Bicyclic Eremophilane-type Petasite Sesquiterpenes Potentiate Peroxisome Proliferator-activated Receptor γ Activator-mediated Inhibition of Dendritic Cells
Received: 21-Sep-2017 / Accepted Date: 20-Nov-2017 / Published Date: 27-Nov-2017
Abstract
Background: Dendritic cell (DC) activation induces expression of co-stimulatory surface molecules, as well as migration into secondary lymphoid organs, where they activate naïve T cells. A family of plant derivatives, eremophilanetype petasite sesquiterpenes can regulate the immune system through DC targeting due to their anti-inflammatory effects. Peroxisome proliferator activated receptor gamma (PPARγ) is involved in inhibition of inflammatory responses and induction of DCs to acquire a mucosal phenotype.
Objective: Since mucosal DCs are central in innate immune responses, we hypothesized that eremophilane-type petasite sesquiterpenes exerted their anti-inflammatory effects by inhibiting DC maturation and activation, through PPARγ.
Methods: This study assessed the bicyclic eremophilane-type petasite sesquiterpene compounds Fukinone and 10βH-8α,12-Epidioxyeremophil-7(11)-en-8β-ol (ZYFDC21 and ZYFDC22), in the maturation and activation of mouse dendritic cells, measured by surface expression of co-stimulatory molecules and cytokine production upon LPS stimulation, in the presence or absence of PPARγ agonists. DCs were generated from mouse bone marrow cells in media supplemented with GM-CSF+IL-4, and were harvested on day 8 and activated for 3 h with bicyclic eremophilanetype petasite sesquiterpenes ZYFDC21 or ZYFDC22 in presence or absence of synthetic PPARγ agonists (GW1929, TGZ), or the natural PPARγ ligand 15d-PGJ2, followed by overnight activation with LPS.
Results: Effects on DC maturation were evaluated by surface expression of the co-stimulatory molecule CD86 by flow cytometry, and for DC activation, by relevant cytokines released in cell-free supernatants measured by ELISAs. We observed differences in the upregulation of surface expression of CD86, along with release levels of TNF, IL-6 and IL-12p70 in DCs stimulated with LPS when using combinations of bicyclic eremophilane-type petasite sesquiterpenes ZYFDC21 or ZYFDC22, and PPARγ agonists, in particular the PPARγ ligand 15d-PGJ2.
Conclusion: These results indicate that bicyclic eremophilane-type petasite sesquiterpenes Fukinone and 10βH- 8α,12-Epidioxyeremophil-7(11)-en-8β-ol inhibit maturation and activation of DC, and this activity is augmented upon PPARγ activation.
Keywords: Inflammation; Plant derivatives; Transcription factor; Peroxisome proliferator activated receptor gamma
Abbreviations
BmDC: Bone-marrow derived Dendritic Cell; DC: Dendritic Cell; ELISA: Enzyme Immunosorbent Assay; LPS: Lipopolysaccharide; MFI: Median Fluorescence Intensity; TGZ: Troglitazone; 15d-PGJ2: 15d-PGJ2-15deoxy-Δ12,14-Prostaglandin J2- PGJ2.
Introduction
Sesquiterpenes have been known to have anti-inflammatory activity in a variety of settings, showing inhibitory effects on nitric oxide production in lipopolysaccaride (LPS)-activated mouse macrophages [1-8]. Some sesquiterpenes inhibit inflammation by targeting dendritic cell (DC) maturation and activation. For example, a sesquiterpene glycoside isolated from Kandelia candel inhibited pro-inflammatory cytokine production from lipopolysaccharide (LPS)-stimulated bone marrow-derived DCs [9], and micheliolide, a sesquiterpene lactone, inhibits the production of IL-6 and TNF from LPS-stimulated primary DCs [10]. While some examples of the anti-inflammatory effects of sesquiterpene on DCs have been demonstrated, the molecular targets of specific sesquiterpenes and their interactions with endogenous inflammatory signaling pathways are unknown.
One possible target of sesquiterpenes in many inflammatory cells is the peroxisome proliferator-activated receptor (PPAR) pathway, which plays an important role in several cellular functions, including maturation and differentiation. Three PPAR subtypes have been identified, alpha, delta and gamma, and are ligand activated nuclear receptors which can be activated by polyunsaturated fatty acids, eicosanoids and various synthetic ligands. PPAR gamma (PPARγ) is primarily expressed in adipose tissue and, to a lesser extent, in the colon, immune system and the retina. PPARγ was first identified as a regulator of adipogenesis, but also plays an important role in cellular differentiation, insulin sensitization, atherosclerosis and cancer. Several sesquiterpenes or terpenoid-like compounds have been shown to either directly activate PPARγ or to modify its response to other ligands. For example, odoratin, an undecanortriterpenoid from Chromolaena odorata, moderately activates PPARγ [11]; tirotundin and tagitinin A, both sesquiterpene lactones, transactivate PPARγ-dependent promoters, including PPRE (PPARγ response element), SHP, and ABCA1 gene promoters in dose-dependent manner, and artemisinic acid, the quintessential sesquiterpene, reduces expression of PPARγ in human adipose tissue-derived mesenchimal stem cells [12]. Altogether, these data suggest that sesquiterpenes may similarly influence DC function through the PPARγ pathway. Recently, our group isolated two novel eremophilane-type sesquiterpene compounds from Petasites and characterized their inhibitory effects on the degranulation of antigenactivated mast cells (manuscript in preparation). We hypothesized that these novel sesquiterpenes would inhibit DC maturation and activation, and that this activity would be augmented in the presence of a PPARγ agonist. In this study, we demonstrate, for the first time, that the novel bicyclic eremophilane-type petasite isolated sesquiterpenes have the ability to efficiently inhibit dendritic cell maturation and activation, and this inhibition is potentiated by the synthetic, as well as naturally occurring, nuclear peroxisome proliferator-activated receptor γ agonists.
Materials and Methods
Plant material
Bicyclic sesquiterpenes Fukinone (ZYFDC21), and 10βH-8α,12- Epidioxyeremophil-7(11)-en-8β-ol (ZYFDC22) were isolated and purified from rhizome of Petasites tatewakianus, at the School of Pharmacy, Shanghai University of Traditional Chinese Medicine as previously described [13].
Generation of bone marrow DCs from C57BL/6 mice
Female C57BL/6 mice (6-10 wk old) were obtained from The Jackson Laboratory. All mice were treated according with protocols approved by the University of Alberta Animal Care and Use Committee. Bone marrow derived DCs (BmDC) were generated using a standard protocol with little modification [14]. Briefly, bone marrow was flushed dispersed and collected from femurs and tibias of female C57BL/6 mice, passed through a 70 μm nylon mesh, and suspended in complete medium (RPMI 1640 containing 5 mM Hepes, 50 U Pen/Strep, 2 mM glutamine, 50 μM 2-ME, 50 mM gentamycin sulfate, 10% FBS) in the presence of GM-CSF and IL-4, 10 ng/mL respectively (Peprotech, Rocky Hill, NJ, USA), and cultured in tissue culture dishes (Thermo Fisher, Carlsbad, CA, USA), in a humidified atmosphere of 5% CO2 in air at 37°C. All media components, except for GM-CSF and IL-4, were obtained from Gibco (Carlsbad, CA, USA). During culture, half of the media was replaced on days 3, and 6. On day 8, BmDC were harvested, and their phenotype was confirmed by microscopical analysis.
Effect of sesquiterpenes and PPARγ agonists on BmDC
Initially, 0.2 × 106 BmDC/mL were deposited, per well, in a 12 well plate, and incubated with either eremophilane-type petasite sesquiterpene, ZYFDC21 (50 μM), ZYFDC22 (25 μM), in presence or absence of synthetic PPARγ agonists troglitazone (TGZ) (5 μM, or 10 μM; Sigma-Aldrich Canada, Oakville, ON, Canada) or GW1929 (40 μM; Cayman Chemical, Ann Arbor, MI, USA), or the physiologically relevant PPARγ natural ligand 15d-PGJ2 (0.5 μM, or 5 μM; Cayman Chemical, Ann Arbor, MI, USA). Cells were then incubated for 20 h at 37°C, and 5% CO2 and viability was assessed by trypan blue exclusion (Gibco Carlsbad, CA, USA). Cells exposed to the petasite sesquiterpenes and synthetic and natural PPARγ ligands were >90% viable after treatment (data not shown). In order to evaluate if the PPARγ pathway and or the petasite sesquiterpenes were involved in the maturation and activation of DCs, BmDC were pre-incubated under each treatment for 3 h at 37°C, and 5% CO2 and, since we were interested in determining the effects on maturity and activation, we additionally incubated with LPS (10 mg/mL) overnight. BmDC stimulated with LPS or complete media alone were included as positive and negative controls respectively. Cell-free supernatants from the different conditions were collected and stored at -20°C for cytokine analysis with commercial ELISAs. Cells were fixed for 5 min in 2% formaldehyde, suspended in cold 1% BSA-flow Buffer (0.05% sodium azide, 0.1% BSA in PBS), incubated overnight at 4°C, and analyzed by flow cytometry.
Flow cytometry of BmDC
After stimulation, 1 × 105 BmDC were analyzed by flow cytometry for the expression of CD80 and CD86 surface molecules. For this, we used FITC-Armenian Hamster IgG Anti-Mouse CD80 (Affymetrix eBioscience, USA), and APC-Rat anti mouse CD86 antibodies (BD Pharmingen, San Diego, CA, USA), along with their isotype controls: FITC-Armenian Hamster IgG (Affymetrix eBioscience, USA) and APC-Rat IgG2a k (BD Pharmingen, San Diego, CA, USA). Cells were incubated for 60 min at 4°C, washed twice and analyzed in a CytoFLEX flow cytometer (Beckman Coulter, USA). Gating was determined according to the APC-Armenian Hamster anti mouse CD11c (BD Pharmingen, San Diego, CA, USA) positive population, while Armenian Hamster IgG (Affymetrix eBioscience, USA) was included as isotype control. Cell analysis was performed using the FlowJo V10 LLC software (Ashland OR, USA). Results were expressed as the median of fluorescence intensity (MFI) ± SEM.
Cytokine release analysis
Concentrations of TNF, IL-6 and IL-12p70 released in the cell-free supernatants were determined by ELISA according to the instructions of the commercial ELISA Kits (Affimetrix eBiosciences, Santa Clara, CA, USA).
