Carbamazepine Causes Fetal Intrauterine Growth Restriction (IUGR) in Rats
Editor assigned: 01-Jan-1970 / Reviewed: 01-Jan-1970 / Revised: 01-Jan-1970 /
Abstract
Purpose: Carbamazepine (CBZ) is widely used in the treatment of trigeminal neuralgia, affective disorders, and mainly as an anticonvulsant, specially by fertile women, due to their need to continuously use CBZ during pregnancy and the lactation period. CBZ crosses the placenta barrier and may impair pregnancy and the embryonic development. The aim of this study was to determine the effect of CBZ on maternal reproductive outcome, besides fetal growth and development in Wistar rats.
Methods: Rat dams received CBZ (20 mg/Kg/day) or propylene glycol (vehicle) via intraperitoneal (i.p.) injection throughout the gestational period. On the 19th day of gestation, the ovary and uterine contents were examined, and the placenta and fetuses were analyzed.
Results: The CBZ exposure during pregnancy caused a reduction in fetal weight, fetal weight classification, and crown-rump distance. CBZ also decreased the implantation index, average number of corpora lutea, fetal weights and crown-rump length and increased the pre and post-implantation loss rate. The CBZ-exposed fetus also presented external congenital malformations.
Conclusion: The results suggest that maternal exposure to CBZ interfered on several maternal reproductive outcomes and can cause severe fetal intrauterine growth restriction (IUGR)
Keywords
Carbamazepine; Fetus; Intrauterine growth restriction; Pregnancy; Rat; Reproductive outcome
Introduction
The gestational period is accompanied by several physiological and anatomical changes, including hormonal oscillations, weight gain, increased plasma volume and decreased plasma proteins, These changes have significant consequences on the pharmacokinetic and pharmacodynamic properties of different therapeutic agents. Increased bioavailability and/or hepatic metabolism induction of medicinal products may occur. Some pregnant women have pre-existing medical conditions, that can compromise fetal development, either by the symptoms themselves or by exposure to potentially teratogenic drugs used in their treatment [1].
Epilepsy is one of the most common neurological disorders that requires continuous treatment during pregnancy. Epileptic women have a 33% risk of increased seizures and two times increase risk of hemorrhage, eclampsia, abruptio placentae, premature labor, miscarriage, stillbirth, developmental delay and major malformations. Antiepileptic drugs (AEDs) used as a monotherapy reduces the risk of adverse outcomes [2,3]. The occurrence of an epileptic seizure in pregnant women may cause depression of the fetal heart rate and hypoxia [4,5]. Pharmacological intervention in the treatment of epilepsy should be continued throughout the gestational period to reduce the risk of convulsions that are harmful to both mother and fetus.
Carbamazepine (CBZ) is an AED and mood-stabilizing drug used in the treatment of epilepsy, bipolar disorder, trigeminal neuralgia, and chronical pain disorder. This drug is widely used by women of childbearing age with epilepsy and it is considered less toxic than, phenobarbital, phenytoin, primidone or valproic acid [6].
CBZ is a lipid-soluble compound that can easily cross the placenta barrier and other biological membranes [7,8]. However, there is some controversy concerning the teratogenic effects of CBZ, various clinical studies reported malformations such as craniofacial defects, heart defects, and neural tube defects, as well as growth retardation and developmental delay. Adherence of extra-embryonic membranes and circulatory changes in the yolk sac were also observed after in vitro exposure to CBZ [8,9]. In addition, epidemiological studies show a higher incidence of spontaneous abortions in pregnant women taking CBZ [10].
In fact, several clinical studies reported the occurrence of congenital malformations after maternal exposure to CBZ. However, few experimental studies have been conducted in order to evaluate maternal reproductive parameters and detailed analysis of the CBZ effects on the embryofetal development. The utilization and comparison between different species used as experimental models is fundamental for a better understanding of the phenomena involved embryonic and postnatal development, under different conditions. In this context, however, the rat is a useful model, since there is strong evidence that, among rodents, this species is most closely related to man [11].
Thus, the purpose of this study was to evaluate reproductive performance and embryofetal development in pregnant rats treated with CBZ throughout pregnancy.
