Bone Tumors with Aneurysmal Content: Decision Algorithm in Primary Surgical Treatment
Received: 27-Dec-2021 / Manuscript No. joo-21-50587 / Editor assigned: 29-Dec-2021 / PreQC No. joo-21-50587 (PQ) / Reviewed: 10-Jan-2022 / QC No. joo-21-50587 / Revised: 12-Jan-2022 / Manuscript No. joo-21-50587 (R) / Accepted Date: 13-Jan-2022 / Published Date: 18-Jan-2022 DOI: 10.35248/2472-016X.1000163
Abstract
Introduction: Several benign and malignant bone tumors have aneurysmal content. Primary ABC (aneurysmal bone cyst), secondary aneurysmal bone cyst, and telangiectatic osteosarcoma are the most common. Confirming diagnosis of biopsy of cystic tumors with aneurysmal content was a dilemma because it was difficult to obtain adequate tissue for pathology and presents risks. Surgeons may choose to remove a lesion without the need for preoperative biopsy. However, the criteria for this are not well defined. The purpose of this study was to develop an algorithm for clinical decision-making using a score based on the lesion’s main characteristics.
Methods: We conducted a retrospective analysis of bone tumors diagnosed as primary ABC (10 cases), secondary ABC (7 cases), and telangiectatic osteosarcoma (2 cases) between 2008 and 2019. A protocol was devised containing age, diagnosis, and imaging. Through literature review and data collected from the devised protocol criteria, including age, location, type of bone destruction, amount of fluid-fluid level, biological behavior, presence of solid component, and pathological fracture, a score between 0 and 8 was generated.
Results: The threshold established to discriminate the need for biopsy was 3.5 (sensitivity 88.9%, specificity 90%, positive predictive value 88.9%, and negative predictive value 90%). Benign tumors, such as primary ABC (10 cases), scored 2 – 4; secondary ABC (7 cases) scored 3-7; and telangiectatic osteosarcoma (2 cases) scored 6.
Conclusion: According to our findings it is possible to remove some tumors with aneurysmal content without prior biopsy using the decision algorithm, whose criteria were based on the features that needed the most attention. The algorithm scores from 0-8, separates lesions into lower and higher aggressiveness, indicates cases that require biopsy and can be used only by evaluating the MRI scan. Tumors scoring ≤ 3 can be treated without prior biopsy with curettage and adjuvant therapy. Tumors with other scores should be biopsied.
Keywords
Fluid-fluid levels; Magnetic resonance imaging; Needle biopsy; Aneurysmal bone cyst; Secondary aneurysmal bone cysts; Telangiectatic osteosarcoma
Introduction
There are reports in the literature of many bone tumors, both benign and malignant, that are associated with aneurysmal content [1-6]. The first description of an aneurysmal cyst was by Jaffé and Lichteintein in 1942 [7]. Aneurysmal bone content are characterized by different sizes spaces filled with blood and separated by connective tissue septa of various thicknesses [5,8]. They are preferentially present in long bones [5, 9]. The main characteristic is the presence of fluid-fluid levels which are the result of the difference in density of the hemorrhagic cellular component and the plasma, the less dense [10]. They are observed in magnetic resonance imaging [9] and computerized tomography [11]. Primary ABC, secondary bone cyst, and telangiectatic osteosarcoma are the most frequently described types [2, 5, 9, 12]. Differentiating them is a challenge, because they show similar clinical, epidemiological, imaging, and pathological characteristics.
Diagnosis of bone tumors is usually achieved through bone biopsy (both percutaneous and surgical) [13]. This type of procedure guides the orthopedic surgeon to make the best treatment decision. In some cases, surgeons may choose to remove aneurysmal content lesion without the need for preoperative biopsy [14] especially in prominent fluid levels, because it is difficult to obtain adequate tissue cores and the result obtained will probably be non-diagnostic [13]. It is a consensus among multidisciplinary teams that characteristics such as volume of the solid component [15], poorly defined margins [9, 16], type of periosteal reaction [16-17], invasion of adjacent soft tissue [18-19], perilesional edema [9, 18], and presence of pathological fracture [20] indicate more aggressive behavior or malignant behavior of the tumor and thus indicate the need for biopsy. In contrast, well-defined margins, sclerosis halo, and absence of periosteal reaction suggest less aggressive behavior, and such lesions are often resected without the need for preoperative biopsy [9]. The decision to biopsy first or directly removes the tumor is based on many factors such as clinical characteristics, epidemiological characteristics, imaging, and the surgeon’s expertise; however, some cases are still controversial.
Incorrect diagnosis can lead to catastrophic consequences because malignant tumors, such as telangiectatic osteosarcomas, present aggressive biological behavior and their treatment requires chemotherapy, in contrast to other lesions with aneurysmal content [21].
Therefore, is it possible to remove tumors with aneurysmal content without previous biopsy? Are there characteristics that help in this decision? The purpose of the current study was to create a scoring system, presented as a «Decision Algorithm,» using epidemiological and imaging characteristics in order to discriminate aneurysmal lesions that should be biopsied from those that can be removed without preoperative biopsy.
Literature Review
Many different tumors, besides primary ABC, have already been described as having aneurysmal content [1, 4-5, 22] (Board 1). The literature reports various characteristics that help in distinguishing benign from malignant aneurysmal lesions, especially telangiectatic osteosarcoma [1, 5, 15, 19, 23-24].
| Giant Cell Tumor | Non Ossifying Fibroma | Telangiectatic Osteosarcoma |
|---|---|---|
| Osteoblastoma | Chondromyxoid Fibroma | Fibrosarcoma |
| Chondroblastoma | Bone Cysts with Fracture | Metastasis of Carcinoma |
| Fibrous Dysplasia | Eosinophilic Granuloma |
Board 1: Bone tumors with aneurysmal content.
Primary Aneurysmal Bone Cyst
Primary ABC is a benign, locally aggressive, expansive, hemorrhagic lesion with variable sized spaces filled with serous-hematic or hematic content delimited by septa that contain bone trabeculae, osteoid, and giant cells [1, 11, 22, 25]. It is currently considered a primary bone tumor due to chromosomal rearrangement of the USP6 gene, located in chromosome 17p13, associated with several types of fusion, mainly with CDH11 [8, 26]. The gene is found only in primary ABC [26-27]. Techniques such as FISH (fluorescent in situ hybridization) and next generation sequencing can differentiate primary ABC from secondary ABC and from telangiectatic osteosarcoma [26].
Primary ABCs affect patients more commonly before 20 years of age [25] and are slightly more common in female patients [2, 28]. They are found anywhere in the skeleton [29], usually in the metaphyseal portion of long bones [22], around the knee [28] and in the posterior elements of the vertebrae [11, 22, 30].
