OHP base: 11 FOLFOX alone: 3 Bmab+FOLFOX: 7 Pmab+FOLFOX: 1 CPT-11 base: 3 FOLFIRI alone: 1 Bmab+FOLFIRI: 1 SU+FOLFIRI: 1 5-FU base: 5 5-FU/LV (RPMI): 1 UZEL/UFT: 1 S-1: 2 capesitabine: 1 |
DIF: Dihydropyrimidine (DPD) Dehydrogenase Inhibitory Fluoropyrimidine (DIF).
UZEL/UFT: Leucovorin/Tegafur・Urasil, Bmab: Bevacizumab, Pmab: Panitumumab; SU: Sunitinib.
Table 1a: Received Regimens of Bevacizumab+DIF group and Other regimens group.
| Bmab+DIF (11) | Other regimens (19) | |
|---|---|---|
| sex | Male: 6, Female: 5 | Male: 10, Female: 9 (N.S.) |
| age (median) | 81 y.o. (75-84) | 77 y.o. (75-85) (N.S.) |
| ECOG-PS | PS 0/1/2=6/5/0 | PS 0/1/2=10/7/2 (N.S.) |
| Disease status | Unrsecatble: 6 (55%) p.o.recurrence: 5 (45%) |
Unrsecatble: 11 (58%) p.o.recurrence: 8 (42%) |
| Recurrence site | Liver: 7 | liver: 5 |
| Lung: 0 | lung: 4 | |
| LN: 3 | LN: 7 | |
| PD: 1 | pd: 6 | |
| LR: 1 | LR: 1 | |
| Bone: 1 (Some cases overlapped) | Bone: 1 (Some cases overlapped) | |
| Multiple: 2 (18%) | Multiple: 4 (17.6%) |
p.o. Recurrence: Post Operative Recurrence, LN: Lymphnode.
PD: Peritoneal Dissemination, LR: Local Recurrence.
Table 1b: Patients characteristics of bevacizumab+DIF group and other regimens.
Response and time-to-event measures
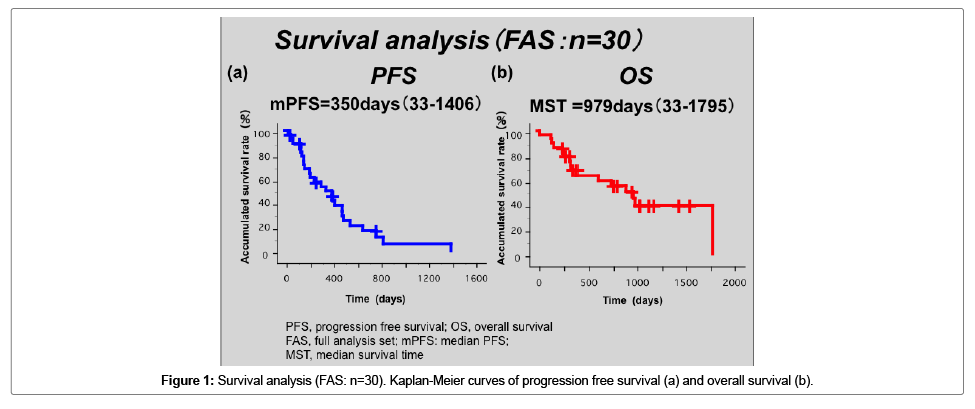
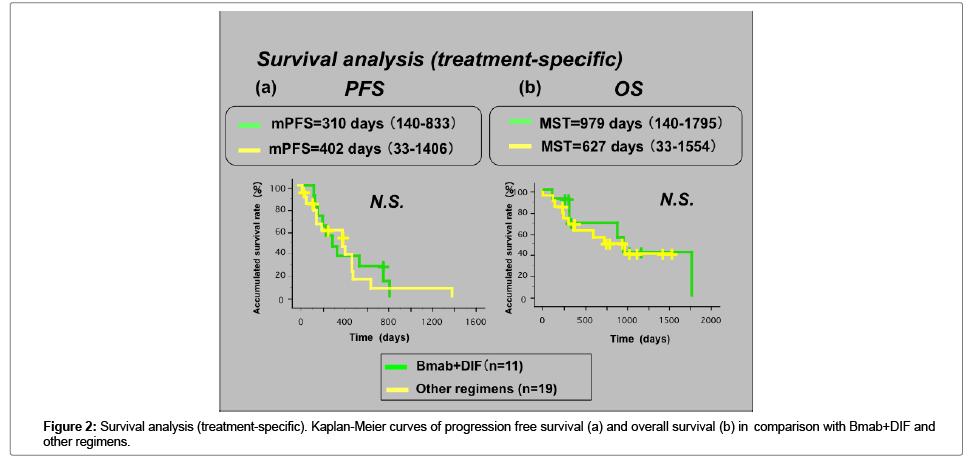
The median follow-up period was 2,635 days (range: 459-3622). The median number of treatment courses was 6 with Bmab+DIF, and 7 with other regimens. The response rate (RR) with Bmab+DIF was 18.2% (CR, n=0; PR, n=2; NC, n=9; PD, n=0), while that for other regimens was 23.5% (CR, n=0; PR, n=5; NC, n=11; PD, n=2; NE, n=1). The disease control rate (DCR) was 100% with Bmab+DIF, and 84.2% with other regimens (Table 1c). There was no significant difference in terms