Materials and Methods
Animals and experimental design
Adult female (n=25) and male (n=13) Wistar rats were housed in polypropylene cages (40 cm × 30 cm × 15 cm) filled with a layer of autoclaved white pine shavings, under controlled conditions: hygiene,photoperiod (12 h light/dark cycle), humidity (60%) and temperature (22-23°C). They had free access to tap water and commercial lab chow (Nuvilab, Nuvital Nutrientes). The females were mated overnight with males (two females per male); every morning, the males were separated from females and vaginal smears of each female were examined. Sperm presence in vaginal wet smears was defined as the first day of gestation (DG 1) [12]. The pregnant rats were housed individually, weighed daily and carefully monitored twice a day (morning and afternoon) for clinical signs of toxicity and possible gestational disorders.
Rat dams received CBZ (C-8981, Sigma Chemical Co., St. Louis, MO; 99.5% purity, 20 mg/Kg/day; CBZ group-n=16) or propylene glycol (vehicle of CBZ; 99.8% purity; density 1.034 g/mL; 1 g/kg b.w.; Control group-C group-n=9), by via intraperitoneal (i.p.) injection, during the whole gestation. The CBZ dose chosen in the current study is the usual anticonvulsive dose used for preventing kindled seizure in Wistar rats [13-18].
The i.p. injection is used in this study since it is an effectively and recognized method of drug administration in rodents, including pregnant animals; in addition, the daily administration of CBZ via gavage, during all pregnancy and lactation period, could be much more stressing to the rat dams and could interfere with the gestation period and delay the embryo development [16,19]. I.p. injections were given preferentially between inner thigh and extern genitals or into the lower left quadrant of the abdomen to ensure no organs were punctured [20].
The experimental protocol followed the ethical principles adopted by the Brazilian College of Animal Experimentation and it was approved by the local Institutional Ethics Committee (Protocol number 2009/11).
Maternal reproductive and offspring performance
On GD 19, the pregnant rats were submitted to euthanasia through CO2 inhalation [21,22]. Then, laparotomy was performed and the uterus was removed and weighted. The numbers of implantations and resorptions were counted. The ovaries were also removed and the corpora lutea computed. The number of live/ dead fetuses, placenta and fetus weight and crown-rump distance were also recorded.
The fetuses were weighed and classified as: adequate (APA) -weights did not diverge more than +1.7 standard deviations (SDs) from the control group (1.4-1.9 g range), small (SPA)-weights were at least 1.7 SD lower than the control group, or large (LPA) -weights were at least 1.7 SDs greater than the control group [23,24].
The fetuses were analyzed for the presence of external anomalies and sexed. The external macroscopic analysis consisted of number (absent, supernumerary, double), shape, position, size (small, enlarged, dilated, narrowed, elongated, distended, short, atresia) and color evaluation of the craniofacial structures (facial clefts, presence of exophthalmos, implantation in both ears, pinna malformations, presence and position of facial appendages, nasal deformities), observation of anterior and posterior extremities (number of fingers; presence, position and size of limbs), thoracic region, abdominal and dorsal (presence of hematoma, spina bifida, gastroschisis) and tail (presence, size and shape).
In sequence, half of the fetuses in each litter were fixed in Bodian's fluid for visceral examination as described by Wilson [25] and Barrow and Taylor [26]. The remaining fetuses were stored in 70% ethanol for skeletal examination after the alizarin red S staining procedure [27,28]. Skeletal examination of all fetuses was made under a dissecting microscope. Anomalies such as absence, abnormal shape and size of the bones were recorded. Examination of the head, which included evaluation of the cranial, facial and palatal bones; examination of the trunk, in which anomalies of the vertebrae, ribs and sternum were recorded; examination of the bones of the forelimbs, including the bones of the metacarpus and phalanges, and examination of the bones of the hindlimbs, which, similarly, included examination of the metatarsal and phalangeal bones.
The following parameters were calculated: Pre-implantation loss rate [(number of corpora lutea-number of implantation sites /number of corpora lutea) x 100]; Post-implantation loss rate [(number of implantations-number of live fetuses/number of implantations) x 100]; Implantation index [(number of implantations/ number of corpora lutea) X 100]; Resorption rate [(number of resorption/number of implantations) x 100]; Sexual index [number of male fetus/ number of female fetus]; Placental index [placental weight/ fetal weight] [29].