The symptoms are similar to those of other benign and malignant bone tumors: pain and volume increase, of up to 6 months’ duration, and pathological fracture, which is less frequent [2, 22, 30]. Acute pain is a symptom of pathological fracture, most commonly observed in the spine (8% of cases) [2]. The tumor in the spine may present pain, torticollis, painful scoliosis, neurological symptoms, and rarely visible mass [22].
These tumors show diverse radiographic characteristics: eccentric, well defined, lytic, sometimes trabeculated, with bone expansion and bone thinning, and appearance of soap bubbles [8, 22, 30]. Central location is frequently observed when short bones are involved, usually in the hands and feet [22]. Other locations and features have also been classified and described: Capanna et al. [31] divided primary COA of the long bones into five morphologycal types: Type I: central lesion, with the entire content within the bone and with no or slight bone expansion; Type II: central lesion occupying the entire bone with expansion and cortical thinning; Type III: eccentric lesion with bone thinning involving only one cortical bone; Type IV: the rarest form, subperiosteal lesion with external extension to the bone, intact cortical or superficially eroded; Type V: subperiosteal lesion with both external and central growth and cortical destruction. Dabska and Buraczewski [32] described different radiographic characteristics depending on which of the four phases the lesion was in. Initial phase: the involved bone shows osteolysis and tenuous periosteal elevation. Growth phase: the tumor shows rapid tumor enlargement with progressive bone destruction and is ill-defined. The external border and septae are radiographically invisible reminiscent of a malignant tumor. Stabilization phase: classic imaging, with thin periosteal bone and ossification of the septae. Healing phase: gradual ossification of the lesion with irregular bone structure [32]. Rapidly enlarging ABC can be ill-defined and irregular, suggesting a malignant lesion such as a lytic osteogenic sarcoma [12].
CT can better define borders of the lesion that were hardly seen on radiography, detect thinning and erosion of the cortex, help predict potential fracture risk, and show fluid levels in 30-35% of cases [11, 22, 30].
MRI has a higher sensitivity than that of CT to demonstrate fluidfluid levels and complements plain radiography [22]. These levels have been reported in both benign and malignant lesions and are not exclusive to primary ABC [5, 15]. Imaging characteristics commonly described are cystic spaces, fluid-fluid levels, internal septae of different thicknesses, bone expansion and lobulations [8, 29-30]. The signal characteristics of aneurysmal cysts on MRI may be different within the same tumor and their distinction may vary with the degree of T2-weighted sequence. If only one sequence is obtained, the fluid levels may not be noted [29]. Gadolinium contrast administration enforces the visualization of the cystic walls and septae [22, 30]. Other characteristics may be present such as extension to adjacent soft tissue with well-defined low signal periosteal margin, similar to those seen on CT images [29-30]. They may have minimal or no solid component [22].
Secondary Aneurysmal Bone Cyst
Secondary ABCs are tumors with aneurysmal content associated with another primary bone tumor [1-5]. There is a paucity of information about secondary ABC in the literature [1, 5]. About 29- 35% of tumors with aneurysmal content are part of a pre-existing primary lesion, with giant cell tumor (GCT) as the most common (19- 39%) [4-5, 30] followed by chondroblastoma [5].
The presence of a solid component of the tumor, best visualized on MRI, is one of the main imaging characteristics that help differentiate a primary from a secondary ABC [15]. O’Donnell & Saifuddin [15] concluded that the larger the volume of the solid component in the aneurysmatic lesions, the greater the probability that it is a secondary or malignant lesion and that the majority of the predominantly cystic lesions were primary ABC [15]. Gutierrez et al. [5] reported in a series of 49 cases of secondary aneurysmal bone cysts that it was not possible to differentiate on imaging the different entities of primary lesions . Secondary tumors present clinical, imaging, and
anatomopathological features similar to those of primary tumors regardless of the presence of aneurysmal content. They are preferentially located in the epiphysis of long bones [5, 9]. GCT, chondroblastoma, fibrous dysplasia, osteoblastoma, osteosarcoma and chondromyxoid fibroma present a higher percentage of association with aneurysmal content than the respective primary lesions without fluid levels [5].
Telangiectatic Osteosarcoma
Telangiectatic osteosarcoma is a rare malignant bone tumor, being one of the subtypes of osteosarcoma [23-24, 33]. It presents multiple aneurysmal cavities separated by septae containing high grade sarcomatous cells [12, 19].
Telangiectatic osteossarcomas make up 2-12% of all osteosarcomas cases [12, 19, 34]. They affect male and female patients in a 3:2 ratio [12]. The main age group is the second decade of life [12, 19, 24, 33]. It is more common in the knee region, mainly in the distal femur and femoral shaft [12,19]. Compared to ABC, the axial skeleton is involved less frequently [9].
More than 90% of the cases present pain and volume increase, and 29% of the cases present pathological fracture, in some phase of the disease during treatment [12].
The literature reports many characteristics on plain radiography such as geographic bone destruction, no sclerotic margin [9, 19], and moth-eaten [24] and permeative patterns [9, 35]. Other features are described such as lytic image, bone expansion with or without cortical destruction, wide transition zone, presence of discrete mineralized matrix [58-76% of cases], and Codman’s triangle [9, 12, 19, 20, 24, 34, 36].
CT shows soft tissue mass and mineralized matrix [20]. It is the best imaging method to detect mineralized areas of the tumor [20], as seen in 85% of cases [34].
MRI is the best method to evaluate telangiectatic osteosarcoma [34]. It has heterogeneous signals in T1 and T2 sequences [34]. In T2, fluid-fluid levels with high signal intensity [19], caused by hemorrhage, are better depicted (89% of cases) [34]. Gao et al. [19] reported on MRI: small to large cystic spaces; fluid-fluid levels; soft tissue mass and postcontrast enhancement of the septae and in the periphery of the cystic cavity. They reported that a large percentage of the cystic cavities in their study were blood-filled (72.7%) and that small cystic spaces were rarely seen in primary ABC compared to telangiectatic osteosarcoma. After contrast media administration, telangiectatic osteosarcoma showed thickened septae and periphery, with or without nodular tissue [19] > 2 mm surrounding them, and similarity to a honeycomb [19, 24, 34].
Telangiectatic osteosarcoma presents a rapid growth pattern that can be confused with ABC both on imaging and in the pathological examination [19-20]. Biopsy must be directed to nodular areas where the malignant mesenchymal cells, which are absent in ABC, are located [34]. There can be cellular atypia, hyperchromasia, atypical mitoses, and pleomorphism [24]. Sangle and Layfield [24] divided telangiectatic osteosarcoma into two types: low grade (mild to moderate nuclear atypia and few mitoses), which makes diagnosis difficult, and high grade (poorly differentiated tumor with marked anaplasia and high mitotic activity). Yin et al. [23] reported that 22% of telangiectatic osteosarcoma cases were misdiagnosed as benign tumors through needle or open biopsy.