Statistical analysis
Experiments were performed in triplicate, with BmDC obtained from at least three biological replicates (n ≥ 3). Values are expressed as mean ± standard error of the median (SEM). Statistical differences in the mean values among treatment groups were determined by using a one-way ANOVA test with post-hoc analysis with Tukey’s multiple comparison tests. In all cases, a value for p
Results
PPARγ activation inhibits DC maturation
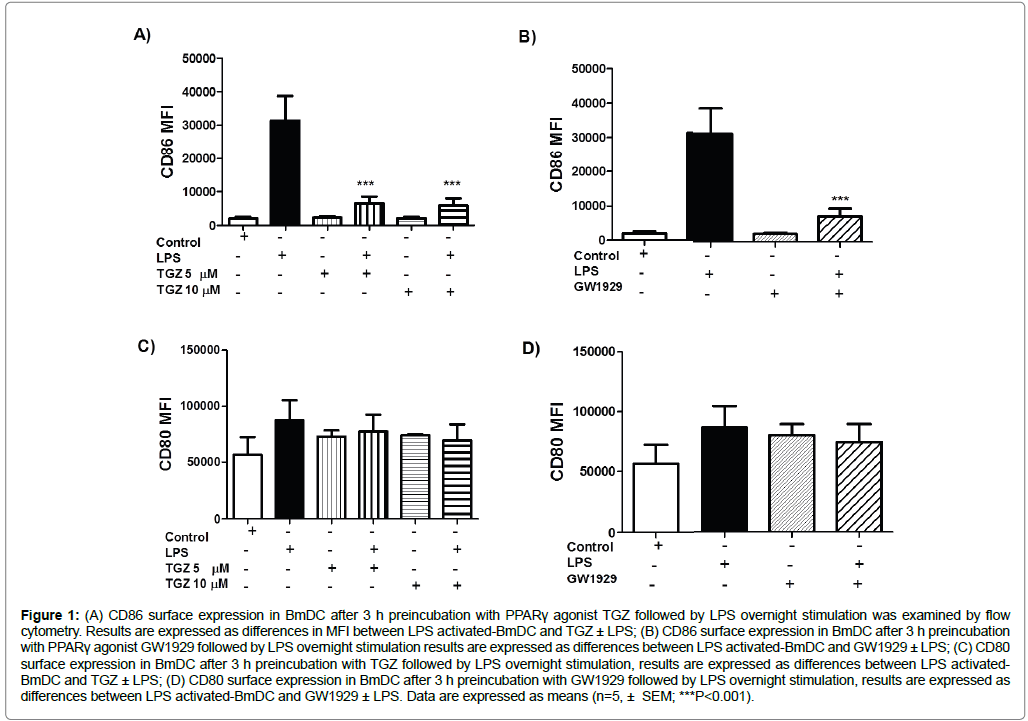
In order to determine whether PPARγ agonists modified the maturation of DC, we first analyzed the surface expression of the maturation marker CD86 on BmDC by flow cytometry. BmDC were treated with the synthetic PPARγ agonists TGZ (5 or 10 μM), or GW1929 (40 μM), and under these conditions, treatment with the PPARγ agonists had no effect on the expression of CD86. Figure 1A shows that BmDC exposed to different concentrations of TGZ (5 and 10 μM) did not show altered expression of CD86, exhibiting levels similar to control untreated cells; yet LPS stimulation induced upregulation of the co-stimulatory molecule CD86 on BmDC (MFI 31431 ± 7316, n=5). Interestingly, when the BmDC were pre-treated with the synthetic PPARγ agonist TGZ (5 or 10 μM for 3 h) followed by 20 h stimulation with LPS, there was a significant inhibition in the CD86 surface expression on BmDC compared to the LPS stimulation alone (MFI 6563 ± 1938 and MFI 5989 ± 2072 for TGZ 5 or 10 μM, respectively; n=5, Figure 1A). The same is true for BmDC exposed to GW1929 for 3 h, followed by 20 h stimulation with LPS, where we observed a 76% inhibition in the CD86 expression response compared to LPS alone (MFI 7459 ± 2317, n=5, Figure 1B). We also examined the expression of CD80 after BmDC were exposed to the synthetic PPARγ agonists, followed by LPS overnight stimulation. We observed a 12-20% inhibition in the expression of CD80 when cells were pretreated with TGZ, and this inhibition was dose-dependent (Figure 1C). Pre-treatment with GW1929 also promoted a 15% inhibition in the expression of CD80 (Figure 1D).
Figure 1: (A) CD86 surface expression in BmDC after 3 h preincubation with PPARγ agonist TGZ followed by LPS overnight stimulation was examined by flow cytometry. Results are expressed as differences in MFI between LPS activated-BmDC and TGZ ± LPS; (B) CD86 surface expression in BmDC after 3 h preincubation with PPARγ agonist GW1929 followed by LPS overnight stimulation results are expressed as differences between LPS activated-BmDC and GW1929 ± LPS; (C) CD80 surface expression in BmDC after 3 h preincubation with TGZ followed by LPS overnight stimulation, results are expressed as differences between LPS activated- BmDC and TGZ ± LPS; (D) CD80 surface expression in BmDC after 3 h preincubation with GW1929 followed by LPS overnight stimulation, results are expressed as differences between LPS activated-BmDC and GW1929 ± LPS. Data are expressed as means (n=5, ± SEM; ***P<0.001).
Natural PPARγ ligand 15d-PGJ2 modulates DC maturation
The cyclopentenone metabolite of PGJ2, 15d-PGJ2, is a naturally occurring derivative of prostaglandin D2 and has been shown to directly activate PPARγ [15-17]
BmDC were preincubated with 15d-PGJ2 (0.5 and 5 μM), for 3 h and the expression of CD86 was evaluated by flow cytometry. As shown in Figure 2A, 15d-PGJ2 alone had no effect on the expression of CD86 at either of the concentrations tested. 15d-PGJ2 treatment for 3 h decreased LPS-induced expression of CD86 by BmDC by 60% and 50% (MFI 12166 ± 1138 at 0.5 μM, and 15147 ± 1376 at 5 μM; n=3, Figure 2). CD80 surface expression was also downregulated (9 to 17% for 0.5 μM and 5 μM, respectively), by LPS-stimulated BmDC that were pretreated for 3 h with the natural PPARγ agonist 15d-PGJ2 (Figure 2B, n=3). BmDC stimulated with LPS or complete media were included as positive and negative controls respectively.
Figure 2: (A) CD86 surface expression in BmDC after 3 h preincubation with the natural PPARγ ligand 15d-PGJ2 followed by LPS overnight stimulation was examined by flow cytometry, results are expressed as differences between LPS activated-BmDC and 15d-PGJ2 ± LPS; (B) CD80 surface expression in BmDC after 3 h preincubation with 15d-PGJ2, results are expressed as differences in MFI between LPS activated-BmDC and 15d-PGJ2 ± LPS. Data are expressed as means (n=3 ± SEM; **P<0.01, ***P<0.001).
PPARγ activation promotes the inhibition of BmDC cytokine secretion
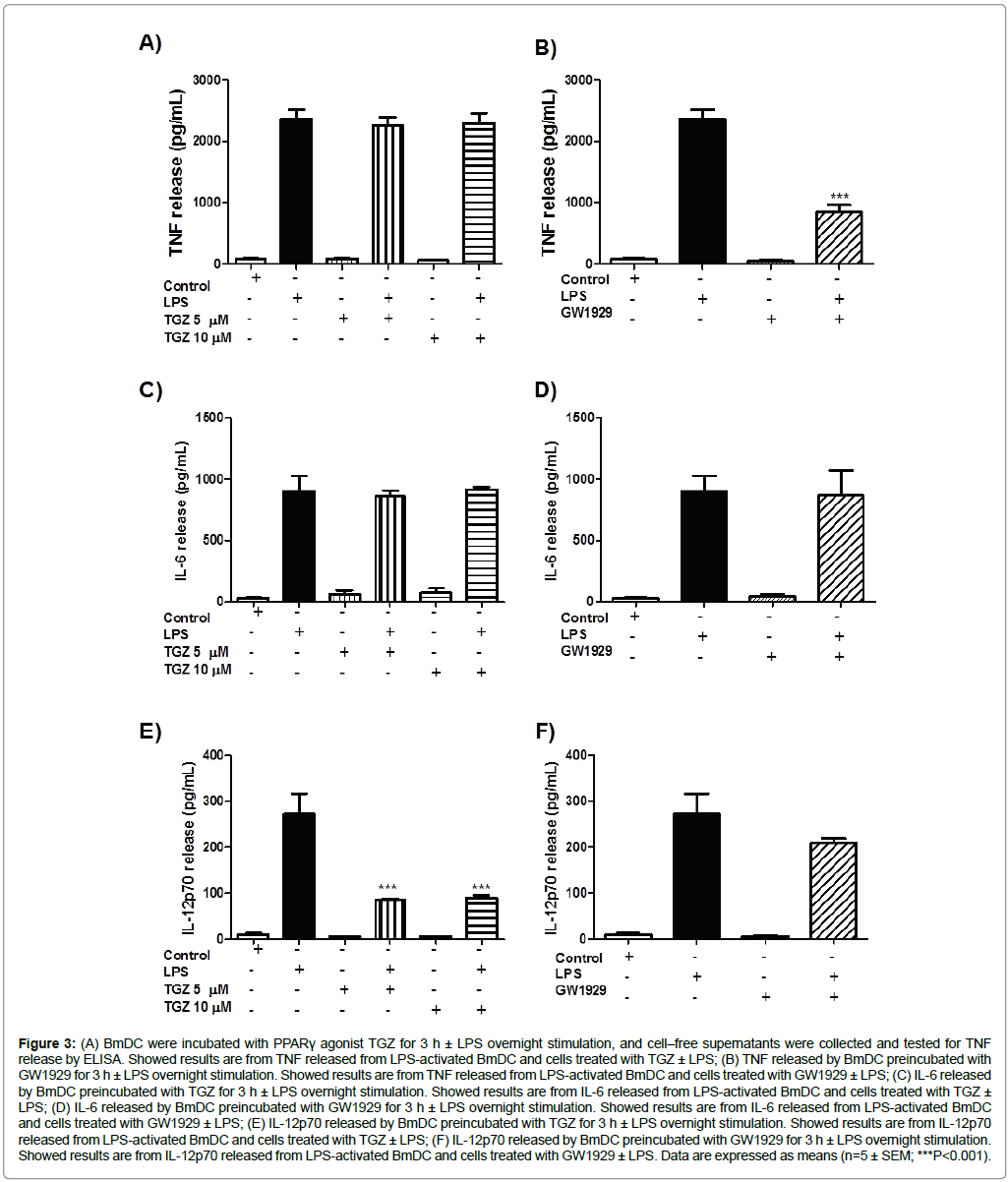
We analyzed the effects of the activation of PPARγon the cytokine secretion of TNF, IL-6 and IL-12p70 released in the cell-free supernatant of BmDC, after the 3 h treatment with TGZ (5 or 10 μM), or GW1929 (40 μM), by commercial ELISA. As shown in Figure 3A, treatment with both concentrations of TGZ slightly inhibited TNF release; pre-treatment with GW1929 significantly inhibited release of TNF (about 65 ± 5% compared to LPS, Figure 3B). However, under the same conditions, BmDC release of IL-6 was unaffected by treatment with the PPARγ agonists, compared to LPS stimulation alone (Figure 3C and Figure 3D). The release of IL-12p70, the bioactive isoform of the cytokine, was also evaluated in the cell-free supernatants of BmDC exposed to 5 μM and 10 μM TGZ with and without LPS stimulation. We found that TGZ significantly inhibited (68 ± 1% and 66 ± 2%, respectively) IL-12p70 production, as shown in Figure 3E. Furthermore, we observed a modest 20 ± 5% inhibition of IL-12p70 released by BmDC exposed to the synthetic PPARγ agonist GW1929 (Figure 3F).