Statistical analysis
The data were submitted to statistical tests using the GraphPad Prism 8.2.1 software. The data that passed the normality test were submitted to t-test (Student’s test) and expressed as mean ± standard deviation (SD). The non-parametric Mann–Whitney’s test was used to compare data that failed the normality test and were expressed as median (interquartile range – IQR). The Fisher's exact test was used for comparison of proportions. Differences were considered significant when p ≤ 0.05.
Results
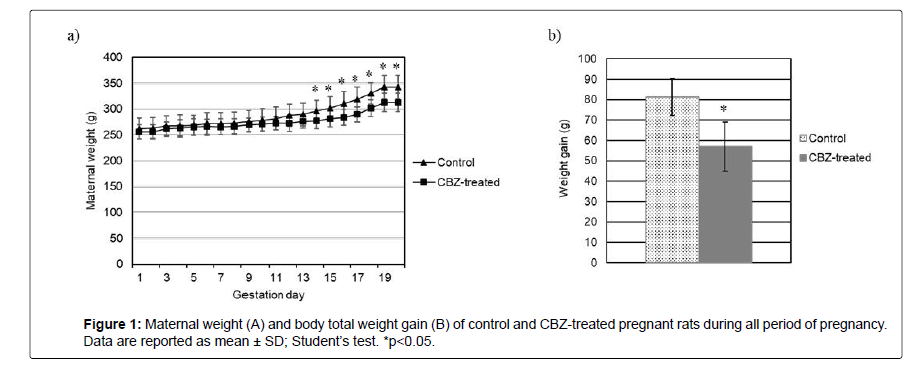
CBZ-treated pregnant rats showed no signs of physical debility or gestational anomalies. All females survived to scheduled study termination on GD 19. However, the CBZ treatment caused a significant decrease in absolute maternal body weight from GD14 until GD19, as well as reducing weight gain in pregnant rats (Figure 1).
The corpora lutea number, pre-implantation and post-implantation loss rate in the CBZ-treated group was greater as compared with the control group (Table 1). No significant differences were found between the CBZ-treated and the control groups for resorption and implantation numbers, post-implantation loss and resorption rates, number of pups per litter, live fetuses and sex ratio. Meanwhile, fetal body weights and fetal crown-rump lengths were smaller after prenatal CBZ exposure. Regarding placental weight and index, the CBZ-treated group did not show statistically significant differences compared to the control group (Table 1).
| Control | CBZ -treated | |
|---|---|---|
| Pregnant females (n) | 9 | 10 |
| Corpora lutea | ||
| Total (n) | 113 | 144 |
| Median (IQR1-IQR3)B | 12 (12.0-13.0) | 14 (13.0-14.8)* |
| Implantation | ||
| Total (n) | 112 | 115 |
| Median (IQR1-IQR3)B | 12 (12.0-13.0) | 12.5 (11.3-13.0) |
| Implantation index (%)C | 98.9 | 81.6* |
| Total fetuses (n) | 108 | 104 |
| Number of fetuses/litterB | 12.0 (11.0-13.0) | 11.0 (9.5-12.0) |
| Live fetuses | ||
| Total (n) | 107 | 96 |
| Median (IQR1-IQR3)B | 12.0 (11.0-13.0) | 11 (7.5-11.8) |
| Dead fetuses | ||
| Total (n) | 1 | 8 |
| Median (IQR1-IQR3)B | 0.0 (0.0-0.0) | 0 (0.0-0.0) |
| Resorptions | ||
| Total (n) | 4 | 10 |
| Median (IQR1-IQR3)B | 0 (0.0-1.0) | 1.0 (1.0-1.0) |
| Resorption rate (%)C | 3.8 | 8.2 |
| Pre-implantation loss (%)C | 1 | 19.2* |
| Post-implantation loss (%)C | 4.6 | 15.6* |
| Fetal body weight (g)A | ||
| Mean ± SD | 1.6 ± 0.2 | 1.4 ± 0.2* |
| %SPAC | 2.8 | 19,2* |
| %APAC | 85.2 | 79,8* |
| %LPAC | 12 | 1* |
| Sex ratio (M/F)C | 1 | 0.87 |
| Fetal crown-rump length (mm)A | ||
| Mean ± SD | 24.2 ± 1.4 | 23.2 ± 1.1* |
| Placental weight (g) A | ||
| Mean ± SD | 0.42 ± 0.05 | 0.41 ± 0.08 |
| Placental indexA | ||
| Mean ± SD | 0.26 ± 0.04 | 0.29 ± 0.05 |
| Note: IQR: Interquartile Range; SD: Standard Deviation; SPA: Small for Pregnancy age; APA: Adequate for Pregnancy Age; LPA: Large for Pregnancy Age. A Student´s t-test; B Mann-Whitney test; C Fisher exact method. *p>0.05. | ||
Table 1: Reproductive Outcomes from control and CBZ-treated groups.