of the antitumor effects or the DCR in the two groups. In the Full set analysis (FAS) group the median PFS was 350 days, and the MST was 976 days (Figure 1a and 1b) shows the treatment-specific PFS and OS. The mPFS with Bmab+DIF was 310 days, while that with other regimens was 402 days (Figure 2a). The MST with Bmab+DIF was 979 days, and that with other regimens was 627 days (Figure 2b). The difference was not statistically significant.
| Bmab+DIF (11) | Other regimens (19) | |
|---|---|---|
| Median follow-up period | 2635 days (459-3622) | |
| Number of received courses (median) | 6 (3-25) | 7 (2-20) (N.S.) |
| Anti-tumor effect (RR) | CR/PR/SD/PD=0/2/9/0 18.2% |
CR/PR/SD/PD/NE=0/5/11/2/1 23.5% (N.S.) |
| DCR | CR/PR/SD/PD=0/2/9/0 100% |
CR/PR/SD/PD/NE=0/5/11/2/1 84.2% (N.S.) |
RR: Response Rate, DCR: Disease Control Rate, CR: Complete Response.
PR: Partial Response, SD: Stable Disease, PD: Progressive Disease NE: Not Evaluable.
Table 1c: Results of anti-tumor effects of Bevacizumab+DIF group and other regimens.
Adverse events
As shown in Table 2a, the overall incidence of hematological toxicities was higher in other regimens than in Bmab+DIF. The opposite was observed with regard to non-hematological toxicities. The specific adverse events are shown in Table 2b. The incidence of digestive tractassociated adverse events in Bmab+DIF was remarkable; however, the severity of all of the adverse events was lower than grade 3. Conjunctivitis and lacrimation (which were associated with a lower QOL) in patients who received Bmab+DIF were exclusively observed with S-1. Two deaths occurred (cerebral infarction (CT), n=1; unknown etiology, n=1) in patients who received Bmab+DIF, and 2 deaths occurred (CT, n=1; unknown etiology, n=1) in patients who received other regimens.