Figure 3: (A) BmDC were incubated with PPARγ agonist TGZ for 3 h ± LPS overnight stimulation, and cell–free supernatants were collected and tested for TNF release by ELISA. Showed results are from TNF released from LPS-activated BmDC and cells treated with TGZ ± LPS; (B) TNF released by BmDC preincubated with GW1929 for 3 h ± LPS overnight stimulation. Showed results are from TNF released from LPS-activated BmDC and cells treated with GW1929 ± LPS; (C) IL-6 released by BmDC preincubated with TGZ for 3 h ± LPS overnight stimulation. Showed results are from IL-6 released from LPS-activated BmDC and cells treated with TGZ ± LPS; (D) IL-6 released by BmDC preincubated with GW1929 for 3 h ± LPS overnight stimulation. Showed results are from IL-6 released from LPS-activated BmDC and cells treated with GW1929 ± LPS; (E) IL-12p70 released by BmDC preincubated with TGZ for 3 h ± LPS overnight stimulation. Showed results are from IL-12p70 released from LPS-activated BmDC and cells treated with TGZ ± LPS; (F) IL-12p70 released by BmDC preincubated with GW1929 for 3 h ± LPS overnight stimulation. Showed results are from IL-12p70 released from LPS-activated BmDC and cells treated with GW1929 ± LPS. Data are expressed as means (n=5 ± SEM; ***P<0.001).
PPARγ ligation skews BmDC cytokine response
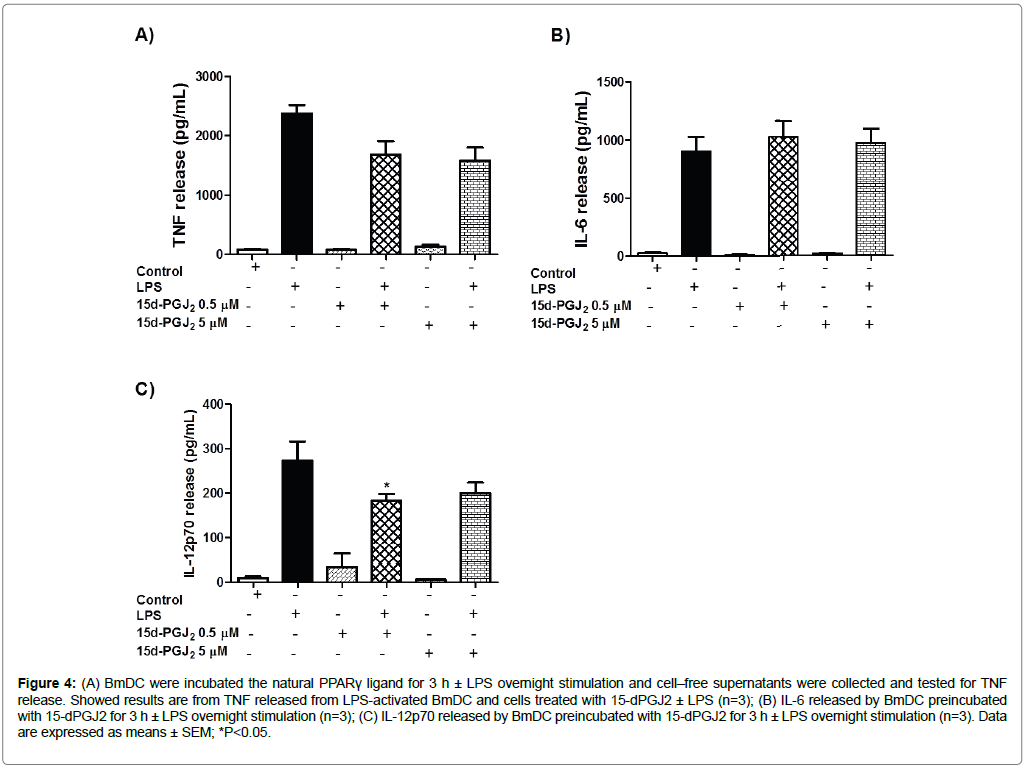
We were interested in studying the response of BmDC to the treatment with the natural PPARγ ligand 15d-PGJ2 (0.5 and 5 μM), and we found that this molecule similarly inhibited TNF release by BmDC. BmDC treated for 3 h with 15d-PGJ2 plus LPS showed a 29 ± 9% and 33 ± 9% inhibition at concentrations of 0.5 and 5 μM of the PPARγ ligand, respectively, in the presence of LPS compared to TNF release of BmDC treated with LPS alone (Figure 4A). Moreover, we observed a slight (10%) increase in IL-6 release in cell-free supernatants of BmDC exposed to 15d-PGJ2 followed to overnight LPS stimulation (Figure 4B) under both conditions, compared to the LPS positive control. IL-12p70 showed a 33 ± 5% and 27 ± 8% inhibition in release, at 0.5 and 5 μM respectively (Figure 4C). Cell-free supernatants of BmDC cultured in complete media were included as negative controls.
Figure 4: (A) BmDC were incubated the natural PPARγ ligand for 3 h ± LPS overnight stimulation and cell–free supernatants were collected and tested for TNF release. Showed results are from TNF released from LPS-activated BmDC and cells treated with 15-dPGJ2 ± LPS (n=3); (B) IL-6 released by BmDC preincubated with 15-dPGJ2 for 3 h ± LPS overnight stimulation (n=3); (C) IL-12p70 released by BmDC preincubated with 15-dPGJ2 for 3 h ± LPS overnight stimulation (n=3). Data are expressed as means ± SEM; *P<0.05.
Bicyclic petasite eremophilane-type sesquiterpenes potentiate the effects of PPARγ agonists on BmDC maturation and activation
Petasite sesquiterpenes have been shown to have anti-inflammatory activity in a variety of settings. We sought to assess the effects of two petasite eremophilane-type sesquiterpene compounds Fukinone (ZYFDC21), and 10βH-8α,12-Epidioxyeremophil-7(11)-en-8β-ol (ZYFDC22), isolated from the rhizome of Petasites tatewakianus, on the maturation and activation of BmDCs. To evaluate the cytotoxic effects of the bicyclic compounds we performed dose-response assays with several cell lines, using the XTT assay kit (Roche, data not shown). We selected sub-toxic doses of ZYFDC21 (50 μM) and ZYFDC22 (25 μM), and further evaluated their cytotoxic effects on BmDC after 1, 3, 24 and 48 h incubation, measuring viability by trypan blue exclusion (data not shown). BmDC viability was ≥ 95% under all tested conditions and, therefore, these concentrations were used for all experiments.
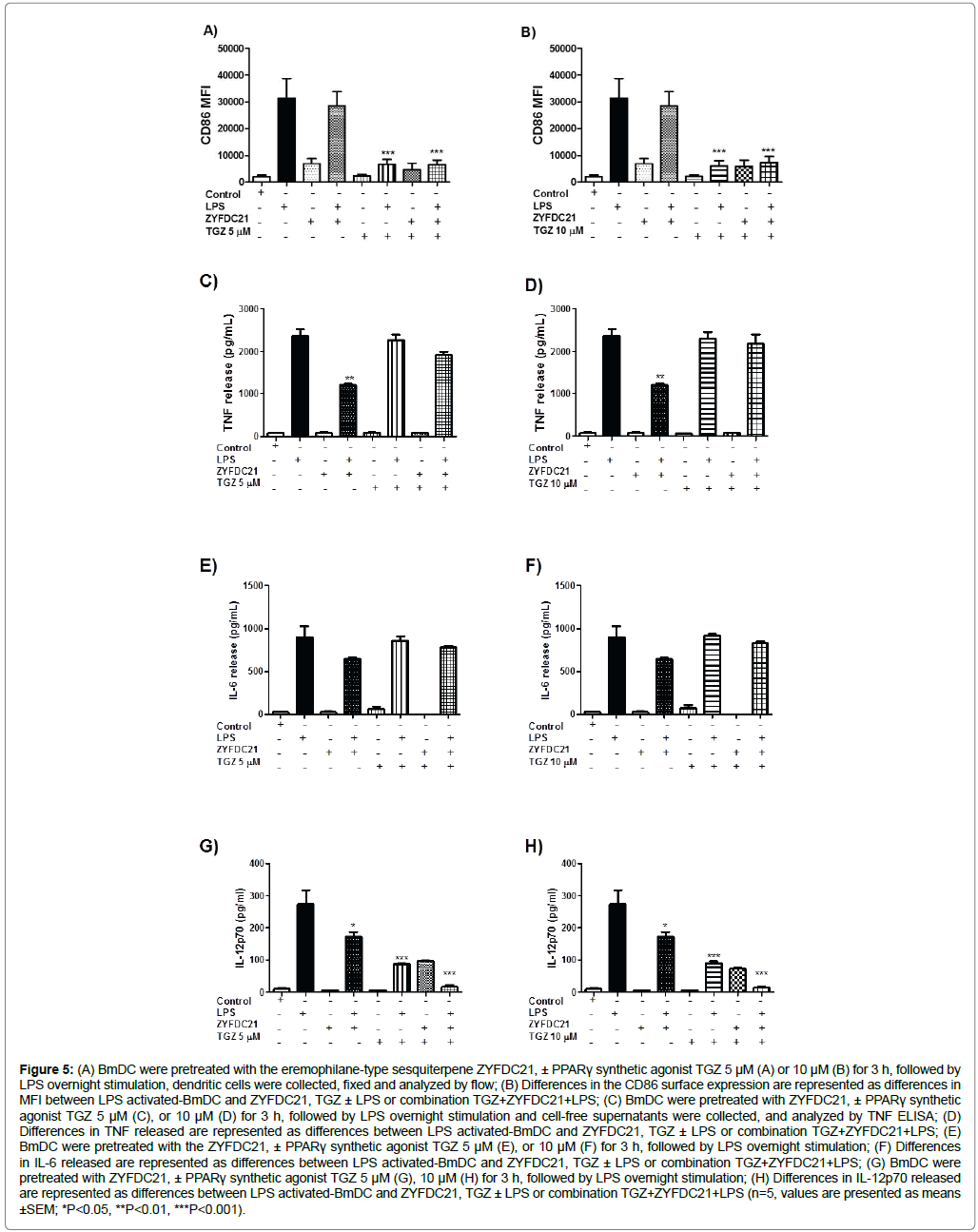
There is evidence that some sesquiterpenes exert anti-diabetic, anticarcinogenic and anti-inflammatory effects, mediated by the PPARγ pathway. We sought to identify whether the sesquiterpenes would inhibit BmDC maturation and activation, and if this inhibitory activity would be augmented by the presence of a PPARγ synthetic agonist. For that purpose, BmDC were exposed to the synthetic PPARγ agonists TGZ (5 or 10 μM) or GW1929 (40 μM), in presence or absence of the petasite sesquiterpenes ZYFDC21 (50 μM) or ZYFDC22 (25 μM) for 3 h, followed by the overnight LPS stimulation. Firstly, we assessed the effects of bicyclic sesquiterpenes on BmDC maturation by flow cytometry. The presence of the sesquiterpene ZYFDC21 (Figure 5A and Figure 5B) and ZYFDC22 (Figure 6A and Figure 6B) alone induced a noticeable increase in CD86 expression (MFI 6985 ± 1825 and 6882 ± 1274 respectively) compared to control, untreated BmDC (MFI 2073 ± 510). Interestingly, when BmDC were incubated with the petasite sesquiterpenes for 3 h, followed by LPS overnight incubation, CD86 expression decreased in about 9% on ZYFDC21-treated cells. Exposure to a combination of ZYFDC21 (50 μM) plus the synthetic PPARγ agonist TGZ (5 or 10 μM), followed by overnight LPS stimulation, resulted in an enhanced downregulation in CD86 surface expression (up to 80% compared to LPS levels alone, Figure 5A and Figure 5B).