Decision Criteria Based On The Literature Review
Some epidemiological and imaging characteristics reported in the literature demonstrated greater aggressiveness or merit greater attention:
1. Age group under 18 years: Telangiectatic osteosarcoma is more common in children and adolescents [9, 12, 19, 23-24, 33].
2. Major sites of telangiectatic osteosarcoma: Most frequent location is the long bones [9,12, 23, 33, 35, 37].
3. Aneurysmal tumors in the spine: In children and young adults with aneurysmal content lesion > 2/3, tumors were probably benign; in patients aged > 50 years who had aneurysmal content lesion < 2/3, the tumors were probably malignant, and malignant tumors were frequent in individuals over 50 years, mostly metastatic [10].
4. Type of bone destruction: The Lodwick criteria [38] describe the greater or lesser aggressiveness of bone tumors on imaging exams: Lesion IA, sharply defined margin and with sclerotic rim; lesion IB, well-defined margin, little or no sclerotic rim and with thinning of the cortex; Lesion IC, ill-defined margin, possible sclerotic rim, cortical destruction possible; Type II moth-eaten, cortical destruction, and Type III permeative destruction. Type IC, II, and III lesions are the most aggressive and related to telangiectatic osteosarcoma [9, 20, 24, 34].
5. Fluid-fluid bone lesions on MRI scans:O’Donnel and Saifuddin [15] reported that lesion with aneurysmal content volume > 2/3 had 89% benignity at diagnosis, lesions with complete fluid-fluid level content were benign, and malignant tumors commonly had fluid-fluid levels < 1/3. Rajeswaran et al. [14] reported that tumors that showed 100% aneurysmal content were almost certain to be benign even with aggressive appearance on imaging exams. Zishan et al. [9] reported that 48% of telangiectatic osteosarcomas had aneurysmal content volume < 1/3 of the lesion.
6. Aggressiveness characteristics of aneurysmal tumors: Cortical bone rupture, presence of periosteal reaction, soft tissue mass, mineralized matrix, and perilesional bone edema have been described [9, 20, 24, 35].
7. Presence of solid content: Primary ABC, secondary ABC, and telangiectatic osteosarcoma are tumors that present solid content and can present diverse volumes in their composition. The literature reports that tumors without solid content were benign [14] and that malignant lesions were frequently filled with 1/3 of the volume of the lesion with aneurysmal content, that is, at least 2/3 of the lesion presented solid content [15]. The greater the volume of the liquid level, the less frequent was the malignancy [15].
8. Pathological fracture: The literature describes the presence of fracture in telangiectatic osteosarcomas in 29-61% of cases [12, 20, 23, 33, 37]. Gutierrez et al. [5] reported 8% of fractures in secondary tumors, the most frequent being GCT. Primary ABC rarely fractures [2, 22, 30].
Material and Methods
A retrospective study was carried out at the “Hospital de Clinicas” of the University of Campinas from 2008 to 2019 and was approved by our institutional review board. We selected all patients with the following anatomopathological diagnoses: “Aneurysmal Bone Cyst”; “Secondary Aneurysmal Bone Cyst”; “Telangiectatic Osteosarcoma”; and other bone tumors that were described in the report “presence of aneurysmal content”. The inclusion criteria were all patients with preoperative images of radiography or MRI. Patients with head tumors, solid ABC, and those with no available data were excluded.
A protocol was prepared containing epidemiological data: age at diagnosis; radiographic characteristics (Lodwick criteria), [38] lesion location, periosteal reaction, calcification, and pathological fracture; MRI scan characteristics (presence of fluid-fluid levels, solid content, type of tumor margin, and presence of peripheral reactive edema).
The images visualized on MRI were the axial, sagittal, and coronal sections in T1-weighted, T2-weighted (SPAIR or SPIR) and postcontrast (T1 SPIR GD) sequences. All the images were analyzed by a radiologist and the histopathological diagnoses were established by a pathologist, both specialists in the musculoskeletal system.
In the protocol the Lodwick criteria [38] was used both for radiography and for MRI classification, which was adapted and described by the authors of the present study in order to evaluate the type of margin of aneurysmal tumors. Both classifications were compared. The type IA, IB, and IC lesions are described in the same way at radiography and MRI: IA lesion, well-defined and margin with sign of sclerosis; IB lesion, well-defined, margin with little sign of sclerosis and with thinning of the cortex; IC lesion, ill-defined, little or no sign of sclerosis and cortical rupture. The type II lesion described at radiography as moth-eaten presented at MRI the description: complete or nearly complete bone marrow invasion, presence of small sparse islands, and irregular cortical invasion. The type III lesion which at radiography presented as permeative was described as: wide transition zone and a more uniform pattern of cortical lysis [39].
Using the decision criteria, an algorithm was created to score (0- 8) the main characteristics of lesions with aneurysmal content. Eight criteria were included: 1) patient age, 2) location in the long bones, 3) tumors in the spine, 4) type of bone destruction, 5) percentage of fluid-fluid level volume, 6) presence of solid content, 7) aggressiveness characteristics on radiography or MRI, 8) presence of fracture in pathologic bone. The scores used were 0, 1, and 2 (Table 1).
| Score | 2 | 1 | 0 |
|---|---|---|---|
| Age | ≤ 18 years old | > 18 years old | |
| Long bones | Yes | No | |
| Tumors in the spine | ≥ 50 years | ||
| Bone destruction (radiographic or MRI) | IC, moth-eaten, permeative | IA- IB | |
| Fluid-fluid level | ≤ 1/3 | 1/3 - 2/3 | ≥ 2/3 |
| Solid content | Yes | No | |
| Aggressiveness characteristics (cortical rupture, periosteal reaction, soft tissue mass, mineralized matrix, perilesional bone edema) | Yes | No | |
| Pathological fracture | Yes | No |
Table 1: Decision algorithm.
Statistical Analysis
Statistical analysis was performed to verify whether the score assigned in the decision algorithm was able to discriminate the need for preoperative biopsy and to establish the best algorithm threshold.
The Mann-Whitney test was utilized to evaluate two independent groups according to the anatomopathological diagnosis. The group that did not require biopsy (score 0) was compared, by median, with the group that required biopsy (score 1). Primary ABC scored 0, and the other diagnoses scored 1.
The receiver operating characteristic (ROC) curve was created with the objective of measuring the performance and establish the best algorithm threshold that could discriminate the necessity of preoperative biopsy. With the threshold the diagnostic measurements were calculated (sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV)) with the respective 95% confidence intervals.
The analyses were performed using the software IBM-SPSS for Windows version 22.0, the results were tabulated using Microsoft- Excel 2010 software, and the tests were performed with a significance level of 5%.