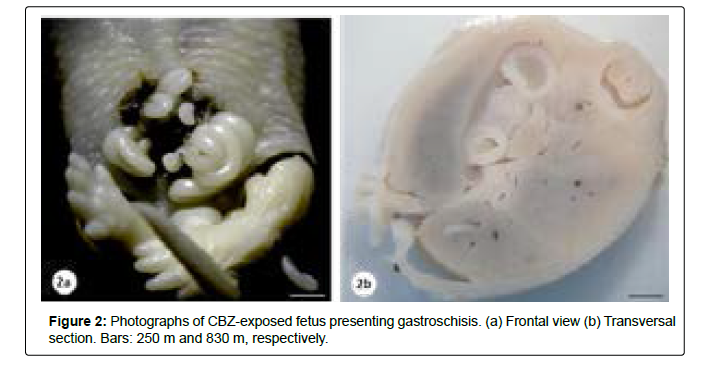
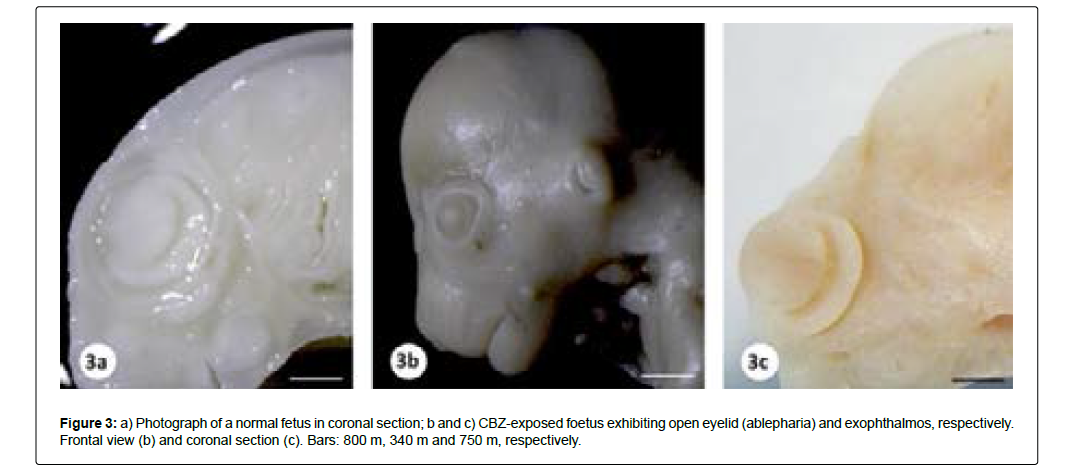
The presence of external malformations such as gastroschisis, open eyelid (ablepharia) and bilateral exophthalmos was considered related to CBZ exposure (Table 2, Figures 2 and 3). Visceral and skeletal anomalies in the head, trunk, forelimbs and hindlimbs did not differ significantly between the control and the CBZ treated groups (Table 2).
