Capsule Endoscopy in the Detection of Non-Small Bowel Lesions Missed By Endoscopy
DOI: 10.4172/2161-069X.1000651
Abstract
Background and aims: Capsule endoscopy (CE) is indicated in cases with obscure gastrointestinal bleeding (OGIB). However, lesions detected by CE are frequently within the reach of conventional Upper or lower GI endoscopy. We evaluated the accuracy of CE in the study of OGIB, examining the incidence of CE detected non-small bowel lesions (NSBL) missed by conventional endoscopy and studying its impact on patient management.
Methods: We retrospectively analyzed 2010 CE procedures performed in a tertiary care center (IBD-Unit Referral Centre in Bologna), comparing the findings on CE to those on prior colonoscopy and upper GI endoscopy performed within 3 months and two weeks of the CE procedure, respectively. We evaluated the impact of CE findings on patient management.
Results: CE revealed abnormal findings in 1608 out of 2010 patients. Previously missed NSBLs were revealed on CE in 283 cases. Of these, 265 pre-CE endoscopic reports were found to not conform to reporting guidelines. NSBLs on CE led to management changes in 271 patients.
Conclusion: This study confirms the utility of CE in patients with OGIB. However, in a considerable number of cases, CE identified lesions missed by conventional endoscopy, suggesting that a second look prior to CE may be appropriate in some patients.
Keywords: Capsule endoscopy; Obscure gastrointestinal bleeding; Polyethylene glycol
Introduction
The European Society of Gastrointestinal Endoscopy (ESGE), the American Gastroenterological Association (AGA), and the American College of Gastroenterology (ACG) recommend that first line evaluation of the small bowel (SB) be performed using small bowel capsule endoscopy (CE) following upper and lower GI endoscopy, though in some scenarios a second look endoscopy may be of use [1-3]. In addition, recent guidelines on the management of iron deficiency anemia (IDA) from the British Society of Gastroenterology (BSG) suggest that examination of the small bowel be performed in cases involving symptoms indicating SB disease or with unsatisfactory response to iron replacement therapy [4]. Evaluation of the small bowel can also be performed by cross sectional imaging or enteroscopy, yet CE has a relatively high diagnostic yield and the benefit of being a minimally invasive endoscopic technique.
Frequently, lesions detected on CE are located in areas of the GI tract that are within the reach of a conventional endoscope. This suggests that in some cases a second look upper or lower GI endoscopy may be indicated. Moreover, CE has been reported to reveal non-small bowel lesions (NSBLs) missed upon prior conventional endoscopy with a detection rate ranging from 3.5% to over 30% [5-6].
Investigation by CE reportedly leads to the identification of a culprit lesion in the small bowel in roughly two thirds of patients with obscure gastrointestinal bleeding (OGIB) [6-13]. The entire small bowel may be evaluated by CE in up to 90% of patients, and CE has a diagnostic yield of 38% to 83% in cases of suspected small bowel bleeding [14]. The positive and negative predictive values of CE in the evaluation of GI bleeding are high (94%-97% and 83%-100%, respectively), and findings on CE lead to changes in patient management in 37% to 87% of cases [15-17]. The primary limits of CE include low specificity and a false negative rate of 10% to 36%, along with lack of visualization of the major duodenal papilla in a substantial number of patients, potentially leading to lower accuracy in the identification of duodenal lesions [18- 22].
The current study evaluated the diagnostic yield of CE in the investigation of OGIB and analyzed the incidence of CE-detected NSBLs that were missed at conventional endoscopy and its impact on patient management in a monocenter case series.
Materials and Methods
This single center retrospective study analyzed data from patients undergoing CE in the IBD Unit Referral Center in Bologna between February 2003 and February 2020. Procedures in which CE failed to reach the cecum before battery life expiration were considered as incomplete and were excluded from the study.
We analyzed patient demographics, indications for CE, procedural data such as gastric and small bowel transit time, findings of the examination, data regarding pre-CE upper and lower endoscopy, diagnosis and management pre and post CE, and patient outcome [23]. NSBLs were defined as any abnormal CE findings detected in the stomach, proximal SB, terminal ileum and colon.