Results
The characteristics of the lesions were collected from MRI and from radiography exams. There were 15 patients with both radiography and MRI scans and 4 patients with only MRI, totaling 19 patients. A comparison between classifications for bone destruction types in radiography and MRI was performed and the results matched in 80% of cases (Table 2).
| Diagnosis n=15 | Age | Location | Classification Radiography | Classification RM | Concordance |
| ABC | 13 | Tibia | IB | IB | Yes |
| ABC | 33 | Tibia | IA | IA | Yes |
| ABC | 18 | Clavicle | IB | IB | Yes |
| ABC | 48 | Humerus | IB | IB | Yes |
| ABC | 12 | Ulna | IB | IB | Yes |
| ABC | 11 | Tibia | IB | IB | Yes |
| ABC | 15 | Column | IC | IB | No |
| ABC | 12 | Fibula | IB | IB | Yes |
| GTC | 19 | Fibula | IC | IC | Yes |
| CMF | 22 | Femur | IB | IB | Yes |
| GCT | 21 | Tibia | II | IC | No |
| GCT | 47 | Femur | IB | IC | No |
| CMF | 22 | Basin | IB | IB | Yes |
| FD | 12 | Femur | IA | IA | Yes |
| TO | 22 | Tibia | III | III | Yes |
Table 2: Comparison between classification for bone destruction types in radiography and MRI.
ABC: aneurysmal bone cyst; GCT: giant cell tumor; CMF: chondromyxoid fibroma; FD: fibrous dysplasia; TO: telangiectatic osteosarcoma
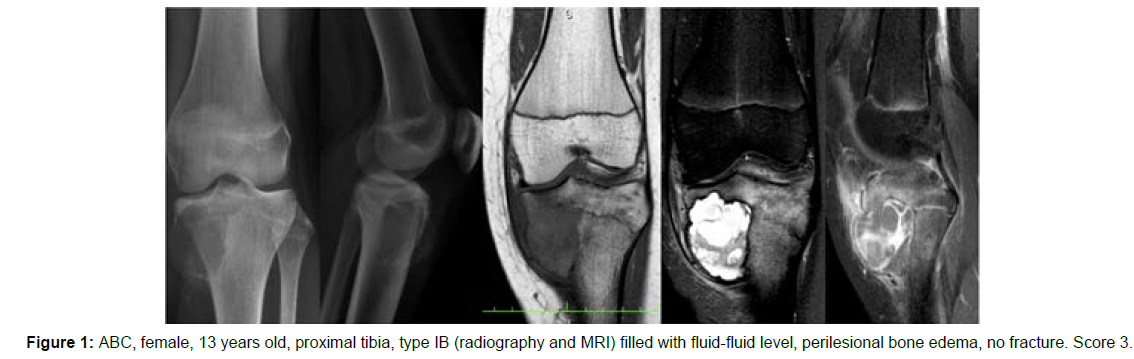
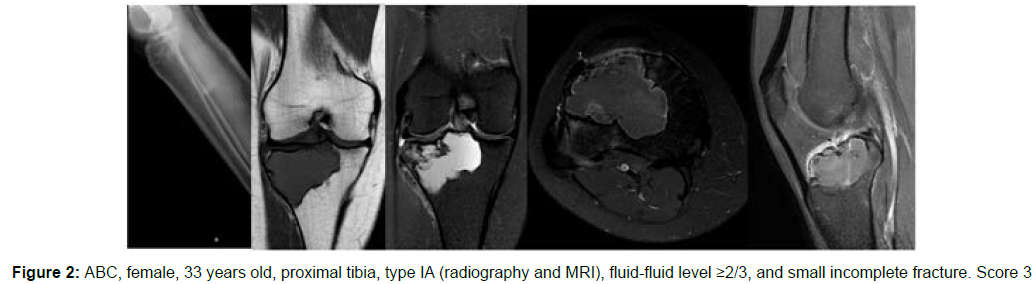
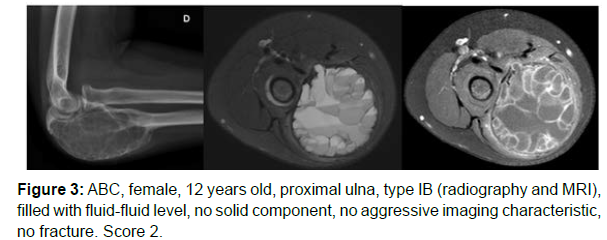
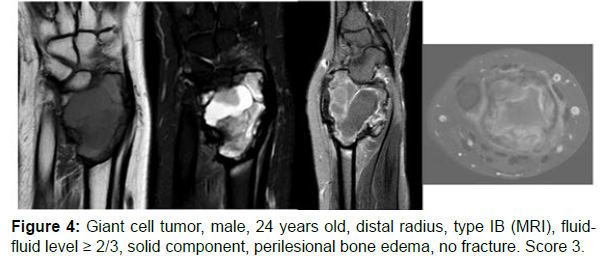
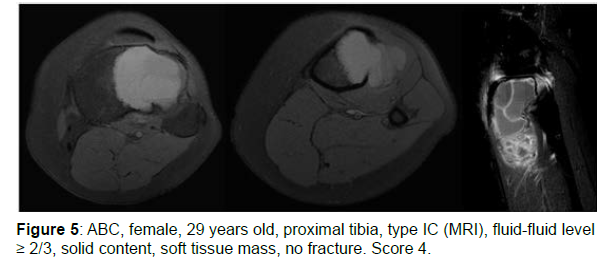
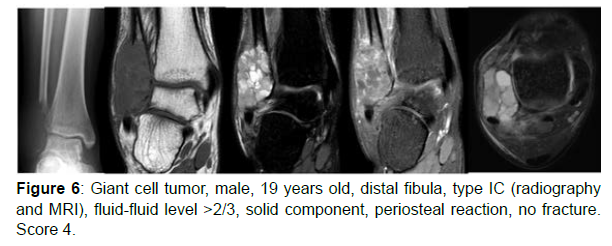
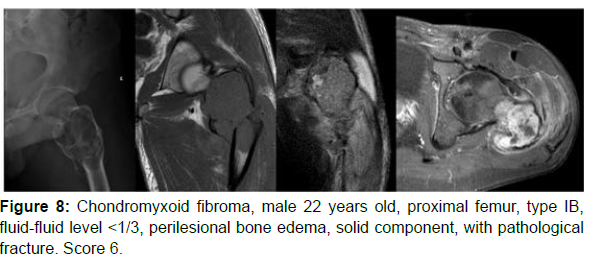
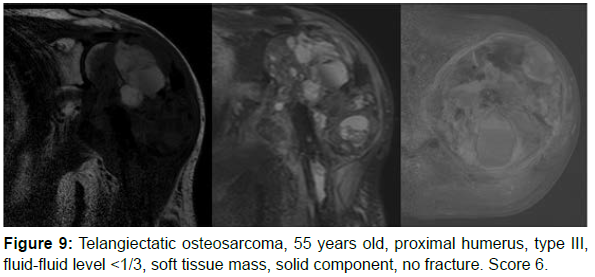
The decision algorithm was used to score all cases (Table 3). Primary ABC (n=10) presented six cases with score 3, three cases with score 2, and one case with score 4 (score of 2-4) (Figures 1-3). Secondary ABC presented three cases with score 4, two cases with score 6, one case with score 7, and one case with score 3 (score of 3-7) (Figures 4-8). Telangiectatic osteosarcoma presented two cases with score 6 (Figure 9). All cases of secondary ABC and telangiectatic osteosarcoma presented solid content, whereas in primary ABC cases this content was absent in six cases and present in four.