| (a) | Hematological Toxicity | Non-hematological Toxicity | ||||||
| Bmab+DIF | Other regimens | Bmab+DIF | Other regimens | |||||
| Total | 13.5% | 30.5% | 21.4% | 11.8% | ||||
| Grade ≦2 | 13.5% | 17.3% | 19.9% | 10.6% | ||||
| Grade ≧3 | 0% | 13.3% | 1.9% | 1.1% | ||||
| (b) Hematological Toxicity | Bmab+DIF | Other regimens | ||||||
| Gr (1+2) | Gr (3+4) | Gr 5 | Total | Gr (1+2) | Gr (3+4) | Gr 5 | Total | |
| Leukopenia | 2 (18%) | 0 (0%) | 0 (0%) | 2 (18%) | 2 (11%) | 4 (22%) | 0 (0%) | 6 (32%) |
| Neutropenia | 2 (18%) | 0 (0%) | 0 (0%) | 2 (18%) | 2 (11%) | 4 (22%) | 0 (0%) | 6 (32%) |
| Anemia | 1 (9%) | 0 (0%) | 0 (0%) | 1 (9%) | 4 (26%) | 2 (11%) | 0 (0%) | 6 (32%) |
| Thrombocytopenia | 1 (9%) | 0 (0%) | 0 (0%) | 1 (9%) | 5 (29%) | 0 (0%) | 0 (0%) | 5 (26%) |
| Non-hematolog-ical Toxicity | Bmab+DIF | Other regimens | ||||||
| Gr (1+2) | Gr (3+4) | Gr 5 | Total | Gr (1+2) | Gr (3+4) | Gr 5 | Total | |
| N and V | 2 (18%) | 0 (0%) | 0 (0%) | 2 (18%) | 6 (32%) | 0 (0%) | 0 (0%) | 6 (32%) |
| Diarrhea | 1 (9%) | 0 (0%) | 0 (0%) | 1 (9%) | 3 (16%) | 0 (0%) | 0 (0%) | 3 (16%) |
| Appetite loss | 3 (27%) | 0 (0%) | 0 (0%) | 3 (27%) | 4 (21%) | 0 (0%) | 0 (0%) | 4 (21%) |
| Oral mucositis | 5 (45%) | 0 (0%) | 0 (0%) | 5 (45%) | 2 (11%) | 0 (0%) | 0 (0%) | 2 (11%) |
| TD | 3 (27%) | 0 (0%) | 0 (0%) | 3 (27%) | 2 (11%) | 0 (0%) | 0 (0%) | 2 (11%) |
| General fatigue | 2 (18%) | 0 (0%) | 0 (0%) | 2 (18%) | 2 (11%) | 1 (5%) | 0 (0%) | 3 (16%) |
| PNJ | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 4 (21%) | 1 (5%) | 0 (0%) | 5 (26%) |
| Protein urea | 8 (73%) | 0 (0%) | 0 (0%) | 8 (73%) | 5 (26%) | 1 (5%) | 0 (0%) | 6 (32%) |
| Pigmentation | 4 (36%) | 0 (0%) | 0 (0%) | 4 (36%) | 2 (11%) | 0 (0%) | 0 (0%) | 2 (11%) |
| Skin rash | 4 (36%) | 0 (0%) | 0 (0%) | 4 (36%) | 1 (5%) | 0 (0%) | 0 (0%) | 1 (5%) |
| Alopesia | 2 (18%) | - | - | 2 (18%) | 1 (5%) | - | - | 1 (5%) |
| H and F | 1 (9%) | 0 (0%) | 0 (0%) | 1 (9%) | 1 (5%) | 0 (0%) | 0 (0%) | 1 (5%) |
| Nasal bleeding | 2 (18%) | 0 (0%) | 0 (0%) | 2 (18%) | 1 (5%) | 0 (0%) | 0 (0%) | 1 (5%) |
| Lacrimation | 2 (18%) | 0 (0%) | 0 (0%) | 2 (18%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Conjunctivitis | 3 (27%) | 0 (0%) | 0 (0%) | 3 (27%) | 2 (11%) | 0 (0%) | 0 (0%) | 2 (11%) |
| Sudden death | 0 (0%) | 0 (0%) | 1 (9%) | 1 (9%) | 0 (0%) | 0 (0%) | 1 (5%) | 1 (5%) |
| Perforation | 0 (0%) | 1 (9%) | 0 (0%) | 1 (9%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Bradycardia | 0 (0%) | 1 (0%) | 0 (0%) | 1 (9%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| CT | 0 (0%) | 0 (0%) | 1 (9%) | 1 (9%) | 0 (0%) | 1 (5%) | 1 (5%) | 2 (11%) |
 :incidence ≧20%,
:incidence ≧20%,  :grade ≧3,
:grade ≧3,
N and V: Nausea/Vomiting; TD: Taste Disturbance; PNI: Peripheral Nerve Injury; H and F: Hand and Foot Syndrome; CT: Cerebral Thrombosis.
Table 2: (a and b) Adverse events of bevacizumab+DIF group and other regimens.