Control |
CBZ-treated | |
|---|---|---|
| External malformations | ||
| Fetuses examined (litter) | 108 (9) | 104 (10) |
| Fetuses with anomalies (%) | 0 (0.0) | 5 (4.81) |
| Gastroschisis | 0/108 (0.0) | 1/104 (0.96) |
| Exophthalmos bilateral | 0/108 (0.0) | 1/104 (0.96) |
| Ablepharia | 0/108 (0.0) | 3/104 (2.88) |
| Visceral malformations | ||
| Fetuses examined (litter) | 54(9) | 52 (10) |
| Fetuses with anomalies (%) | 0 (0.0) | 2 (3.85) |
| Macrophthalmia | 0 (0.0) | 1/52 (1.92) |
| Ectopic testis | 0 (0.0) | 1/52 (1.92) |
| Skeletal malformations | ||
| Fetuses examined (litter) | 54 (9) | 52 (10) |
| Fetuses with anomalies (%) | 0 (0.0) | 1 (1.92) |
| Unossified hindlimbs | 0/54 | 1/52 (1.92) |
| Fisher exact test. | ||
Table 2: Incidences of external, visceral and skeletal anomalies in fetuses from control and CBZ-treated groups.
Discussion
Mammals reproduction is a complex and prolonged process that has become susceptible to exposure to external agents; the gestational period is one of the most sensitive phases. Thus, it can be considered that maternal exposure to chemical agents, can result in important effects on the embryonic-fetal organism [30]. Both epilepsy and AEDs, such CBZ, have been associated with adverse pregnancy, fetal, and neonatal outcomes [31-38].
However, it should be considered that the toxicokinetic of xenobiotic substances may be modified during pregnancy. In fact, women with epilepsy have an increased frequency of seizures during pregnancy due to declining serum levels of AEDs, requiring readjustment and increased doses of these drugs [39-42]. This increase is mainly due to the reduction of CBZ plasma binding proteins, the presence of free CBZ in blood and the acceleration of its metabolism and elimination. The decrease in binding of CBZ to plasma proteins may lead to increased free blood fraction [43,44].
Obstetric and perinatal complications such as increased incidence of preterm delivery, perinatal morbidity and mortality are frequent in women with epilepsy [43]. Thus, the risk-benefit of the need for a treatment with anticonvulsant drugs to control seizures should be considered. The recommendation is to continue treatment with anticonvulsant drugs during pregnancy [45,46]. There is no consensus that anticonvulsants are safer in pregnancy, since they all have teratogenic potential. However, CBZ is still considered the recommended drug for women at risk of pregnancy. Although the CBZ is considered a human teratogen, it is an important drug option for women with epilepsy during their childbearing years because of its low cost, wide availability, relative safety for fetal outcomes and effectiveness during pregnancy [8,32,39,42,47-51].
The female reproductive performance is a very important parameter for the analysis of the perinatal toxicity of drugs [52-54]. Maternal toxicity related to xenobiotic agents can be assessed by body weight gain during pregnancy, clinical toxicity signs or gestational alterations [55]. Body weight is a sensitive indicator of potentially toxic chemicals in the study of reproductive toxicity [56-58].
The CBZ dose used in this study, which is equivalent to the therapeutic dose in humans, led to a reduction in body weight gain in CBZ-treated rats from DG13, evidencing that CBZ does cause maternal toxicity; nevertheless, the rats exhibited no signs of physical weakness or gestational problems. But, the change in maternal body weight, as an indicator of maternal toxicity, is not necessarily accompanied by embryofetal toxicity such as decreased fetal weight and increased malformations incidence, i.e., developmental effects are not always associated with maternal toxicity; the possibility remains that at least in some cases maternal toxicity may exacerbate the direct effects of a teratogen [55,59,60].
It was observed in this study that CBZ interfered on several reproductive outcomes including reduction in the implantation index, average number of corpora lutea, fetal weights and crown-rump length and an increase of pre and post-implantation loss rate. A reduction in the number of corpora lutea and in the implantation index, together with an increase in the pre-and/or post-implantation loss are critical indexes for the evaluation of female fertility and should also be considered an adverse reproductive effect [61]. Meanwhile, resorption rate, litter size, number of live/ dead fetuses, sex ratio, placental weight and index did not demonstrate significant alterations in CBZ-exposed pregnant rats. Pre-and post-implantation loss rates allow identify whether an environmental factor interferes with events that occurred prior to implantation or that caused changes in the post-implantation period, i.e., the maintenance of pregnancy [62].