Figure 5: (A) BmDC were pretreated with the eremophilane-type sesquiterpene ZYFDC21, ± PPARγ synthetic agonist TGZ 5 μM (A) or 10 μM (B) for 3 h, followed by LPS overnight stimulation, dendritic cells were collected, fixed and analyzed by flow; (B) Differences in the CD86 surface expression are represented as differences in MFI between LPS activated-BmDC and ZYFDC21, TGZ ± LPS or combination TGZ+ZYFDC21+LPS; (C) BmDC were pretreated with ZYFDC21, ± PPARγ synthetic agonist TGZ 5 μM (C), or 10 μM (D) for 3 h, followed by LPS overnight stimulation and cell-free supernatants were collected, and analyzed by TNF ELISA; (D) Differences in TNF released are represented as differences between LPS activated-BmDC and ZYFDC21, TGZ ± LPS or combination TGZ+ZYFDC21+LPS; (E) BmDC were pretreated with the ZYFDC21, ± PPARγ synthetic agonist TGZ 5 μM (E), or 10 μM (F) for 3 h, followed by LPS overnight stimulation; (F) Differences in IL-6 released are represented as differences between LPS activated-BmDC and ZYFDC21, TGZ ± LPS or combination TGZ+ZYFDC21+LPS; (G) BmDC were pretreated with ZYFDC21, ± PPARγ synthetic agonist TGZ 5 μM (G), 10 μM (H) for 3 h, followed by LPS overnight stimulation; (H) Differences in IL-12p70 released are represented as differences between LPS activated-BmDC and ZYFDC21, TGZ ± LPS or combination TGZ+ZYFDC21+LPS (n=5, values are presented as means ±SEM; *P<0.05, **P<0.01, ***P<0.001).
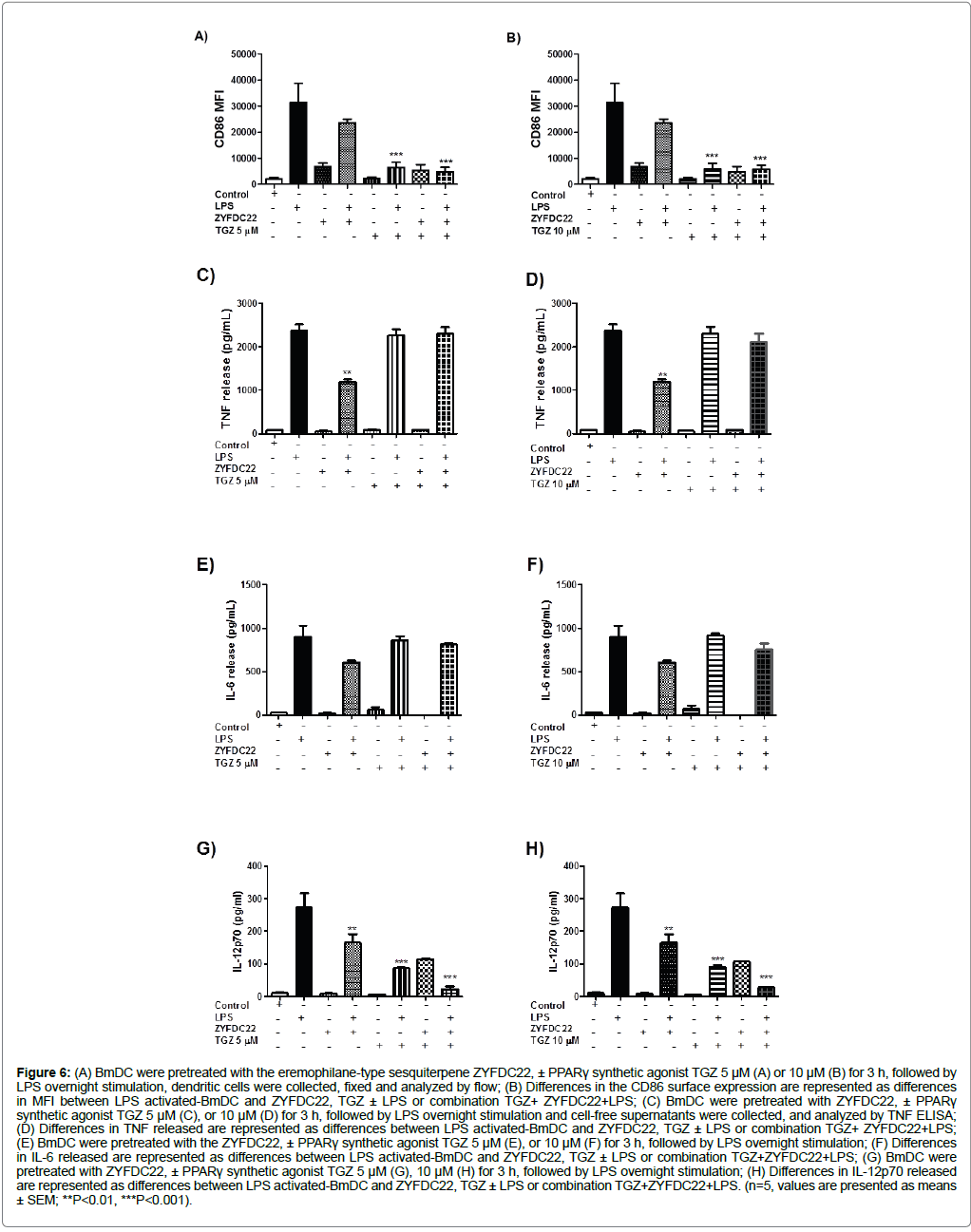
ZYFDC22 (25 μM), was more effective in inhibiting CD86 expression, decreasing CD86 expression up to 25% in BmDC treated with ZYFDC22 alone. The combination of the sesquiterpene with TGZ (5 or 10 μM), followed by the overnight LPS activation, resulted in up to 85% inhibition in CD86 surface expression (Figure 6A and Figure 6B).
Figure 6: (A) BmDC were pretreated with the eremophilane-type sesquiterpene ZYFDC22, ± PPARγ synthetic agonist TGZ 5 μM (A) or 10 μM (B) for 3 h, followed by LPS overnight stimulation, dendritic cells were collected, fixed and analyzed by flow; (B) Differences in the CD86 surface expression are represented as differences in MFI between LPS activated-BmDC and ZYFDC22, TGZ ± LPS or combination TGZ+ ZYFDC22+LPS; (C) BmDC were pretreated with ZYFDC22, ± PPARγ synthetic agonist TGZ 5 μM (C), or 10 μM (D) for 3 h, followed by LPS overnight stimulation and cell-free supernatants were collected, and analyzed by TNF ELISA; (D) Differences in TNF released are represented as differences between LPS activated-BmDC and ZYFDC22, TGZ ± LPS or combination TGZ+ ZYFDC22+LPS; (E) BmDC were pretreated with the ZYFDC22, ± PPARγ synthetic agonist TGZ 5 μM (E), or 10 μM (F) for 3 h, followed by LPS overnight stimulation; (F) Differences in IL-6 released are represented as differences between LPS activated-BmDC and ZYFDC22, TGZ ± LPS or combination TGZ+ZYFDC22+LPS; (G) BmDC were pretreated with ZYFDC22, ± PPARγ synthetic agonist TGZ 5 μM (G), 10 μM (H) for 3 h, followed by LPS overnight stimulation; (H) Differences in IL-12p70 released are represented as differences between LPS activated-BmDC and ZYFDC22, TGZ ± LPS or combination TGZ+ZYFDC22+LPS. (n=5, values are presented as means ± SEM; **P<0.01, ***P<0.001).
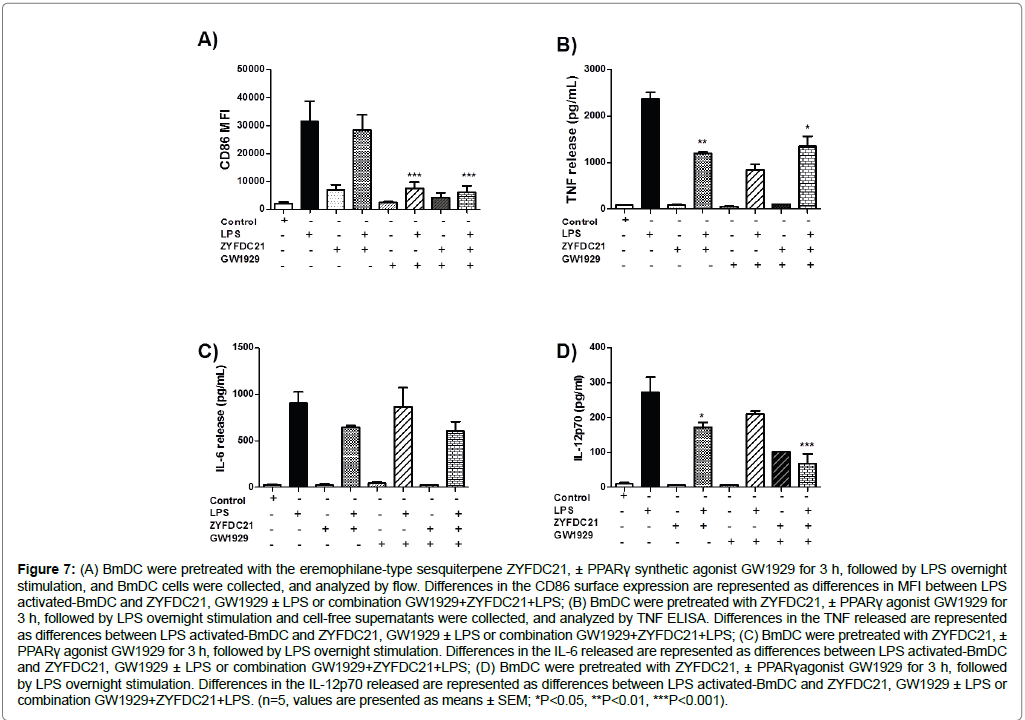
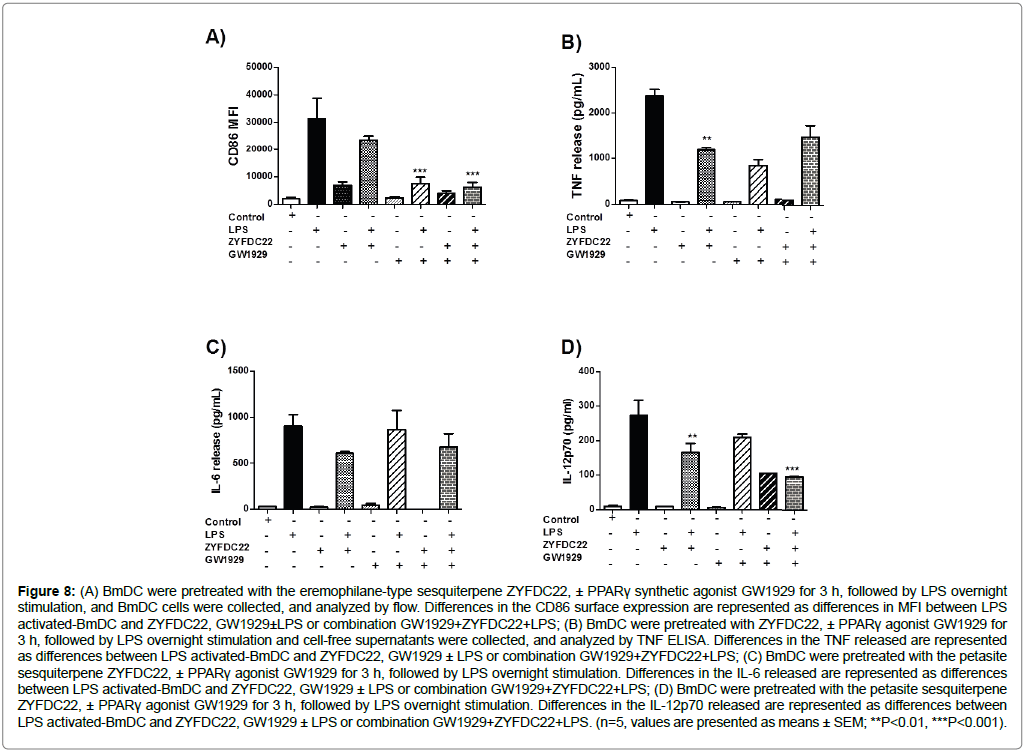
In regard to the synthetic PPARγ agonist GW1929, we found that it also downregulated CD86 expression of BmDC after the 3 h pretreatment (40 μM) in combination with the sesquiterpenes ZYFDC21 (50 μM) or ZYFDC22 (25 μM), and following LPS overnight stimulation (80% reduction, Figure 7A and Figure 8A).