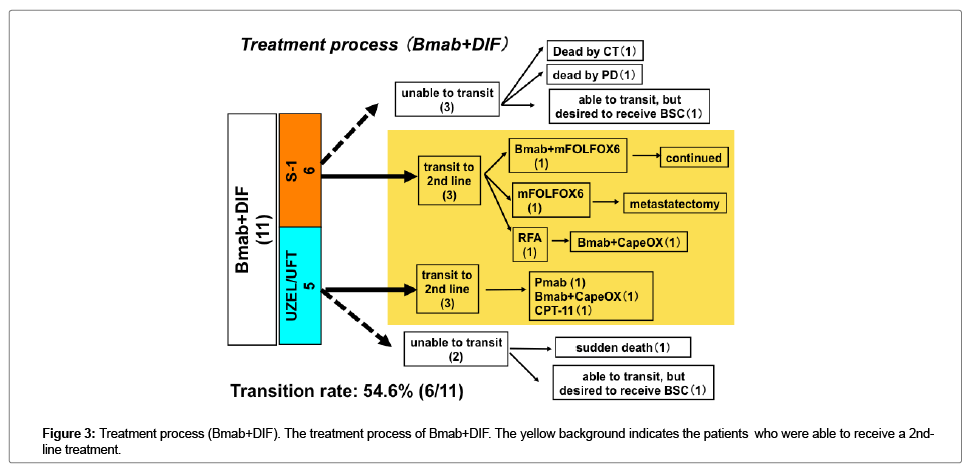
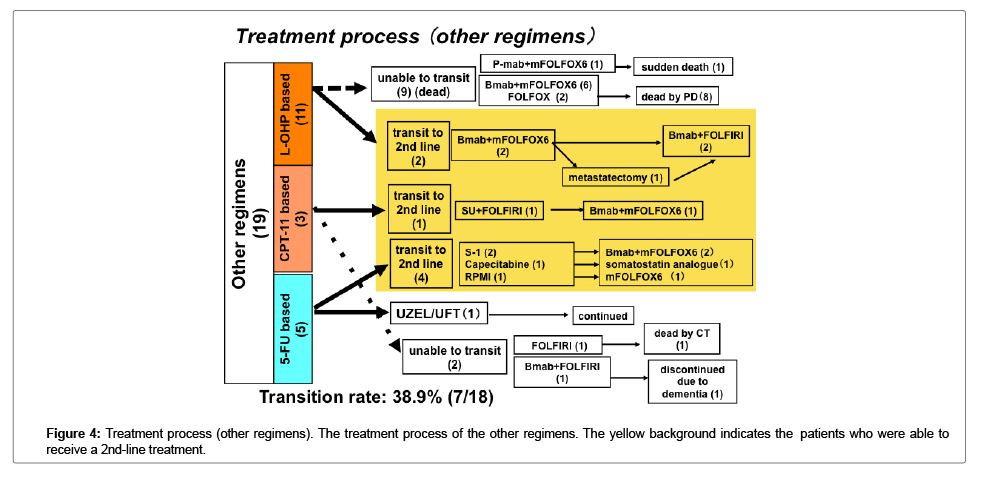
Transition to a 2nd-line treatment
The transition rate to 2nd line treatments was higher in the patients who received Bmab+DIF than in the patients who received other regimens; however, the difference was not statistically significant (Figures 3 and 4).