CE procedure
After obtaining informed consent, all patients were given standardized instructions. Patients were instructed to stop iron supplementation seven days before the procedure and to start a diet low in fiber three days prior to CE, followed by a fasting period starting at midnight before the procedure. All patients received 4 L of polyethylene glycol (PEG) as bowel preparation, administered as 3 L the evening before the procedure and 1 L the morning of the procedure. The capsule (Pillcam SB2 and 3; Medtronic) was swallowed in the morning. Patients were allowed to drink liquids after three hours and to consume a light meal after five hours.
All CE procedures were reviewed by two gastroenterologists experienced in capsule endoscopy (CC, DG). Discrepancies in findings were discussed in order to reach a consensus on the final diagnosis. Findings on CE were documented and categorized using standard terminology.
Diagnostic yield was calculated as the number of procedures in which clinically significant lesions were identified divided by the total number of procedures performed.
Definitions
• Endoscopy pre-CE: only colonoscopy and upper GI endoscopy (UGIE) performed within three months and two weeks before the CE procedure, respectively, was considered.
• Additional findings: gastric lesions (GL) and colonic lesions (CL) differing from those identified on previous endoscopy.
• New findings: novel GL and CL not detected by previous endoscopy (i.e., patients with negative findings on previous endoscopy).
• Clinical impact: percentage of patients in which CE findings led to a diagnostic change.
• Therapeutic impact: percentage of patients in which CE findings led to change in treatment.
Evaluation of previous endoscopic report
Previous UGIE and colonoscopy reports were evaluated to ascertain their conformity to endoscopy reporting guidelines as a surrogate marker of the quality of the endoscopic examination itself.
Statistical analysis
Quantitative data following a normal distribution are presented as mean, standard deviation (SD) and range. Non-normally distributed quantitative data are presented as median and interquartile range. Qualitative data are presented as proportions, and comparison of qualitative data was performed using the Chi squared test. P<0.05 were considered to be statistically significant. All statistical analysis was performed using IBM SPSS Statistics, version 20.0 (IBM Corp., Armonk, N.Y., USA).
Results
Patient demographics
During the study period, 2271 CE procedures were performed in 2242 patients.
Two CE procedures were excluded from analysis due to technical issues, and CE was considered incomplete in 230 cases (10.27%). As a result, 2010 procedures were included in the analysis; 1201 patients were male (58.75%) and the mean age was 61.5 ± 19.6 years (range 22 years-94 years).
CE revealed normal findings in 402 patients (20%), whereas abnormalities were found in 1608 patients (80%).
Abnormalities revealed were: angiodysplasias, 39.5% (n=620); erosions/ulcers, 53.5% (n=838); tumors, 4% (n=62); and active bleeding, 3% (n=48).
1685 patients (83.8%) underwent the CE procedure within 10 days following first level conventional endoscopic examination; 325 patients (16.2%) underwent the procedure between 8 and 28 days following first level endoscopy. CE was performed the same day as colonoscopy in 32 patients (1.6%). Mean lag time between first level endoscopy and CE was 7.8 days.
The mean small bowel transit time was 4 hours and 51 minutes (range 18 minutes 15 hours, 33 minutes). Small bowel cleansing was good in 1507 patients (75%), adequate in 262 patients (13%) and poor in 241 patients (12%) (Table 1).
| Mean age | 61.5 ± 19.6 |
| Years (range, year) | 22-94 |
| Gender n (%) | Male 1201 (58.75) |
| Female 809 (41.25) | |
| Lesions at CE (%) | 1608 (80) |
| No lesions at CE | 402 (20) |
| Lesion(s) | |
| Vascular | 660 (41) |
| Ulcers/erosions | 838 (52) |
| Tumors | 62 (4) |
| Active bleeding whiteout evidence of lesions | 48 (3) |
| NSBL detection (%) | 283 (15.8) |
| Vascular | 140 (49.5) |
| Ulcers/erosions | 102 (36.0) |
| Tumors | 22 (7.8) |
| Active bleeding | 19 (6.7) |
| Site of NSBL | |
| Stomach | 49 (17.3) |
| Duodenum | 89 (31.4) |
| Terminal ileum | 17 (6) |
| Colon | 128 (45.3) |
Table 1: Demographic characteristics of study population.