Figure 7: (A) BmDC were pretreated with the eremophilane-type sesquiterpene ZYFDC21, ± PPARγ synthetic agonist GW1929 for 3 h, followed by LPS overnight stimulation, and BmDC cells were collected, and analyzed by flow. Differences in the CD86 surface expression are represented as differences in MFI between LPS activated-BmDC and ZYFDC21, GW1929 ± LPS or combination GW1929+ZYFDC21+LPS; (B) BmDC were pretreated with ZYFDC21, ± PPARγ agonist GW1929 for 3 h, followed by LPS overnight stimulation and cell-free supernatants were collected, and analyzed by TNF ELISA. Differences in the TNF released are represented as differences between LPS activated-BmDC and ZYFDC21, GW1929 ± LPS or combination GW1929+ZYFDC21+LPS; (C) BmDC were pretreated with ZYFDC21, ± PPARγ agonist GW1929 for 3 h, followed by LPS overnight stimulation. Differences in the IL-6 released are represented as differences between LPS activated-BmDC and ZYFDC21, GW1929 ± LPS or combination GW1929+ZYFDC21+LPS; (D) BmDC were pretreated with ZYFDC21, ± PPARγagonist GW1929 for 3 h, followed by LPS overnight stimulation. Differences in the IL-12p70 released are represented as differences between LPS activated-BmDC and ZYFDC21, GW1929 ± LPS or combination GW1929+ZYFDC21+LPS. (n=5, values are presented as means ± SEM; *P<0.05, **P<0.01, ***P<0.001).
Figure 8: (A) BmDC were pretreated with the eremophilane-type sesquiterpene ZYFDC22, ± PPARγ synthetic agonist GW1929 for 3 h, followed by LPS overnight stimulation, and BmDC cells were collected, and analyzed by flow. Differences in the CD86 surface expression are represented as differences in MFI between LPS activated-BmDC and ZYFDC22, GW1929±LPS or combination GW1929+ZYFDC22+LPS; (B) BmDC were pretreated with ZYFDC22, ± PPARγ agonist GW1929 for 3 h, followed by LPS overnight stimulation and cell-free supernatants were collected, and analyzed by TNF ELISA. Differences in the TNF released are represented as differences between LPS activated-BmDC and ZYFDC22, GW1929 ± LPS or combination GW1929+ZYFDC22+LPS; (C) BmDC were pretreated with the petasite sesquiterpene ZYFDC22, ± PPARγ agonist GW1929 for 3 h, followed by LPS overnight stimulation. Differences in the IL-6 released are represented as differences between LPS activated-BmDC and ZYFDC22, GW1929 ± LPS or combination GW1929+ZYFDC22+LPS; (D) BmDC were pretreated with the petasite sesquiterpene ZYFDC22, ± PPARγ agonist GW1929 for 3 h, followed by LPS overnight stimulation. Differences in the IL-12p70 released are represented as differences between LPS activated-BmDC and ZYFDC22, GW1929 ± LPS or combination GW1929+ZYFDC22+LPS. (n=5, values are presented as means ± SEM; **P<0.01, ***P<0.001).
Secondly, we evaluated the activation of the immune response by the presence of the pro-inflammatory mediators TNF, IL-6 and IL- 12p70 released in the cell-free supernatants of BmDC treated for 3 h with the synthetic PPARγ agonists (TGZ or GW1929), in combination with the petasite sesquiterpene (ZYFDC21 or ZYFDC22), followed by LPS overnight stimulation. Synthetic PPARγ agonist TGZ stimulated a differential response: BmDC exposed to TGZ in combination with petasite sesquiterpene ZYFDC21 (50 μM) for 3 h, followed by LPS overnight stimulation showed a dose-response inhibitory effect, when 5 μM of the PPARγ agonist was used. As shown in Figure 5C and Figure 5E, we obtained 19% and 14% inhibition in TNF and IL-6 release, respectively; however, when we increased the concentration of the PPARγ agonist to 10 μM, the observed inhibition was only 8% (Figure 5D and Figure 5F), for both cytokines.
In these studies, we found that IL-12p70, the bioactive isoform of IL- 12, seems to be involved in the PPARγ/petasine sesquiterpene pathway. In this regard, BmDC exposed to the sesquiterpene ZYFDC21 for 3 h, followed by overnight stimulation with LPS showed a 35% of IL-12p70 inhibition compared to LPS alone; when BmDC were pretreated with ZYFDC21 in combination with the synthetic PPARγ agonist TGZ (5 or 10 μM), followed by LPS overnight stimulation, we found that almost 95% of the IL-12 response was supressed (Figure 5G and Figure 5H).
The same response was seen for the sesquiterpene ZYFDC22; however, in this case, the cytokine release inhibition was decreasing as the concentration of the TGZ was increasing, with 3 and 10% TNF release inhibition (Figure 6C and Figure 6D), and 10 and 15% IL-6 release inhibition at 5 and 10 μM, respectively (Figure 6E and Figure 6F). The IL-12p70 release profile was also inhibited by about 35% for ZYFDC22 followed by LPS overnight stimulation. Finally, the combination of sesquiterpene ZYFDC22 (25 μM) with TGZ (5 or 10 μM) followed by overnight LPS stimulation inhibited around 90% the IL-12 release (Figure 6G and Figure 6H).
As shown in Figure 7A and Figure 8A, when BmDC were treated with the PPARγ agonist GW1929 (40 μM), along with ZYFDC21 or ZYFDC22, results were similar, exhibiting downregulation in CD86 surface expression of CD86 (80% ± 7%), as well as inhibition in release of TNF (43% and 37%, Figure 7B and Figure 8B), and IL-6 (33% and 25%, Figure 7C and Figure 8C). IL-12p70 release seemed to be less affected by the combination of PPARγ agonist/petasite sesquiterpenes, resulting in around 75% inhibition when using ZYFDC21, and 64% for ZYFDC22 (Figure 7D and Figure 8D).
Petasite sesquiterpenes potentiate the effects of PGD2 metabolites on BmDC maturation and activation
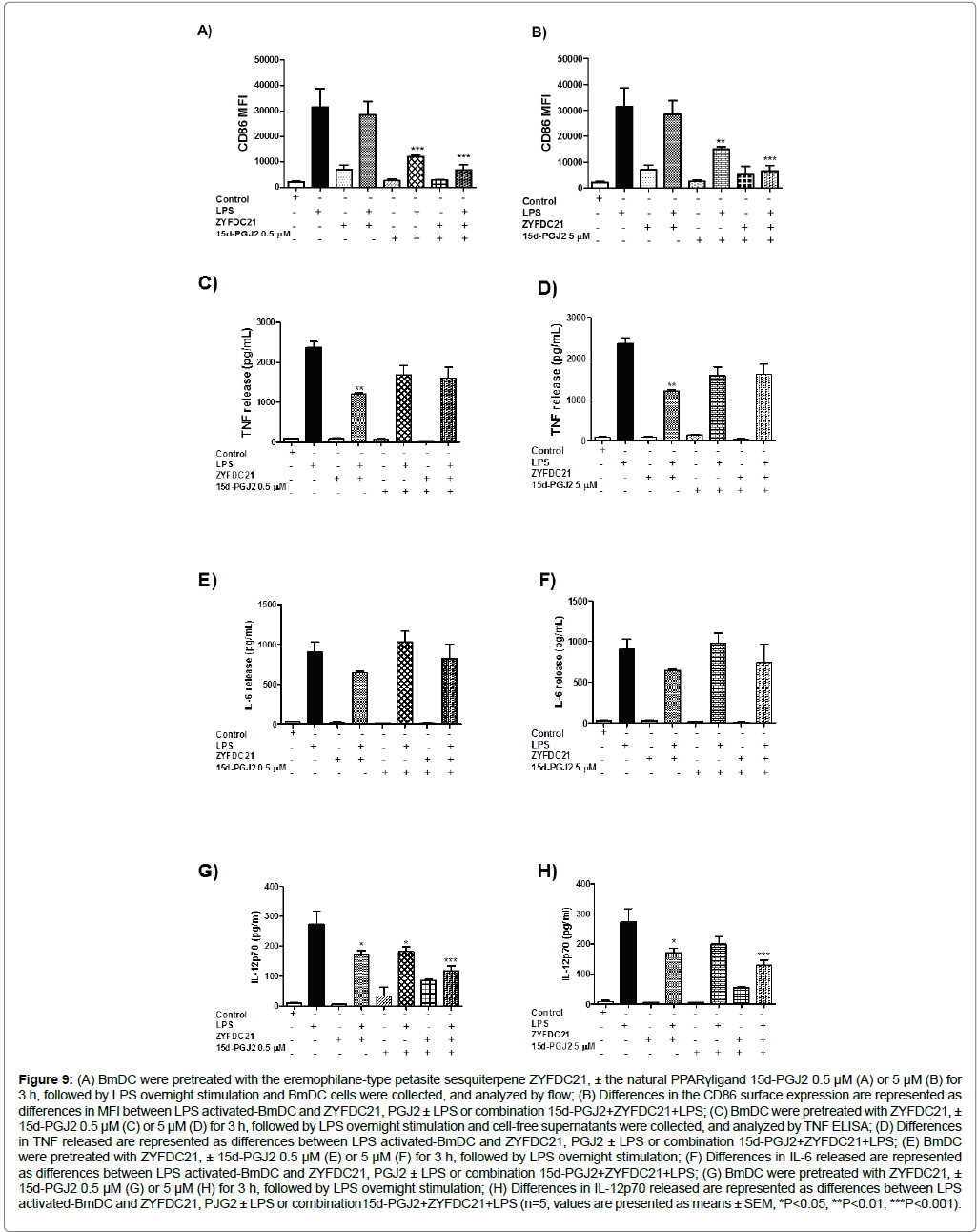
BmDC were exposed to the natural PPARγ ligand (15d-PGJ2, 0.5 and 5 μM) for 3 h in combination with eremophilane sesquiterpenes followed by LPS overnight stimulation. We observed a robust inhibition in the co-stimulatory molecule CD86. Cells incubated in the presence of 15d-PGJ2 and sesquiterpene ZYFDC21 (50 μM) plus LPS showed downregulation (78 ± 6%) in the expression of CD86 with 0.5 and 5 μM of 15d-PGJ2 (Figure 9A and Figure 9B respectively).