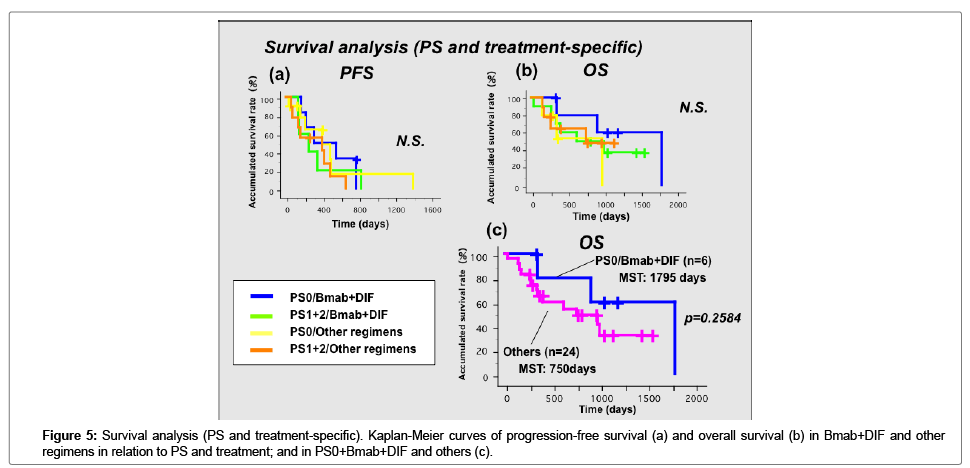
Survival analysis (PS and treatment-specific)
We examined the associations between survival and PS and treatment (Figure 5). As shown in Figure 5a, PFS was not associated with either PS or treatment. However, the OS in patients with PS0 who received Bmab+DIF seemed to be better than that of patients with a higher PS and in the patients who were treated with other regimens (Figure 5b). We created a Kaplan-Meier curve divided by PS0 and Bmab+DIF and included the other PS statuses and treatments. As shown in Figure 5c the OS tended to be longer in the patients with PS0 who received Bmab+DIF than in the other groups; however, the difference was not statistically significant.
Discussion
In the present study, we achieved an MST of 979 days and an mPFS of 350 days. This showed that chemotherapy benefits the survival of late-stage-elderly patients with ARCC. The RR was higher with other regimens than with Bmab+DIF. In addition, metastatectomy and radio frequency ablation (RFA) therapy were achieved in two patients with liver metastasis who received other regimens. Thus, when aiming for conversion therapy, intensive chemotherapy including L-OHP, CPT- 11, and a molecular targeted agents might be appropriate. With regard to the lack of a significant difference in the PFS and OS of patients who received Bmab+DIF and those who received other regimens, it was concluded that Bmab+DIF was not the only optimal regimen for late-stage-elderly patients with ARCC. However, according to the high DCR of 100%, and the high transition rate to 2nd line treatment, Bmab+DIF might be useful as a treatment of choice for late-stageelderly patients. Based on the results of the present study, Bmab+DIF might be the first choice for patients with PS0, if the treatment strategy does not include the provision of conversion therapy. Bmab+DIF might also have a role as a prelude to intensive chemotherapy in view of the high transition rate. Bmab+DIF treatment was associated with good compliance and the adverse effects were either tolerable or controllable. The usefulness of Bmab+S-1 in patients older than 65 years of age and of Bmab+UZEL/UFT in patients older than 75 years of age was shown in the BASIC [6] and J-BLUE [7] trials, respectively. The addition of L-OHP to 5-FU was reported to achieve no survival benefit in elderly patients with stage III cancer after curative resection [8]. However, age alone did not contraindicate intensive chemotherapy such as Bmab+FOLFOX/FOLFIRI in elderly patients with ARCC [9]. Combination chemotherapies were reported to achieve a longer PFS in elderly patients; however, such therapies tended to be associated with worse toxicities, especially with the addition of CPT-11 [ 10]. A doublet chemotherapy including Bmab was also found to be tolerable by elderly patients; however, such patients should be carefully monitored to detect the development of arterial or venous thrombosis [11]. In the present study, various combination chemotherapies were administered and survival was extended in some patients.
The uniqueness of this study is aimed to be an integrated analysis of Bmab+DIF, in other words, combined results of BASIC [6] and J-BLUE study [7] by limiting the late-stage elderly patients with ARCC. The limitation of this study is focused on only age without geriatric assessments. We will intend the next study focusing on not only age but also frailty by geriatric assessment tools.
Conclusions
Although we could not confirm a single regimen that was appropriate for the treatment of late-stage elderly patients with ARCC, Bmab+DIF was suggested to be a candidate treatment of choice due to the 100% disease control rate, good feasibility and smooth transition to 2nd-line chemotherapy.