Non-Small Bowel lesions
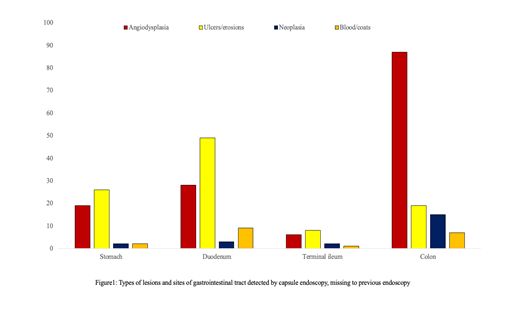
CE detected 49 gastric lesions and 89 duodenal lesions that were missed at previous gastroscopy. In particular, gastric lesions included gastric erosions in 43% (n=21) of cases, vascular lesions in 39% (n=19), gastric ulcers in 10% (n=5), active bleeding in 4% (n=2) and gastric tumors in 4% (n=2) of cases.
Duodenal lesions (DL) were duodenal erosions/ulcers in 55% (n=49) of cases, vascular lesions in 31.5% (n=28), tumors in 3.3% (n=3) and active bleeding in 10.2% (n=9) of cases.
CE detected 17 lesions in the terminal ileum and 128 lesions in the colon that were missed at previous colonoscopy.
These CL were: 64.1% vascular lesions (n=93), 18.6% ileocolonic ulcers (n=27), 11.8% tumors (n=17), 5.5% active bleeding (n=8).
Fifteen patients presented lesions in both the stomach or duodenal bulb and the small bowel, and twelve patients presented lesions both in the small bowel and the colon.
Overall, CE detected NSBLs not seen during pre-CE endoscopy in 283 patients (14.1%) (Figure 1).
The types of lesions identified in the gastrointestinal tract on CE are shown in (Figure 2).
Clinical and therapeutic impact
NSBL findings on CE led to a change in diagnosis in 271 patients, resulting in a clinical impact of 13.5%. A second endoscopy was required in 279 patients (143 colonoscopy, 136 gastroscopy).
The treatment of choice was pharmacological therapy in 252 patients, therapeutic endoscopy in 99 patients and surgery in 15 patients. 165 patients received iron supplements and 84 coagulation for vascular lesions (angiodysplasias).
We reviewed all endoscopic reports for patients in which discrepancies with CE were found. In particular, of 283 NSBL detected at CE, 265 (95.6%) pre-CE endoscopic reports (110 colonoscopy and 155 gastroscopy) did not conform to reporting guidelines [24-25]. Overall, 13.2% of endoscopy reports were found to not conform to reporting guidelines [26].
Discussion
In keeping with previous reports, our study corroborates the high diagnostic yield and safety of CE in the examination of the GI tract [27-28]. CE demonstrated a diagnostic yield of 80% in our study population and identified clinically significant NSBLs not seen by conventional endoscopy in 16% of cases. Approximately 49% and 51% of lesions were found in the upper and lower GI tract, respectively.
The difference in NSLBs found on CE with respect to other studies could be due to shorter time between endoscopy and CE as a general protocol adopted by our center. In fact, in our protocol we accepted only colonoscopy and UGIE performed three months and two weeks before the CE procedure, respectively.
Several studies have reported the ability of CE to detect lesions missed on upper and lower GI endoscopy [5, 22-24,26-28]. It is unclear why these lesions are missed during initial conventional endoscopy, although possible explanations have been hypothesized. For example, some lesions may have characteristics that make them more difficult to identify, such as size or atypical location. In addition, factors relating to the endoscopic procedures themselves such as quality of the exploration, rate of exam completion or endoscopist experience may play a role [26]. Indeed, digestive endoscopy is an operator dependent procedure, and operator experience is an important factor in the detection of small lesions. Patient intolerance of the endoscopic procedure may also compromise the outcome of the examination. However, time of withdrawal is not always described in the endoscopy reports. In a significant portion of patients, endoscopy reports reported that the examination was not completed due to intolerance of the patient or the presence of solid feces, or quality of the endoscopic report is poor. Furthermore, the terminal ileum was not always explored for a number of reasons, including poor bowel cleansing, patient intolerance, or operator dependency of the examination. In addition, excessive air insufflation may also cause NSBLs to be missed as it causes flat lesions to be harder to detect, and the hypotensive effects of sedative drugs may conceal angiodysplasias.