| Diagnosis | Age | Location | MRI Classification | Fluid-fluid level | Aggressiveness characteristics | Solid content | Fracture | Score |
|---|---|---|---|---|---|---|---|---|
| ABC | 13 | Tibia | IB | Complete | Present (perilesional bone edema) | Absent | Absent | 3 |
| ABC | 33 | Tibia | IA | ≥2/3 | Absent | Present | Present | 3 |
| ABC | 18 | Clavicle | IB | Complete | Present (perilesional bone edema) | Absent | Absent | 3 |
| ABC | 29 | Tibia | IC | ≥2/3 | Present (soft tissue mass) | Present | Absent | 4 |
| ABC | 8 | Spine | IC | Complete | Present (perilesional bone edema) | Absent | Absent | 3 |
| ABC | 48 | Humerus | IB | ≥2/3 | Present (perilesional bone edema) | Present | Absent | 3 |
| ABC | 12 | Ulna | IB | Complete | Absent | Absent | Absent | 2 |
| ABC | 11 | Tibia | IB | Complete | Absent | Absent | Absent | 2 |
| ABC | 15 | Spine | IB | Complete | Present (perilesional bone edema) | Absent | Absent | 2 |
| ABC | 12 | Fibula | IB | ≥2/3 | Absent | Present | Absent | 3 |
| GCT | 19 | Fibula | IC | ≥2/3 | Present (periosteal reaction) | Present | Absent | 4 |
| CMF | 22 | Femur | IB | ≤1/3 | Present (perilesional bone edema) | Present | Absent | 6 |
| GCT | 24 | Radio | IB | ≥2/3 | Present (perilesional bone edema) | Present | Absent | 3 |
| GCT | 21 | Tibia | IC | ≤1/3 | Present (soft tissue mass) | Present | Absent | 6 |
| GCT | 47 | Femur | IC | ≤1/3 | Present (perilesional bone edema) | Present | Present | 7 |
| CMF | 22 | Pelvis | IB | ≤1/3 | Present (perilesional bone edema) | Present | Absent | 4 |
| FD | 12 | Femur | IA | 1/3-2/3 | Absent | Present | Absent | 4 |
| OT | 69 | Humerus | III | ≤1/3 | Present (soft tissue mass) | Present | Absent | 6 |
Table 3: Decision algorithm of primary ABC, secondary ABC, and telangiectatic osteosarcoma cases.
The Mann-Whitney test (Table 4) showed that the decision algorithm score was significantly lower in patients who did not require biopsy and higher in patients who required biopsy (p<0.001). The primary ABC group presented a median of 3 and the secondary ABC and telangiectatic osteosarcoma groups presented a median of 6.
| Variable | Need for Biopsy | Total (N = 19) | p | |
|---|---|---|---|---|
| No (N = 10) | Yes (N = 9) | |||
| Score | <0.001 | |||
| median (min.; max.) | 3 (2; 4) | 6 (3; 7) | 3 (2; 7) | |
Table 4: Obtained score versus the need for biopsy.
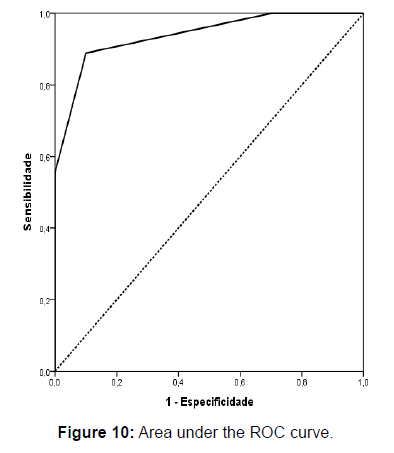
The area under the ROC curve (AUC) was 0.939 (Figure 10). The threshold that better determined the need for biopsy was 3.5, giving a sensitivity of 88.9%, specificity of 90%, PPV of 88.9%, and NPV of 90%., i.e., the tumors that did not require biopsy scored 0-3 (Table 5).
| AUC | CI (95%) | Threshold | Sens. (%) | Spec. (%) | PPV | NPV | |
|---|---|---|---|---|---|---|---|
| Lower | Upper | CI (95%) | CI (95%) | CI (95%) | CI (95%) | ||
| 0.939 | 0.829 | 1 | 3.5 | 88.9 | 90 | 88.9 | 90 |
| (51.8; 99.7) | (55.5; 99.7) | (51.8; 99.7) | (55.5; 99.7) | ||||
Table 5: Diagnostic measures of the threshold.
AUC: Area under the Curve; CI: Confidence Interval; Sens.: Sensitivity; Spec: Specificity; PPV: Positive Predictive Value; NPV: Negative Predictive Value
Discussion
Among all lesions with aneurysmal content, primary ABC is the most frequent [15], accounting for about 70% of cases [9]. Some similar tumors with aneurysmal content may be confused with primary ABC on the basis of information such as patient’s history, signs and symptoms, epidemiology, characteristics of imaging exams, laboratory tests, and anatomopathological exams [5, 8-9, 40]. Diagnostic errors and treatment failures have occurred and have also been reported in the literature [19,41]. Making the differential diagnosis between these tumors, especially with malignant tumors such as telangiectatic osteosarcoma, is an importante challenge [1,9], because telangiectatic osteosarcoma requires chemotherapy and has a worse prognosis [21].
Percutaneous or open biopsy is the procedure frequently performed to diagnose bone lesions with different content [13]. In cases of tumors with predominantly aneurysmal content, its indication is controversial [9], and there is no consensus on whether to perform preoperative biopsy or primary surgery.