Acknowledgements
Any substantial contribution when provided by a person different from the author and list all other persons who do not fulfill authorship criteria. We thank for the assistance by the medical writing expert, Brian Quinn in the Japan Medical Communication. This study has been accomplished by only all the authors contributions. The authors declare that they have no conflict of interest. The findings of this study was ordinary clinical practice covered by national medical insurance, thus we do not receive any funds nor any financial support.
References
- Cancer Statistics in Japan 2014 Edited by The Editorial Board of the Cancer Statistics in Japan Date of publication: March, 2015 Foundation for Promotion of Cancer Research (FPCR) c/o National Cancer Center.
- Cunningham D, Lang I, Marcuello E, Lorusso V, Ocvirk J, et al. (2013) Bevacizumab plus capecitabine versus capecitabine alone in elderly patients with previously untreated metastatic colorectal cancer (AVEX): an open-label, randomized phase 3 trial. Lancet Oncol 14: 1077-1085.
- Watanabe T, Itabashi M, Shimada Y, Tanaka S, Ito Y, et al. (2015) Japanese Society for Cancer of the Colon and Rectum (JSCCR) Guidelines 2014 for treatment of colorectal cancer. Int J Clin Oncol 20: 207-239.
- Shirasaka T (2009) Development History and Concept of an Oral Anticancer Agent S-1 (TS-1®): Its Clinical Usefulness and Future Vistas. Jpn J Clin Oncol 39: 2-15.
- Douillard JY, Hoff PM, Skillings JR, Eisenberg P, Davidson N, et al. (2002) Multicenter Phase III Study of Uracil/Tegafur and Oral Leucovorin Versus Fluorouracil and Leucovorin in Patients with Previously Untreated Metastatic Colorectal Cancer. J Clin Oncol 20: 3605-3616.
- Yoshida M, Muro K, Tsuji A, Hamamoto Y, Yoshino T, et al. (2015) Combination chemotherapy with bevacizumab and S-1 for elderly patients with metastatic colorectal cancer (BASIC trial). Eur J Cancer 51: 935-941.
- Nishina T, Moriwaki T, Shimada M, Higashijima J, Sakai Y, et al. (2015) Uracil-Tegafur and Oral Leucovorin Combined With Bevacizumab in Elderly Patients (Aged ≧ 75 Years) With Metastatic Colorectal Cancer:A Multicenter, Phase II Trial (Joint Study of Bevacizumab, Oral Leucovorin, and Uracil-Tegafur in Elderly Patients [J-BLUE] Study) Clinical Colorectal Cancer 15: 236-242.
- Kim CAK, Spratlin JL, Armstrong DE, Ghosh S, Mulder KE (2014) Efficacy and Safety of Single Agent or Combination Adjuvant Chemotherapy in Elderly Patients With Colon Cancer: A Canadian Cancer Institute Experience. Clin Colorectal Cancer 13: 199-206.
- Papamichae D, Audisio RA, Glimelius B, de Gramont A, Glynne-Jones R, et al. (2015) Treatment of colorectal cancer in older patients: International Society of Geriatric Oncology (SIOG) consensus recommendations 2013. Ann Oncol 26: 463-476.
- Landre T, Uzzan B, Nicolas P, Aparicio T, Zelek L, et al. (2015) Doublet chemotherapy vs. single-agent therapy with 5FU in elderly patients with metastatic colorectal cancer. a meta-analysis. Int J Colorectal Dis 30: 1305-1310.
- Bosse D, Vickers M, Lemay F, Beaudoin A (2015) Palliative chemotherapy for patients 70 years of age and older with metastatic colorectal cancer: a single-centre experience. Curr Oncol 22: e349-e356
Citation: Kobayashi K, Fujita F, Inoue Y, Sakimura C, Kuba S, et al. (2018) Bevacizumab (Bmab)+Dihydropyrimidine Dehydrogenase (DPD) Inhibitory Fluoropyrimidine (DIF) Combined Chemotherapy for the Treatment of Late-Stage Elderly Patients with Advanced/Recurent Colorectal Cancer (ARCC). J Oncol Res Treat 3: 119.
Copyright: © 2018 Kobayashi K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Usage
- Total views: 4398
- [From(publication date): 0-2018 - Jul 15, 2025]
- Breakdown by view type
- HTML page views: 3515
- PDF downloads: 883