As upper and lower GI lesions are typically detectable using traditional endoscopy, in some cases an endoscopic second look may be warranted before proceeding with CE. Previous studies have reported the diagnostic yield of a second look as 35% to 75% for the upper GI tract and 6% for the lower GI tract [5,26-28]. Although there is currently a lack of a strong recommendation, we believe that the high percentage of NSBLs suggests that a second look endoscopy is of value in patients with unreliable first level exams, particularly in patients with persistent bleeding. Furthermore, in the event of poor intestinal preparation, patient intolerance, or incomplete examinations, first level procedures should be repeated before proceeding to CE examination.
One of the main limitations of the current study is its retrospective design, and a prospective follow up is necessary in order to confirm that the detected lesions are not incidental findings. Moreover, in the absence of a clear marker of endoscopy quality, we selected the conformity of the endoscopy reports to reporting guidelines as a surrogate, as the quality of the endoscopic report is directly linked to the quality of the endoscopic examination itself.
Conclusion
Our study confirms the importance of capsule endoscopy in the diagnostic work up of obscure gastrointestinal bleeding. However, the study reveals that in a considerable number of patients, capsule endoscopy identifies lesions within the reach of conventional endoscopic techniques. This suggests that capsule endoscopy may be unnecessary in some cases, in particular when first level endoscopy is unreliable. The strengthening of first level endoscopic procedures and the judicious use of conventional endoscopy as a second look could lead to a larger percentage of early diagnoses, with a substantial economic impact.
Acknowledgment
None of the authors have a financial relationship with a commercial entity that produces relevant healthcare products and/or services relevant to this article.
Ethics
The study protocol was approved by the Comitato Etico Indipendente dell’AOU di Bologna (n°173/2017/O/OssN). Informed consent was obtained from all patients.
References
- Pennazio M, Spada C, Eliakim R (2015) Small-bowel capsule endoscopy and device assisted enteroscopy for diagnosis and treatment of small bowel disorders: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy 47:352-376.
- Raju GS, Gerson L, Das A, Lewis B, Association AG (2007) American Gastroenterological Association (AGA) Institute technical review on obscure gastrointestinal bleeding. Gastroenterology 33:1697-1717.
- Gerson LB, Fidler JL, Cave DR, Leighton JA (2015) ACG clinical guideline: Diagnosis and management of small bowel bleeding. Am J Gastroenterol 110:1265-1287.
- Goddard AF, James MW, McIntyre AS, Scott BB (2011) British society of gastroenterology. Guidelines for the management of iron deficiency anaemia. Gut 60:1309-1316.
- Riccioni ME, Urgesi R, Cianci R, Marmo C, Galasso D, et al. (2014) Obscure recurrent gastrointestinal bleeding: a revealed mystery? Scand J Gastroenterol 49:1020-1026.
- Spiller RC, Parkins RA (1983) recurrent gastrointestinal bleeding of obscure origin: Report of 17 cases and a guide to logical management. Br J Surg 70:489-93.
- Scapa E, Jacob H, Lewkowicz S (2002) Initial experience of wireless capsule endoscopy for evaluating occult gastrointestinal bleeding and suspected small bowel pathology. Am J Gastroenterol 97:2776-9.
- Costamagna G, Shah SK, Riccioni ME (2002) A prospective trial comparing small bowel radiographs and video capsule endoscopy for suspected small bowel disease. Gastroenterology 123:999-1005.
- Lewis BS, Swain P (2002) Capsule endoscopy in the evaluation of patients with suspected small intestinal bleeding: Results of a pilot study. Gastrointest Endosc 56:349-53.
- Ell C, Remke S, May A, Helou L, Henrich R, et al. (2002) The first prospective controlled trial comparing wireless capsule endoscopy with push enteroscopy in chronic gastrointestinal bleeding. Endoscopy 34:685-9.