Rajeswaran et al. [14] studied the accuracy of needle-guided biopsy results in 53 tumors with 100% aneurysmal content and reported: 36% of the lesions obtained diagnosis; of lesions that underwent surgical curettage, 96% obtained diagnosis; all lesions were benign and could be treated with primary curettage without the need for biopsy. Jelinek et al. [13] studied the results of percutaneous biopsy guided by CT of 110 bone tumors, of which 21 were predominantly cystic, and reported: a total of 9 patients needed to undergo open biopsy being 7 cystic lesions because they were not obtained by percutaneous biopsy; there were 13 tumors with incorrect diagnosis or no diagnosis, 6 of which were cystic lesions with aneurysmal content showing in these cases an accuracy of 71%, and 100% in sclerotic, and 89% in solid tumors. They reported that biopsy was infrequently performed in cystic tumors; the diagnosis of tumors with cystic content was a challenge for both percutaneous and open biopsy; the indication of biopsy of cystic tumors with aneurysmal content was a dilemma because it was difficult to obtain adequate tissue for pathology; and finally, biopsying predominantly cystic tumors, particularly with fluid-fluid levels, will probably not obtain diagnostic results. Zishan et al. [9] cited a high rate of error and diagnostic failure in needle-guided biopsies of primary ABC and telangiectatic osteosarcoma. In aneurysmal tumors in the spine, Singla et al. [10] reported that of 42 patients who underwent needle-guided biopsy, 38% of cases were undiagnosed, and that of these undiagnosed cases 72% were primary ABC. On the other hand, Hegde et al. [42] reported that there is reluctance of physicians to perform biopsy of primary ABC due to the diagnostic accuracy of the test; however, in their study of 73 patients with benign and malignant aneurysmal tumors, there was 93% accuracy and no complications. Deventer et al. [27] , through a literature review on the treatment of primary ABC, reported that biopsy was mandatory to confirm the diagnosis of these cases.
Biopsy is not a procedure without risks, since it is aggressive and generates increased cost not only to the patient but also to the health service. Jelinek et al. [13] reported that open biopsies result in delayed wound healing, increase cost 3-7 times, and have a 2-20% rate of complications like infection, hematoma, and nerve injury. Hegde et al. [42] reported that in biopsies in general, complications like local pain, superficial and deep infection, fractures, hematomas, intercompartmental contamination, and even neurovascular bundle injury can occur. Therefore, biopsy is a surgical procedure that should be well indicated, planned, performed, and if possible avoided.
Some surgeons, according to their experience and knowing the diagnostic difficulty of aneurysmal tumors, choose to operate on these tumors without preoperative biopsy. However, misdiagnosis in relation to telangiectatic osteosarcoma and its inadequate treatment can lead to catastrophic consequences.
Primary ABC, secondary ABC, and telangiectatic osteosarcoma are reported in the literature in different forms, locations, and with different degrees of aggressiveness and similar characteristics are described in all of them: presence of solid content, perilesional bone edema, soft tissue edema, extracortical mass, periosteal reaction, foci of calcification, and pathological fracture [5, 9, 19-20, 24, 33, 35, 37]. But the main characteristic that differentiates benign from malignant tumors is the proportion of fluid-fluid level and the presence of solid content, that is, the greater the proportion of fluid-fluid level volume the greater the possibility of benignity [15].
O’Donnel and Saifuddin [15] studying 83 tumors with aneurysmal content concluded that: 89% of the tumors with fluid-fluid level > 2/3 were benign; all tumors with 100% aneurysmal content were benign; the larger the volume of the solid component, the higher the possibility of a secondary lesion; and approximately 69% of the cases with aneurysmal content less than 1/3 were malignant. Rajeswaram et al. [14] studying tumors with 100% aneurysmal content reported that they were almost certainly benign and that the lesions could be treated with curettage without the need for biopsy. Zishan, et al. [9] studying 152 cases of primary ABC and 31 cases of telangiectatic osteosarcoma concluded: tumors with 100% aneurysmatic content with Lodwick criteria IA and IB [38] were primary ABC and did not need to be biopsied; IC, motheaten and permeative lesions with solid content should be biopsied; no telangiectatic osteosarcoma presented 100% aneurysmal content; 74% of cases with fluid-fluid level volume > 2/3 were primary ABC; and 95% of telangiectatic osteosarcomas presented aneurysmatic content less than 2/3. Regarding spinal tumors, Singla et al. [10] studied 42 patients with aneurysmal tumors and found that in children and adolescents who presented tumors with aneurysmal volume greater than 2/3, the tumors were probably benign, while in patients aged over 50 years with tumors with aneurysmatic content < 2/3, the tumors were probably malignant.
The literature is rich in criteria trying to differentiate malignant from benign aneurysmal tumors and citing the results and diagnostic failures of biopsied cases. Sasaki, et al. [1] created a flowchart to facilitate the differentiation between primary ABC, secondary ABC, and telangiectatic osteosarcoma: patients in the second decade of life with metaphyseal or metaepiphyseal lesion presenting intact cortical bone were found to have primary ABC. If these patients presented cortical bone destruction with soft tissue mass and normal serum alkaline phosphatase, they had GCT, while if the alkaline phosphatase was elevated they had telangiectatic osteossarcoma. Patients in the second decade of life with epiphyseal and diaphyseal lesions and patients in the third decade of life with diaphyseal or metaphyseal lesions associated with cortical bone destruction, soft tissue mass, and normal serum alkaline phosphatase probably had secondary ABC. Gao et al. [19] (2 studied 26 patients with telangiectatic osteosarcoma and reported: nine biopsied cases (35%) obtained an incorrect anatomopathological result of primary ABC, since a malignant tumor can be easily confused on imaging and anatomopathological examination with primary ABC; features such as aggressive growth pattern, mineralized matrix, and presence of thick and nodular septa around the cavities of fluidfluid levels were typical of telangiectatic osteosarcoma. Yin at al. [23] studied 51 cases of telangiectatic osteosarcoma in order to develop a model that would differentiate this tumor from primary ABC and reported: 11 patients (22%) were misdiagnosed with benign tumors on biopsy; 54 percutaneous biopsies and 5 open biopsies were required to obtain an initial preoperative diagnosis; features such as age below 18 years, pathological fracture, and increased serum levels of lactate dehydrogenase, alkaline phosphatase, white cells, and platelets can be used as a model for differential diagnosis. Murphey, et al. [20] analyzed CT and MRI of 40 telangiectatic osteosarcomas to distinguish them from primary ABC and reported some characteristics such as: wide hemorrhagic or necrotic spaces with fluid-fluid levels; thick nodular tissue around cavities that were well visualized on post-contrast examinations; presence of mineralized matrix and aggressive growth pattern with extra-cortical mass without pseudocapsule. The primary ABC presented the thickness of the periphery of the lesion and thin septa.