Pre and post-implantation loss rates allow the identification of whether an environmental factor interferes with events that occurred prior to implantation or caused changes in the post-implantation period, i.e., the maintenance of pregnancy. The rate of pre-implantation losses establishes the relation between two variables, the number of corpora lutea and the number of implantations and corresponds to the number of unfertilized oocytes and/or the embryonic losses before the endometrial implantation. An increase in pre-implantation loss may indicate an adverse effect on gamete transport, fertilization, zygote/blastula, and/or the process of implantation itself. Thus, a reduction in the implantation index is associated with an increase in the pre-implantation loss rate. CBZ may have caused alterations in the fertilization processes, implantation of blastocyst, inducing pre and/ or peri-implantation losses. Changes in pre-implantation embryonic development may be caused by direct action of the xenobiotic agent on the conceptus or indirectly, due to changes in tubal secretion or modification of transport time to the uterus caused by the toxic agent, impairing the necessary timing of implantation [62]. Late embryonicfetal viability damage is evidenced by an increase in post-implantation loss [63].
This drug is transferred by the placenta and can accumulate in fetal tissue, inducing intrauterine growth restriction (IUGR) [64- 66]. Clinical evidences demonstrated an increase in free CBZ blood levels, further facilitating its passage through the placental barrier [50]. There is a characteristic relationship between CBZ use during pregnancy, developmental defects and fetal CBZ syndrome according to epidemiological and experimental studies [8,67,68].
In developmental toxicity studies, a reduction in mean fetal body weight is usually considered to be a result of prenatal growth retardation [69]. In this study, exposure to CBZ throughout the gestational period caused a reduction in fetal crown-rump length and body weight, along with an increase in the percentage of SPA fetuses as well as decreased rates of APA and LPA fetuses, indicating changes in intrauterine fetal growth. IUGR is the leading cause of fetal, neonatal and perinatal morbidity and mortality [70-72]. The intrauterine development in mammals is the period of active cell proliferation and differentiation and it is highly sensitive to chemical insults. A number of effects, ranging from growth retardation to severe organ anomalies and functional defects, have been reported as a result of chemical exposure to embryos [73].
CBZ has effects on fetal growth and development. Experimental studies reported decreased fetal body weight and crown-rump length due to CBZ exposure, indicating IUGR and possible relation to fetal ossification retardation [74-76].
Regarding the impact of CBZ on fetal growth and organogenesis, IUGR is inversely proportional to the CBZ dose, besides incomplete ossification of the skull, ribs and hind limbs, absence of maxilla ossification and scoliosis [75,76]. Although it was observed a reduction in fetal weight and length due to prenatal exposure to CBZ, no significant changes were observed during the examination of skeletal anomalies. A reduction in fetal body weight may be related to changes in the ossification process, especially later ossification centers such as sternebrae V and VI of the prenatal sternum, anterior and posterior phalangeal segments of the paws, and growth retardation [77].
Although skeletal examination is further confused by the differences between mouse and rat, the time of laparotomy coincides with the period of peak osteogenesis. The ossification of rodent fetal bones occurs rapidly during the last 48 hours of gestation. Because of these factors, the skeletal system in rodent fetuses is immature when it is evaluated. So, reduced degree of ossification of some fetal skeleton bones does not necessarily imply that a growth retardation process has occurred. Although bone ossification advances with gestational age, an impairment of calcification of a particular bone is not necessarily secondary to a slower development of the skeleton as a whole.
The delay in prenatal ossification may be transient and does not persists in the postnatal phase [78]. It should also be considered that the delay in ossification depends on the age at which the fetuses were collected [79]. In addition, delayed ossification typically occurs in association with other effects on fetal growth, particularly decreased fetal weight, as found in this study. Usually, delayed ossification and decreased fetal weight are indicatives of widespread effect on fetal maturation [79]. In this study, cesarean sections were performed on the 20th day of gestation and, therefore, the skeletal changes observed in animals belonging to the CBZ-treated group should be interpreted with caution, and further studies are needed to complement this assessment.
The IUGR may be associated with oxidative stress-induced adverse intrauterine environment to the embryofetal development [80-82]. Oxidative stress causes embryos injury due to peroxidation of membrane phospholipids and chemical modifications of different types of biomolecules [83].