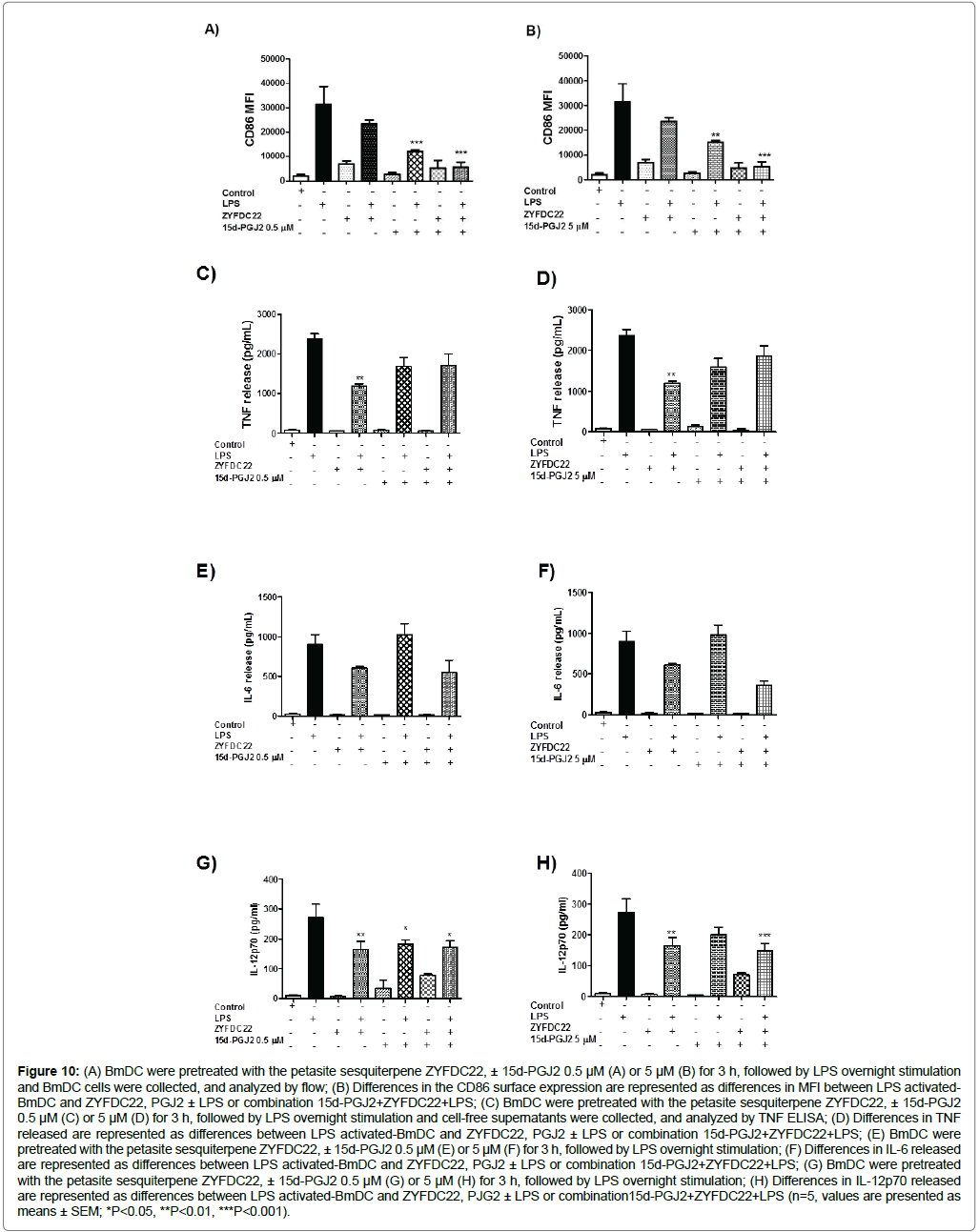
The same is true for the sesquiterpene ZYFDC22 (25 μM), which exhibited CD86 surface expression inhibition (82 ± 6%) with 0.5 and 5 μM 15d-PGJ2 (Figure 10A and Figure 10B respectively). Similarly, BmDC activation was also affected by the treatment with the natural PPARγ ligand, where TNF release by BmDC was inhibited after pretreatment with the combination of the PPARγ ligand (15d-PGJ2, 0.5 and 5 μM) sesquiterpenes, plus overnight incubation with LPS (30 ± 10% for ZYFDC21, Figure 9C and Figure 9D; and, 25 ± 10% for ZYFDC22, Figure 10C and Figure 10D) compared to LPS alone. When BmDC were exposed to petasite sesquiterpenes plus LPS there was a 50 ± 2% inhibition in the TNF release. In addition, the combination of the natural PPARγ ligand pre-treatment plus petasite sesquiterpenes and LPS overnight stimulation promoted a modest IL-6 inhibition of 9%, and 18% for ZYFDC21 (Figure 9E and Figure 9F); but a solid 39, 60% IL-6 inhibition following a dose-dependent PPARγ ligand pattern (Figure 10E and Figure 10F). Both sesquiterpenes have the ability to inhibit IL-6 release by BmDC by around 30% after 3 h pre-treatment followed for LPS stimulation. The release of IL-12 was also inhibited (50 ± 10%) with the combination of sesquiterpene ZYFDC21 and both tested concentrations of the natural PPARγ ligand (0.5 and 5 μM) plus LPS stimulation (Figure 9G and Figure 9H). However, when we tested the sesquiterpene ZYFDC22 in combination of 15-PJG2, we found that the inhibition was dose-dependent (30 ± 10% and 50 ± 10% IL-12 release inhibition at 0.5 and 5 μM 15-PJG2, respectively. Figure 10G and Figure 10H). Both sesquiterpenes were able to inhibit about 35% of IL-12 release after LPS stimulation.
Figure 9: (A) BmDC were pretreated with the eremophilane-type petasite sesquiterpene ZYFDC21, ± the natural PPARγligand 15d-PGJ2 0.5 μM (A) or 5 μM (B) for 3 h, followed by LPS overnight stimulation and BmDC cells were collected, and analyzed by flow; (B) Differences in the CD86 surface expression are represented as differences in MFI between LPS activated-BmDC and ZYFDC21, PGJ2 ± LPS or combination 15d-PGJ2+ZYFDC21+LPS; (C) BmDC were pretreated with ZYFDC21, ± 15d-PGJ2 0.5 μM (C) or 5 μM (D) for 3 h, followed by LPS overnight stimulation and cell-free supernatants were collected, and analyzed by TNF ELISA; (D) Differences in TNF released are represented as differences between LPS activated-BmDC and ZYFDC21, PGJ2 ± LPS or combination 15d-PGJ2+ZYFDC21+LPS; (E) BmDC were pretreated with ZYFDC21, ± 15d-PGJ2 0.5 μM (E) or 5 μM (F) for 3 h, followed by LPS overnight stimulation; (F) Differences in IL-6 released are represented as differences between LPS activated-BmDC and ZYFDC21, PGJ2 ± LPS or combination 15d-PGJ2+ZYFDC21+LPS; (G) BmDC were pretreated with ZYFDC21, ± 15d-PGJ2 0.5 μM (G) or 5 μM (H) for 3 h, followed by LPS overnight stimulation; (H) Differences in IL-12p70 released are represented as differences between LPS activated-BmDC and ZYFDC21, PJG2 ± LPS or combination15d-PGJ2+ZYFDC21+LPS (n=5, values are presented as means ± SEM; *P<0.05, **P<0.01, ***P<0.001).
Figure 10: (A) BmDC were pretreated with the petasite sesquiterpene ZYFDC22, ± 15d-PGJ2 0.5 μM (A) or 5 μM (B) for 3 h, followed by LPS overnight stimulation and BmDC cells were collected, and analyzed by flow; (B) Differences in the CD86 surface expression are represented as differences in MFI between LPS activated- BmDC and ZYFDC22, PGJ2 ± LPS or combination 15d-PGJ2+ZYFDC22+LPS; (C) BmDC were pretreated with the petasite sesquiterpene ZYFDC22, ± 15d-PGJ2 0.5 μM (C) or 5 μM (D) for 3 h, followed by LPS overnight stimulation and cell-free supernatants were collected, and analyzed by TNF ELISA; (D) Differences in TNF released are represented as differences between LPS activated-BmDC and ZYFDC22, PGJ2 ± LPS or combination 15d-PGJ2+ZYFDC22+LPS; (E) BmDC were pretreated with the petasite sesquiterpene ZYFDC22, ± 15d-PGJ2 0.5 μM (E) or 5 μM (F) for 3 h, followed by LPS overnight stimulation; (F) Differences in IL-6 released are represented as differences between LPS activated-BmDC and ZYFDC22, PGJ2 ± LPS or combination 15d-PGJ2+ZYFDC22+LPS; (G) BmDC were pretreated with the petasite sesquiterpene ZYFDC22, ± 15d-PGJ2 0.5 μM (G) or 5 μM (H) for 3 h, followed by LPS overnight stimulation; (H) Differences in IL-12p70 released are represented as differences between LPS activated-BmDC and ZYFDC22, PJG2 ± LPS or combination15d-PGJ2+ZYFDC22+LPS (n=5, values are presented as means ± SEM; *P<0.05, **P<0.01, ***P<0.001).
Discussion
Peroxisome proliferator activator receptors (PPARs) were initially identified as receptors that controlled physiological responses to dietary intake of fatty acids [18,19], in particular, PPARγ is involved in adipocyte differentiation, and glucose metabolism [20]. It has been shown that PPARγ ligands have anti-inflammatory effects on mast cells, monocytes, macrophages and dendritic cells, by modulating expression of costimulatory and adhesion molecules, altering their phenotype and leading to an impaired expression of pro-inflammatory cytokines/chemokine factors involved in T-cell activation and recruitment [21-25].