- Mylonaki M, Fritscher-Ravens A, Swain P (2003) Wireless capsule endoscopy: A comparison with push enteroscopy in patients with gastroscopy and colonoscopy negative gastrointestinal bleeding. Gut 52:1122-6.
- Chong AK, Taylor AC, Miller AM, Desmond PV (2003) Initial experience with capsule endoscopy at a major referral hospital. Med J Aust 178:537-40.
- Selby W (2004) Can clinical features predict the likelihood of finding abnormalities when using capsule endoscopy in patients with GI bleeding of obscure origin? Gastrointest Endosc 59:782-7.
- Adler DG, Knipschield M, Gostout C (2004) A prospective comparison of capsule endoscopy and push enteroscopy in patients with GI bleeding of obscure origin. Gastrointest Endosc 59:492-8.
- Rondonotti E, Villa F, Mulder CJ, Jacobs MA, de Franchis R (2007) Small bowel capsule endoscopy in 2007: indications, risks and limitations. World J Gastroenterol 13:6140-9.
- Delvaux M, Fassler I, Gay G (2004) Clinical usefulness of the endoscopic video capsule as the initial intestinal investigation in patients with obscure digestive bleeding: Validation of a diagnostic strategy based on the patient outcome after 12 months. Endoscopy 36:1067-73.
- Pennazio M, Santucci R, Rondonotti E, Abbiati C, Beccari G, et al. (2004) Outcome of patients with obscure gastrointestinal bleeding after capsule endoscopy: Report of 100 consecutive cases. Gastroenterology 126:643-53.
- Ben Soussan E, Antonietti M, Hervé S, Savoye G, Ramirez S, et al. (2004) Diagnostic yield and therapeutic implications of capsule endoscopy in obscure gastrointestinal bleeding. Gastroenterol Clin Biol 28:1068-73.
- Goldstein JL, Eisen GM, Lewis B, Gralnek IM, Zlotnick S, et al. (2005) Video capsule endoscopy to prospectively assess small bowel injury with celecoxib, naproxen plus omeprazole, and placebo. Clin Gastroenterol Hepatol 3:133-41.
- Lewis BS, Eisen GM, Friedman S (2005) A pooled analysis to evaluate results of capsule endoscopy trials. Endoscopy 37:960-5.
- Appleyard M, Fireman Z, Glukhovsky A (2000) A randomized trial comparing wireless capsule endoscopy with push enteroscopy for the detection of small bowel lesions. Gastroenterology 119:1431-8.
- Kong H, Kim YS, Hyun JJ (2006) Limited ability of capsule endoscopy to detect normally positioned duodenal papilla. Gastrointest Endosc 64:538-41.
- Clarke JO, Giday SA, Magno P, Shin EJ, Buscaglia JM, et al. (2008) How good is capsule endoscopy for detection of periampullary lesions? Results of a tertiary-referral center. Gastrointest Endosc 68:267-72.
- Beg S, Ragunath K, Wyman A (2017) Quality standards in upper gastrointestinal endoscopy: A position statement of the British Society of Gastroenterology (BSG) and Association of Upper Gastrointestinal Surgeons of Great Britain and Ireland (AUGIS). Gut 66:1886-99.
- Valori R, Cortas G, de Lange T (2019) Performance measures for endoscopy services: A European Society of Gastrointestinal Endoscopy (ESGE) quality improvement initiative. United European Gastroenterol J 7:21-44.
- Juanmartiñena Fernández JF, Fernández-Urien Sainz I, Zabalza Ollo B (2018) Gastroduodenal lesions detected during small bowel capsule endoscopy: Incidence, diagnostic and therapeutic impact. Rev Esp Enferm Dig 110:102-8.
- Innocenti T, Dragoni G, Roselli J, Macrì G, Mello T, et al (2020) Non-small-bowel lesions identification by capsule endoscopy: A single centre retrospective study. Clin Res Hepatol Gastroenterol 45:30083-8.
- Kitiyakara T, Selby W (2005) Non-small-bowel lesions detected by capsule endoscopy in patients with obscure GI bleeding. Gastrointest Endosc 62:234-8.
Select your language of interest to view the total content in your interested language