The consequences of these damages include mitochondrial activity, embryonic development and apoptosis [84]. Nonetheless, excessive oxidative stress is strongly associated with embryotoxicity. Over the course of the development, the delicate balance between oxidants and antioxidants can be disrupted by exogenous agents that induce reactive oxygen species (ROS) production and lead to oxidative stress. Fetal and embryonic periods are highly susceptible to tissue and organ damage by ROS. In addition, gestation itself is a physiological condition with increased metabolic demand and increased tissue oxygen requirements and, in the event of abnormalities in pregnancy, oxidative imbalance may occur, and excess free radicals can promote damage to the fetus, which already has an antioxidant defense system.
It is important to emphasize that the main intracellular antioxidant component, glutathione, only reaches its maximum production at the end of gestation [85-88].
In fact, CBZ can induce oxidative stress, resulting in excessive production of ROS and/or insufficient removal of these compounds by the antioxidant defense system. Anticonvulsant drugs may still cause direct mitochondrial damage, interfering with oxidative phosphorylation, the respiratory chain and β-oxidation, leading to decreased intracellular ATP levels, and promoting mitochondrial membrane rupture [89].
CBZ also has anti-proliferative, cytotoxic and genotoxic effects revealed by a decrease in the cytokinesis-block proliferation index [90, 91]; therefore, these events could also be involved in IUGR.
Thus, fetuses exposed to anticonvulsant drugs, including CBZ, are more susceptible to congenital malformations, such as cardiovascular, genitourinary tract and craniofacial anomalies, neural tube defects, limb anomalies, neurobehavioral alterations and IUGR [2,8,9,47,49,51,74,92- 100].
CBZ teratogenicity mechanisms appear to be related to the inhibition of K+ fast channel activation, folic acid antagonism, reduction of retinoic acid and formation of toxic metabolites such as methyl sulfonyl epoxide-CBZ (CBZE) [101-103].
The teratogenicity of CBZ was investigated in Sprague-Dawley rats at doses of 0, 200, 400, and 600 mg/kg administered by gavage, from DG 7-18. The 200 mg/kg dose of CBZ did not cause fetal abnormalities but reduced fetal body weight. However, the 400 and 600 mg/kg CBZ doses caused maternal toxicity evidenced by reduced body weight gain during pregnancy, reduced live fetal weight and increased visceral abnormalities. Skeletal anomalies only occurred at the highest dose. The authors suggested that CBZ is not potent at inducing malformations in rats because they only occurred at concentrations above the therapeutic range [104].
Afshar, et al. [67] observed that i.p. CBZ administration in pregnant mice from DG 6 to 15, at doses of 15 mg/kg/day and 30 mg/ kg/day, induced several external and skeletal malformations, including premature eye opening, mild to severe exophthalmos, brachygnathia, vertebral and calvarial deformities, brachydactyly, short tail and growth retardation. In this study, the occurrence of eye malformations was prevalent, mainly premature opening of both eyes. Other studies also reported congenital eye malformations (bilateral anophthalmia, bilateral microphthalmia, unilateral optic nerve coloboma) associated with the treatment of pregnant women with CBZ [105-107]. However, there was no significant alteration in the proportion of external and visceral anomalies, which could characterize the teratogenic effect. It has been reported that CBZ induces a pattern of minor anomalies [108].
Nonetheless, the risk associated with prenatal CBZ exposure is of considerable importance, as this medication has a variety of clinical applications. So, the prescription of CBZ must be monitored to maintain therapeutic effects for the mother but, at the same time, to minimize fetal drug exposure. In this context, human trials are limited, and experimental animal models can be used in biological research, being of great importance for medical science. Whereas recognition drugs and other agents as potential teratogens, especially in cases where toxic effects are not as significant or where malformations occur spontaneously and frequently in the general population, experimental trials, such as this study, are essential for the risk determination in embryofetal development and may help in the interpretation of clinical studies [109,110].
Conclusion
The results suggest that maternal exposure to CBZ interfered on several maternal reproductive outcomes and can cause severe fetal Intrauterine Growth Restriction (IUGR). The CBZ exposure during pregnancy caused a reduction in fetal weight, fetal weight classification, and crown-rump distance. CBZ also decreased the implantation index, average number of corpora lutea, fetal weights and crown-rump length and increased the pre and post-implantation loss rate. The CBZexposed fetus also presented external congenital malformations.