Dendritic cells are the most potent antigen presenting cells (APCs), and are involved in initiating the adaptive immune responses. The expression of surface adhesion (CD40), costimulatory (CD80, CD86) and major histocompatibility complex (MHC) class-II molecules promote the contact between dendritic cells and T cells, while costimulatory molecules signal T cells to proliferate and differentiate [26]. In the mouse, CD86 is the main activation marker of bonemarrow derived DC, being strongly up-regulated after maturation, while CD80 expression is less relevant for murine DC [26,27]. In this context, our studies demonstrated that LPS stimulation upregulated CD80 and CD86 expression on BmDC. We also confirmed that the use of synthetic (Troglitazone and GW1929), as well as natural (15d-PGJ2) PPARγ ligands decreased the expression of CD80 and CD86 after LPS stimulation. Further, we observed a significant reduction on TNF cytokine release with the GW1929 and 15d-PGJ2. IL-6 release exhibited no change with all PPARγ agonists used, while IL-12p70 production was attenuated by the PPARγ agonist. All of these results were similar to previous reports [22,23,28-31].
Advances in the investigation of plant–derived chemicals used in alternative medicine for the treatment of several chronic diseases have shown that Petasite species from petasite sesquiterpenes possess anti-inflammatory properties [32-34]. Due to their anti-inflammatory effects mediated via leukotriene synthesis inhibition, sesquiterpenes have been used for the treatment of inflammatory diseases such as arthritis, migraine, as well as asthma and allergy [35-37].
The anti-inflammatory effect of Petasite sesquiterpenes is based on their ability to block Ca2+ channels, decreasing intracellular Ca2+ concentration, inhibiting leukotriene B4 and cysteinyl leukotrienes synthesis in eosinophils and neutrophils [12,32,35,38-43]. The active components are sesquiterpene esters of the eremophilane-type, and their bioactivity is attributed to petasine and isopetasine [44,45]. Studies by Shimoda [46] showed that the effective constituent in the extract of Petasites japonicus was petasine, which had inhibitory effects on leukotriene synthesis [38] and bronchoconstriction [47]. Another eremophilane-type sesquiterpene ketone, namely, fukinones (1 and 3) (Naya and Kotake), exerted suppressive mechanisms in a type I hypersensitivity model in rats and IgE-sensitized RBL-2H3 cells, through inhibition of smooth muscle constriction and inhibition of degranulation, leukotriene release, and TNF production by mast cells [32,46]. In this context [32], also reported anti-allergic and antiinflammatory effects of several compounds obtained from plants of the petasites genus in an ovalbumin-induced asthma model. Use of Bakkenolide B isolated from P. japonicus results in inhibited mast cell degranulation as well as, expression induction of inducible nitric oxide synthase and cyclooxygenase 2. The bronchoalveolar lavage fluid of their ovalbumin-induced asthma model showed that Bakkenolide B inhibited the migration of eosinophils, macrophages, and lymphocytes to the lungs. In this case, however, the authors mentioned that Bakkenolide B did not prevent maturation of immature dendritic cells. Previous studies from our lab showed that different extracts of petasites could inhibit type I and type IV hypersensitivity in mouse models of homogeneous and heterogeneous passive cutaneous anaphylaxis [48].
Elegant studies evaluated the agonistic activity of the sesquiterpene lactones tirotundin and targitining A, isolated from Tithonia diversifolia against PPARs. For this, they used a transient transfection reporter assay with HepG2 cells, and found that tirotundin and targitining A transactivated PPARγ-dependent promoters including PPRE (PPARγ response element), SHP and ABCA1 gene promoters, and that both sesquiterpene lactones transactivated PPARγ by directly binding to the PPARγ ligand binding domain (LBD). In this context [11], showed that five isolated components of Chromolaena odorata, another plant used in traditional medicine for their anti-inflammatory activities, had a transactivation effect on PPARγ. More recent studies by [49] demonstrated by luciferase reporter assay in HEK293 cells, that the bicyclic sesquiterpene trans-Caryophyllene aroma compound of plant foods and teas activates PPARγ through direct interaction with the ligand binding domain of PPARγ. However, trans-caryophyllene showed no binding affinity for, or transactivation of PPARγ.
On another hand [44], demonstrated that petasin derived from P. japonicus activates AMPK in the liver, skeletal muscle and adipose tissue of mice, via phosphorylation of AMPK. AMPK activation enhanced the transcription of the proliferator-activated receptor-γ coactivator-1α (PGC-1α), which regulates the genes involved in energy metabolism including mitochondrial biogenesis.
Our studies showed that the eremophilane-type sesquiterpenes ZYFDC21 and ZYFDC22 noticeable increased CD80 and CD86 surface expression in non-stimulated BmDC. In contrast, when the cells were pre-treated with sesquiterpene ZYFDC21 (Fukinone) followed by LPS, we observed a decrease in CD86 surface expression This inhibition was amplified up to 80% by the presence of the PPARγ agonists TGZ, GW1929 and 15d-PGJ2. The inhibitory effect was also observed when we used ZYFDC22 (10βH-8α,12-Epidioxyeremophil-7(11)-en-8β-ol) in combination with TGZ, GW1929 or 15-d PGJ2, followed by LPS stimulation, where 85% of CD86 surface expression was significantly inhibited by PPARγ agonist treatment. The absence of costimulatory molecules, such as CD86, influences dendritic cell function, altering their maturation, and varying the expression of the necessary signals required for the activation and differentiation of naïve T cells into type 1 (IL-12, IFNγ) or type 2 (IL-4, IL-5, IL-10) cytokine producing cells. In this context, our studies showed that both sesquiterpenes ZYFCD21 and ZYFDC22 inhibited the secretion of the soluble factors TNF and IL-6, after LPS stimulation. These results are comparable to those obtained by [50], who demonstrated that the sesquiterpene lactone parthenolide inhibited dendritic cell maturation and cytokine secretion induced by LPS.
The level of IL-12 secreted by dendritic cells induced by microbial pathogens, such as LPS, during the immunological synapse is a key factor in the outcome of immune responses. IL-12 is a critical Th1-skewing cytokine that elicits IFNγ production by T cells and by NK cells [51], favoring a Th2/Th3 response and inhibiting T cell recruitment [52]. PPARγ is an important modulator on B and T lymphocytes as well as dendritic cells [22,53,54] and PPARγ ligands include a class of antidiabetic drugs, thiazolidinediones (TZD); as well as naturally produced Prostaglandin D2 (PGD2) and its metabolite 15-dideoxy-Δ PGJ2 (15d-PGJ2), which associate irreversibly to the receptor through covalent binding, mediating their effects by activation of PPARγ-dependent and independent pathways [21,55]. Prostaglandins production result in activation of PPARγ-mediated transcription, leading to the inhibition of differentiation, migration and cytokine secretion by antigen-presenting cells, such as dendritic cells or macrophages, hence, affecting the priming and effector functions of T lymphocytes [21].
Our studies showed for the first time, that dendritic cells exposed to the PPARγ ligands TGZ, GW1929 and 15d-PGJ2, in presence of these novel isolated bicyclic eremophilane-type petasite sesquiterpenes ZYFDC21 and ZYFDC22 followed by LPS stimulation exhibited a significant reduction (up to 95%) in the production of the bioactive isoform of IL-12 (IL-12p70). In this regard, it has been documented that 15d-PGJ2 abrogates IL-12 production by directly inhibiting the function of IαB kinase, therefore, preventing the translocation of NF-αB to the nucleus [56-58]. Our results showed that sesquiterpenes reduced LPS-induced DC maturation, and inhibited TNF and IL-6 release, as well as the production of the bioactive isoform of IL-12p70, presumably through the direct activation of PPARγ. Since it is well known that the transcription factor NF-αB plays a key role in the activation of PPARγ in the inflammatory response, it would be of interest to determine whether sesquiterpenes bind directly to the PPARγ receptors, thereby inhibiting IαB kinase (IKK), and to analyze the downstream signaling cascades that would prevent the translocation of NF-αB to the nucleus, interfering with the inflammatory response.
Conclusions
In summary, our results suggest that the novel Fukinone and 10βH- 8α,12-Epidioxyeremophil-7(11)-en-8β-ol sesquiterpenes derived from Petasite tatewakianus inhibit the maturation of dendritic cells, as well as the production of TNF, IL-6 and IL-12p70 after LPS stimulation. These events seem to be mediated and potentiated by the activation of PPARγ.Petasite sesquiterpenes are compounds with significant potential value for the treatment of inflammatory disorders.
Acknowledgements
The authors would like to thank Dr. Ramses Ilarraza for his comments on the manuscript revision. The authors acknowledge the Canadian Institutes of Health Research (CIHR), the National Research Council of Canada (Intramural Funding), and the Natural Science Foundation of China (Canada-China Joint Health Research Initiatives Grant 81261120567).
Conflict of Interest Disclosure
The authors declare no conflict of interest.
References
- Cheng Z, Zhao J, Liu D, Proksch P, Zhao Z, et al. (2016) Eremophilane-Type Sesquiterpenoids from an Acremonium sp. Fungus Isolated from Deep-Sea Sediments. J Nat Prod 79: 1035-1047.
- Gao S, Xia G, Wang L, Zhou L, Zhao F, et al. (2017) Sesquiterpenes from Curcuma wenyujin with their inhibitory activities on nitric oxide production in RAW 264.7 cells. Nat Prod Res 31: 548-554.
- Hou C, Kulka M, Zhang J, Li Y, Guo F (2014)Occurrence and biological activities of eremophilane-type sesquiterpenes. Mini Rev Med Chem 14: 664-677.
- Qin ZB, Zhang J, Wu XD, He J, Ding LF, et al. (2014) Sesquiterpenoids from Tussilago farfara and their inhibitory effects on nitric oxide production. Planta Med 80: 703-709.
- Wu XD, Ding LF, Tu WC, Yang H, Su J, et al. (2016) Bioactive sesquiterpenoids from the flowers of Inula japonica. Phytochemistry 129: 68-76.
- Zhang M, Zhao JL, Liu JM, Chen RD, Xie KB, et al. (2016) Neural anti-inflammatory sesquiterpenoids from the endophytic fungus Trichoderma sp. Xy24. J Asian Nat Prod Res 19: 1-8.
- Zhao H, Peng Q, Han Z, Yang L, Wang Z (2016) Three New Sesquiterpenoids and One New Sesquiterpenoid Derivative from Chinese Eaglewood. Molecules 21: 281.
- Zhao JH, Shen T, Yang X, Zhao H, Li X, et al. (2012) Sesquiterpenoids from Farfugium japonicum and their inhibitory activity on NO production in RAW264.7 cells. Arch Pharm Res 35: 1153-1158.
- Dat le D, Thao NP, Tai BH, Luyen BT, Kim S, et al. (2015) Chemical constituents from Kandelia candel with their inhibitory effects on pro-inflammatory cytokines production in LPS-stimulated bone marrow-derived dendritic cells (BMDCs). Bioorg Med Chem Lett 25: 1412-1416.
- Qin X, Jiang X, Wang Y, Miao Z, He W, et al. (2016) Micheliolide inhibits LPS-induced inflammatory response and protects mice from LPS challenge. Sci Rep 6: 23240.
- Zhang ML, Irwin D, Li XN, Sauriol F, Shi XW, et al. (2012) PPARgamma agonist from Chromolaena odorata. J Nat Prod 75: 2076-2081.