Through the literature review it was possible to identify characteristics that differentiate benign from malignant lesions. The benign tumors presented well-defined Lodwick type IA and IB lesions, aneurysmal content volume > 2/3, presence of thin septa, and absence of fractures, periosteal reaction, perilesional edema, soft tissue mass, and foci of calcification. The malignant lesions presented IC, motheaten, or permeative Lodwick characteristics, volume of aneurysmatic content < 1/3, presence of thick septa, and presence of fracture, periosteal reaction, perilesional edema, soft tissue mass, and foci of calcification.
Most of the literature on aneurysmal content tumors reports that these lesions should be biopsied to obtain the diagnosis, including primary ABC. In contrast, the study by Zishan et al. [9] was the first to demonstrate that some specific types of primary ABC (types IA and IB with 100% aneurysmal content) did not require biopsy for diagnostic confirmation, because they were certain to be primary ABC. The decision criteria organize in an algorithm the main characteristics of tumors with aneurysmal content, and through this score can guide the treatment of these tumors in a different way from those previously reported in the literature. The score, using data taken from MRI scans, distinguishes aneurysmal tumors that should be biopsied from those that can be operated on without biopsy.
The decision criteria score is from 0-8, and according to the statistical analysis the ideal cutoff point would be 3.5 with 93.9% of diagnostic accuracy and PPV and NPV close to 90%. Scores from 0-3 were observed in the imaging examinations to be of lesions with low local aggressiveness, mostly primary ABC; lesions with score 4 were of moderate aggressiveness, including primary and secondary ABC; and those with score 5-8 were the most aggressive, some secondary ABC and others telangiectatic osteosarcoma. The criteria separated the less aggressive tumors (up to score 3) from the more aggressive ones. Among the lesions that scored up to 3, we obtained type IA, IB, and IC lesions with fluid-fluid levels ≥2/3 or complete. All type IA and IB completely filled with fluid-fluid levels were primary ABC, corroborating with Zishan, et al. [9]. Tumors that scored 4 were benign lesions of intermediate aggressiveness, i.e., type IC lesions with higher aneurysmal content and type IA and IB lesions with aneurysmal content < 2/3. The tumors that scored above 5 were aggressive lesions type IC and III with aneurysmal content < 1/3. Criteria such as volume of aneurysmal content and type of bone destruction associated with other criteria such as age, location of the lesion, presence of fracture, and signs of aggressiveness assist in scoring and guide the medical team in making a decision regarding the need for biopsy.
The treatment of lesions with aneurysmal content is diverse. Deventer, et al. [8] reviewing the treatment of 74 cases of primary ABC comparing the 3 most common treatments (intralesional curettage, percutaneous infiltration with polidocanol, and en bloc resection) reported that: percutaneous infiltration was as effective as intralesional curettage, but there was a need for multiple infiltrations that might require curettage or en bloc resection; intralesional curettage was still the most common treatment; and the use of adjuvants such as phenol, electrocautery, hydrogen peroxide and polymethylmethacrylate helped to decrease lesion recurrence rates. Secondary ABCs are treated as the accompanying primary tumors and when they are less aggressive they are treated with intralesional curettage and use of adjuvants [5, 43-47], as this traditional procedure is a treatment option for aneurysmal lesions of low aggressiveness that do not require biopsy.
Conclusion
According to our findings, it is possible to operate on some tumors with aneurysmal content without preoperative biopsy using the decision algorithm, whose criteria were based on the characteristics requiring more attention. The algorithm scores from 0-8, separates lesions into lower and higher aggressiveness, indicates cases that require biopsy, and can be used by evaluating only the MRI scan. Tumors with score ≤ 3 can be operated without prior biopsy with curettage and adjuvant therapy. Lesions with other scores should be biopsied.
References
- Sasaki H, Nagano S, Shimada H, Yokouchi M, Setoguchi T, et al. (2017) Diagnosing and discriminating between primary and secondary aneurysmal bone cysts. Oncol Lett 13: 2290–6.
- Dios AM, Vergel De, Bond RJ, Shives CT, McLeod AR, et al.(1991) A clinicopathologic study of 238 cases. Cancer 69: 2921-2931
- Mankin JH, Hornicek JF, Eduardo Ortiz-Cruz, Villafuerte J, Gebhardt CM (2005) Aneurysmal bone cyst: A review of 150 patients. J Clin Oncol. 23:6756–6
- Kransdorf MJ, Sweet DE (1995) Aneurysmal bone cyst: Concept, controversy, clinical presentation, and imaging. Am J Roentgenol 164:573-80.
- Gutierrez BL, Link MT, Horvai EA, Joseph BG, O’Donnell JR, et al. (2020) Secondary aneurysmal bone cysts and associated primary lesions: imaging features of 49 cases. Clin Imaging 62: 23–32
- Martinez V, Sissons HA (1988) Aneurysmal bone cyst. A review of 123 cases including primary lesions and those secondary to other bone pathology. Cancer. 61:2291–304
- Jaffe HL, Lichtenstein L (1942) Solitary unicameral bone cyst: Whith emphasis on roentgen picture, the patologic appearence and the pathogenesis. Arch Surg 44: 1004-25.
- Deventer N, Schulze M, Gosheger G, de Vaal M, Deventer N (2021) Primary aneurysmal bone cyst and its recent treatment options: A comparative review of 74 cases . Cancers 13:1–12
- Zishan US, Pressney I, Khoo M, Saifuddin A (2020) The differentiation between aneurysmal bone cyst and telangiectatic osteosarcoma: a clinical, radiographic and MRI study. Skeletal Radiol 49:1375–86.
- Singla N, Junaid SE, Siddiqui M, Malhotra K, Saifuddin A (2020) An assessment of fluid–fluid levels on magnetic resonance imaging of spinal tumours. Skeletal Radiol 50: 771-780
- Hudson TM (1984) Fluid Levels in Aneurysmal Bone Cysts : A CT Feature. AJR 142:1001–4.
- Huvos AG, Rosen G, Bretsky SS, Butler A (1982) Telangiectatic Osteogenic Sarcoma: A Clinicopathologic Study of 124 Patients. Cancer 49: 1679–89.
- Jelinek JS, Murphey MD, Welker JA, Henshaw RM, Kransdorf MJ, et al. (2002) Diagnosis of primary bone tumors with image-guided percutaneous biopsy: Experience with 110 tumors. Radiology 223: 731–7.
- Rajeswaran G, Malik Q, Saifuddin A (2013) The role of needle biopsy for focal bone lesions with complete fluid-fluid levels on magnetic resonance imaging. Skeletal Radiol. 42: 765–9.
- O’Donnell P, Saifuddin A (2004) The prevalence and diagnostic significance of fluid-fluid levels in focal lesions of bone. Skeletal Radiol. 33: 330–6.
- Costelloe CM, Madewell JE (2013) Radiography in the initial diagnosis of primary bone tumors. Am J Roentgenol. 200: 3–7.