- Lee J, Kim MH, Lee JH, Jung E, Yoo ES, et al. (2012) Artemisinic acid is a regulator of adipocyte differentiation and C/EBP delta expression. J Cell Biochem 113: 2488-2499.
- Hou CJ, Wang GY, Li YM, Guo FJ (2016) Sesquiterpenes from the roots of Petasites tatewakianus Kitam. Chin Traditional Patent Med 38: 1970-1974.
- Labeur MS, Roters B, Pers B, Mehling A, Luger TA, et al. (1999) Generation of tumor immunity by bone marrow-derived dendritic cells correlates with dendritic cell maturation stage. J Immunol 162: 168-175.
- Forman BM, Tontonoz P, Chen J, Brun RP, Spiegelman BM, et al. (1995) 15-Deoxy-delta 12, 14-prostaglandin J2 is a ligand for the adipocyte determination factor PPAR gamma. Cell 83: 803-812.
- Ide T, Egan K, Bell-Parikh LC, FitzGerald GA (2003) Activation of nuclear receptors by prostaglandins. Thromb Res 110: 311-315.
- Scher JU, Pillinger MH (2005) 15d-PGJ2: the anti-inflammatory prostaglandin? Clin Immunol 114: 100-109.
- Guerre-Millo M, Gervois P, Raspe E, Madsen L, Poulain P, et al. (2000) Peroxisome proliferator-activated receptor alpha activators improve insulin sensitivity and reduce adiposity. J Biol Chem 275: 16638-16642.
- Issemann I, Green S (1990) Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature 347: 645-650.
- Willson TM, Lambert MH, Kliewer SA (2001) Peroxisome proliferator-activated receptor gamma and metabolic disease. Annu Rev Biochem 70: 341-367.
- Appel S, Mirakaj V, Bringmann A, Weck MM, Grunebach F, et al. (2005) PPAR-gamma agonists inhibit toll-like receptor-mediated activation of dendritic cells via the MAP kinase and NF-kappaB pathways. Blood 106: 3888-3894.
- Gosset P, Charbonnier AS, Delerive P, Fontaine J, Staels B, et al. (2001) Peroxisome proliferator-activated receptor gamma activators affect the maturation of human monocyte-derived dendritic cells. Eur J Immunol 31: 2857-2865.
- Kock G, Bringmann A, Held SA, Daecke S, Heine A, et al. (2011) Regulation of dectin-1-mediated dendritic cell activation by peroxisome proliferator-activated receptor-gamma ligand troglitazone. Blood 117: 3569-3574.
- Nencioni A, Grunebach F, Zobywlaski A, Denzlinger C, Brugger W, et al. (2002) Dendritic cell immunogenicity is regulated by peroxisome proliferator-activated receptor gamma. J Immunol 169: 1228-1235.
- Zhang G, Yang J, Li P, Cao J, Nie H (2014) Rosiglitazone inhibits HMC-1 cell migration and adhesion through a peroxisome proliferator-activated receptor gamma-dependent mechanism. Iran J Allergy Asthma Immunol 13: 11-18.
- Inaba K, Witmer-Pack M, Inaba M, Hathcock KS, Sakuta H, et al. (1994) The tissue distribution of the B7-2 costimulator in mice: abundant expression on dendritic cells in situ and during maturation in vitro. J Exp Med 180: 1849-1860.
- Inaba K, Inaba M, Romani N, Aya H, Deguchi M, et al. (1992) Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med 176: 1693-1702.
- Faveeuw C, Gosset P, Bureau F, Angeli V, Hirai H, et al. (2003) Prostaglandin D2 inhibits the production of interleukin-12 in murine dendritic cells through multiple signaling pathways. Eur J Immunol 33: 889-898.
- Marion-Letellier R, Butler M, Dechelotte P, Playford RJ, Ghosh S (2008) Comparison of cytokine modulation by natural peroxisome proliferator-activated receptor gamma ligands with synthetic ligands in intestinal-like Caco-2 cells and human dendritic cells--potential for dietary modulation of peroxisome proliferator-activated receptor gamma in intestinal inflammation. Am J Clin Nutr 87: 939-948.
- Nencioni A, Grunebach F, Zobywlaski A, Denzlinger C, Brugger W, et al. (2002) Dendritic cell immunogenicity is regulated by peroxisome proliferator-activated receptor gamma. J Immunol 169: 1228-1235.
- Thieringer R, Fenyk-Melody JE, Le Grand CB, Shelton BA, Detmers PA, et al. (2000) Activation of peroxisome proliferator-activated receptor gamma does not inhibit IL-6 or TNF-alpha responses of macrophages to lipopolysaccharide in vitro or in vivo. J Immunol 164: 1046-1054.
- Lee KP, Kang S, Park SJ, Choi YW, Lee YG, et al. (2013) Anti-allergic and anti-inflammatory effects of bakkenolide B isolated from Petasites japonicus leaves. J Ethnopharmacol 148: 890-894.
- Sok DE, Oh SH, Kim YB, Kang HG, Kim MR (2006) Neuroprotection by extract of Petasites japonicus leaves, a traditional vegetable, against oxidative stress in brain of mice challenged with kainic acid. Eur J Nutr 45: 61-69.
- Ziment I, Tashkin DP (2000) Alternative medicine for allergy and asthma. J Allergy Clin Immunol 106: 603-614.
- Dong L, Qiao H, Zhang X, Wang C, Wang L, et al. (2013) Parthenolide is neuroprotective in rat experimental stroke model: downregulating NF-kappaB, phospho-p38MAPK, and caspase-1 and ameliorating BBB permeability. Mediators Inflamm 2013: 370804.
- Heptinstall S, White A, Williamson L, Mitchell JR (1985) Extracts of feverfew inhibit granule secretion in blood platelets and polymorphonuclear leucocytes. Lancet 1: 1071-1074.
- Johnson ES, Kadam NP, Hylands DM, Hylands PJ (1985) Efficacy of feverfew as prophylactic treatment of migraine. Br Med J (Clin Res Ed) 291: 569-573.
- Bickel D, Roder T, Bestmann HJ, Brune K (1994) Identification and characterization of inhibitors of peptido-leukotriene-synthesis from Petasites hybridus. Planta Med 60: 318-322.
- Fiebich BL, Grozdeva M, Hess S, Hull M, Danesch U, et al. (2005) Petasites hybridus extracts in vitro inhibit COX-2 and PGE2 release by direct interaction with the enzyme and by preventing p42/44 MAP kinase activation in rat primary microglial cells. Planta Med 71: 12-9.
- Lee KP, Kang S, Noh MS, Park SJ, Kim JM, et al. (2015) Therapeutic effects of s-petasin on disease models of asthma and peritonitis. Biomol Ther (Seoul) 23: 45-52.
- Resnati M, Pallavicini I, Wang JM, Oppenheim J, Serhan CN, et al. (2002) The fibrinolytic receptor for urokinase activates the G protein-coupled chemotactic receptor FPRL1/LXA4R. Proc Natl Acad Sci U S A 99: 1359-1364.
- Thomet OA, Wiesmann UN, Schapowal A, Bizer C, Simon HU (2001) Role of petasin in the potential anti-inflammatory activity of a plant extract of petasites hybridus. Biochem Pharmacol 61: 1041-1047.
- Wu C, Chen F, Rushing JW, Wang X, Kim HJ, et al. (2006) Antiproliferative activities of parthenolide and golden feverfew extract against three human cancer cell lines. J Med Food 9: 55-61.
- Adachi Y, Kanbayashi Y, Harata I, Ubagai R, Takimoto T, et al. (2014) Petasin activates AMP-activated protein kinase and modulates glucose metabolism. J Nat Prod 77: 1262-1269.
- Chizzola R, Langer T, Franz C (2006) An approach to the inheritance of the sesquiterpene chemotypes within Petasites hybridus. Planta Med 72: 1254-1256.
- Shimoda H, Tanaka J, Yamada E, Morikawa T, Kasajima N, et al. (2006) Anti type I allergic property of Japanese butterbur extract and its mast cell degranulation inhibitory ingredients. J Agric Food Chem 54: 2915-2920.
- Ko WC, Lei CB, Lin YL, Chen CF (2000) Relaxant effects of petasins in isolated guinea pig trachea and their structure-activity relationships. Planta Med 66: 650-652.
- Zheng Q, Wu X, Li H, Li Y (2011) Experimental study of anti-allergy effects of bioactive fraction of Petasites japonicas. J Shanghai Univ Tradit Chin Med 25: 79-82.
- Wu C, Jia Y, Lee JH, Jun HJ, Lee HS, et al. (2014) Trans-Caryophyllene is a natural agonistic ligand for peroxisome proliferator-activated receptor-alpha. Bioorg Med Chem Lett 24: 3168-3174.
- Uchi H, Arrighi JF, Aubry JP, Furue M, Hauser C (2002) The sesquiterpene lactone parthenolide inhibits LPS- but not TNF-alpha-induced maturation of human monocyte-derived dendritic cells by inhibition of the p38 mitogen-activated protein kinase pathway. J Allergy Clin Immunol 110: 269-76.
- Heufler C, Koch F, Stanzl U, Topar G, Wysocka M, et al. (1996) Interleukin-12 is produced by dendritic cells and mediates T helper 1 development as well as interferon-gamma production by T helper 1 cells. Eur J Immunol 26: 659-668.
- Asseman C, Mauze S, Leach MW, Coffman RL, Powrie F (1999) An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J Exp Med 190: 995-1004.
- Gelman L, Fruchart JC, Auwerx J (1999) An update on the mechanisms of action of the peroxisome proliferator-activated receptors (PPARs) and their roles in inflammation and cancer. Cell Mol Life Sci 55: 932-943.
- Nencioni A, Wesselborg S, Brossart P (2003) Role of peroxisome proliferator-activated receptor gamma and its ligands in the control of immune responses. Crit Rev Immunol 23: 1-13.
- Shiraki T, Kamiya N, Shiki S, Kodama TS, Kakizuka A, et al. (2005) Alpha,beta-unsaturated ketone is a core moiety of natural ligands for covalent binding to peroxisome proliferator-activated receptor gamma. J Biol Chem 280: 14145-14153.
- Ghosh S, May MJ, Kopp EB (1998) NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol 16: 225-260.
- Rossi A, Kapahi P, Natoli G, Takahashi T, Chen Y, et al. (2000) Anti-inflammatory cyclopentenone prostaglandins are direct inhibitors of IkappaB kinase. Nature 403: 103-108.
- Straus DS, Pascual G, Li M, Welch JS, Ricote M, et al. (2000) 15-deoxy-delta 12,14-prostaglandin J2 inhibits multiple steps in the NF-kappa B signaling pathway. Proc Natl Acad Sci U S A 97: 4844-4849.
Citation: Arizmendi N, Hou C, Guo F, Li Y, Kulka M (2018) Bicyclic Eremophilane-type Petasite Sesquiterpenes Potentiate Peroxisome Proliferator-activated Receptor γ Activator-mediated Inhibition of Dendritic Cells. J Mol Immunol 3: 115.
Copyright: © 2017 Arizmendi N, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Usage
- Total views: 5927
- [From(publication date): 0-2018 - Oct 04, 2025]
- Breakdown by view type
- HTML page views: 4905
- PDF downloads: 1022