- Gillentine MA, Berry LN, Goin-Kochel RP, Ali MA, Ge J, et al. (2017) Update on Survival in Osteosarcoma. Futur Oncol 13:1–12
- Bluemke DA (1998) Differentiation of benign from malignant musculoskeletal lesions using MR imaging: pitfalls in MR evaluation of lesions with a cystic appearance. Image 170: 1251–8.
- Gao ZH, Yin JQ, Liu DW, Meng QF, Li JP (2013) Preoperative easily misdiagnosed telangiectatic osteosarcoma: Clinical-radiologic-pathologic correlations. Cancer Imaging. 13: 520–6.
- Murphey MD, Wan Jaovisidha S, Temple HT, Gannon FH, Jelinek JS, et al. (2003) Telangiectatic Osteosarcoma: Radiologic-Pathologic Comparison. Radiology. 229: 545–53.
- Liu JJ, Liu S, Wang JG, Zhu W, Hua YQ, et al. (2013) Telangiectatic osteosarcoma: A review of literature. Onco Targets Ther 6: 593–602.
- Mascard E, Gomez-Brouchet A, Lambot K (2015) Bone cysts: Unicameral and aneurysmal bone cyst. Orthop Traumatol Surg Res 101: S119–27.
- Yin JQ, Fu YW, Xie XB, Cheng XY, Yang XY, et al. (2018) Telangiectatic osteosarcoma: Outcome analyses and a diagnostic model for differentiation from aneurysmal bone cyst. J Bone Oncol 11:10–6.
- Sangle NA, Layfield LJ (2012) Telangiectatic osteosarcoma. Arch Pathol Lab Med. 136: 572–6.
- Grahneis F, Klein A, Baur-Melnyk A, Knösel T, Birkenmaier C, et al. (2019) Aneurysmal bone cyst: A review of 65 patients. J Bone Oncol 18: 1–6.
- Baumhoer D, Amary F, Flanagan AM (2019) An update of molecular pathology of bone tumors. Lessons learned from investigating samples by next generation sequencing. Genes Chromosom. Cancer 58: 88–99.
- Deventer N, Deventer N, Gosheger G, de Vaal M, Vogt B, et al. (2021) Current strategies for the treatment of solitary and aneurysmal bone cysts: A review of the literature. J Bone Oncol 30:100384.
- Camargo OP, Croci AT, Oliveira NRB, Oliviera CRGMC, Etchebehere M, et al. (1997) Cisto ósseo aneurismático: Análise retorospectiva de 98 casos tratados no IOT-HC-FMusp de 1950 a 1997. Rev Bras Ortop 32:1–6.
- Munk P, Helrns C, Holt R, Johnston J, Steinbach L, et al. (1989) Imaging of Aneurysmal Cysts. Radiology. 153: 99–101
- Mahnken AH, Nolte-Ernsting CCA, Wildberger JE, Heussen N, Adam G, Wirtz DC, et al. (2003) Aneurysmal bone cyst: Value of MR imaging and conventional radiography. Eur Radiol 13:1118–24.
- Capanna R, Bettelli G, Biagini R, Ruggieri P, Bertoni F,et al. (1985) Aneurysmal Cysts of Long Bones. Ital J Orthop Traumatol 11: 409–17.
- Dabska M, Buraczewski J (1969) Aneurysmal bone cyst. Pathology, clinical course and radiologic appearances. Cancer 23: 371–89.
- Angelini A, Mavrogenis AF, Trovarelli G, Ferrari S, Picci P,et al (2016) Telangiectatic osteosarcoma: a review of 87 cases. J Cancer Res Clin Oncol 142: 2197–207.
- Discepola F, Powell TI, Nahal A (2009) Best cases from the AFIP: Telangiectatic osteosarcoma: Radiologic and pathologic findings. Radiographics 29: 380–3.
- Vanel D, Tcheng S, Contesso G, Zafrani B, Kalifa C, et al. (1987) The radiological appearances of telangiectatic osteosarcoma. Skeletal Radiol 16: 196–200.
- Limaiem F, Khaddour K (2021) Telangiectatic Osteosarcoma. In: Stat Pearls. Treasure Island (FL): StatPearls Publishing.
- Weiss A, Khoury JD, Hoffer FA, Wu J, Billups CA, et al (2007) Telangiectatic osteosarcoma: The St. Jude Children’s Research Hospital’s experience. Cancer 109: 1627–37.
- Lodwick GS, Wilson AJ, Farrell C, Virtama P, Dittrich F (1980) Determining growth rates of focal lesions of bone from radiographs. Radiology 134: 577–83.
- Costelloe CM, Madewell JE (2016) Clinical considerations and imaging of bone tumors. In: Czerniak B. Dorfman and Czerniak's bone tumor.
- Kaufman RA, Towbin RB (1981) Telangiectatic osteosarcoma simulating the appearance of an aneurysmal bone cyst. Pediatr Radiol 11: 102–4.
- Saito T, Oda Y, Kawaguchi KI, Tanaka K, Matsuda S, et al. (2005) Five-year evolution of a telangiectatic osteosarcoma initially managed as an aneurysmal bone cyst. Skeletal Radiol 34: 290–4.
- Hegde V, Burke ZDC, Park HY, Zoller SD, Johansen D, et al. (2018) Is core needle biopsy reliable in differentiating between aggressive benign and malignant radiolucent bone tumors?. Clin Orthop Relat Res 476: 568–77.
- Zheng J, Niu N, Shi J, Zhang X, Zhu X, et al. (2021) Chondroblastoma of the patella with secondary aneurysmal bone cyst, an easily misdiagnosed bone tumor: a case report with literature review .BMC Musculoskelet Disord 22: 1–8.
- Marudanayagam A, Gnanadoss JJ (2006) Secondary aneurysmal bone cyst of the patella: a case report. Iowa Orthop J 26:144–6.
- Reda B (2018) Cystic bone tumors of the foot and ankle. J Surg Oncol 117: 1786–98.
- Chen W, DiFrancesco LM (2017) Chondroblastoma an update. Arch Pathol Lab Med 141: 867–71.
- Angelini A, Varela-Osorio AF, Trovarelli G, Berizzi A, Zanotti G,et al. Osteoblastoma of the elbow: analysis of 13 patients and literature review. Eur J Orthop Surg Traumatol 27: 787–95.
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar CrossRef
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Pubmed
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Citation: Voltan K , Paiva DN, Hanasilo CEH, Venancio AFF, Urquiza FF, et al. (2022) Bone Tumors with Aneurysmal Content: Decision Algorithm in Primary Surgical Treatment. J Orthop Oncol 8: 158. DOI: 10.35248/2472-016X.1000163
Copyright: © 2022 Voltan K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 7345
- [From(publication date): 0-2022 - Dec 14, 2025]
- Breakdown by view type
- HTML page views: 6593
- PDF downloads: 752