Acknowledgements
The authors thank CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) and FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo -Processo 2012/05905-9) for financial support.
Author Contributions
MN and Felipe DCS performed the experimental work and analyzed the data. RRA performed the experimental work. SMM designed experiments. SUO designed experiments, oversaw the study and reviewed data. The first draft of the manuscript was written by MN and SUO and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Considerations
All animal experiments were conducted following the national and international guidelines and the relevant national laws on the protection of animals.
References
- Costantine MM (2014) Physiologic and pharmacokinetic changes in pregnancy. Front Pharmacol 5: 65.
- Holmes LB (2002) The teratogenicity of anticonvulsant drugs: A progress report. J Med Genet 39: 245-7.
- Voinescu PE (2015) Pennell PB. Management of epilepsy during pregnancy. Expert Rev Neurother 15: 1171-87.
- Pennell PB (2013) Pregnancy, epilepsy, and women's issues. Continuum (Minneap Minn) 19: 697-714.
- Yerby MS (1994) Pregnancy, teratogenesis, and epilepsy. Neurol Clin 12: 749-71.
- Fritz H, Müller D, Hess R (1976) Comparative study of the teratogenicity of phenobarbitone, diphenylhydantoin and carbamazepine in mice. Toxicology 6: 323-30.
- Artama M, Auvinen A, Raudaskoski T, Isojärvi I, Isojärvi J (2005) Antiepileptic drug use of women with epilepsy and congenital malformations in offspring. Neurology 64: 1874-8.
- Matalon S, Schechtman S, Goldzweig G, Ornoy A (2002) The teratogenic effect of carbamazepine: A meta-analysis of 1255 exposures. Reprod Toxicol 16: 9-17.
- Piersma AH, Verhoef A, Opperhuizen A, Klaassen R, van Eijkeren J, et al. (1998) Embryotoxicity of carbamazepine in rat postimplantation embryo culture after in vitro exposure via three different routes. Reprod Toxicol 12: 161-8.
- Bech BH, Kjaersgaard MI, Pedersen HS, Howards PP, Sørensen MJ, et al. (2014) Use of antiepileptic drugs during pregnancy and risk of spontaneous abortion and stillbirth: Population-based cohort study. BMJ 349: g5159.
- Zhao S, Shetty J, Hou L, Delcher A, Zhu B (2004) Human, mouse, and rat genome large-scale rearrangements: Stability versus speciation. Genome Res 14: 1851-60.
- Witschi E (1962) Biological Handbook of Federation of American Societies for Experimental Biology. Federation of American Societies for Experimental Biology 304-314.
- Cohn DF, Axelrod T, Homonnai ZT, Paz G, Streifler M, et al. (1978) Effect of diphenylhydantoin on the reproductive function of the male rat. J Neurol Neurosurg Psychiatry 41:858-60.
- de Feo MR, Mecarelli O, Ricci G, Rina MF (1991) Effects of carbamazepine on bicuculline-and pentylenetetrazol-induced seizures in developing rats. Brain Dev 13:343-7.
- Lahtinen H, Ylinen A, Lukkarinen U, Sirviö J, Miettinen R, et al. (1996) Failure of carbamazepine to prevent behavioural and histopathological sequels of experimentally induced status epilepticus. Eur J Pharmacol 297:213-8.
- Manent JB, Jorquera I, Mazzucchelli I, Depaulis A, Perucca E, et al. (2007) Fetal exposure to GABA-acting antiepileptic drugs generates hippocampal and cortical dysplasias. Epilepsia 48:684-93.
- McLean KJ, O'Brien TJ, Cook MJ, Vajda FJ (2004) The influence of gender on the aggravation of absence seizures by carbamazepine in the low-dose pentylenetetrazol rat model. Seizure 13:208-16.
- Otsuki K, Morimoto K, Sato K, Yamada N, Kuroda S (1998) Effects of lamotrigine and conventional antiepileptic drugs on amygdala- and hippocampal-kindled seizures in rats. Epilepsy Res 31:101-12.
- Ahmed RG, El-Gareib AW (2017) Maternal carbamazepine alters fetal neuroendocrine-cytokines axis. Toxicology 382:59-66.
- Nebendahl K (2000) Routes of Administration. The laboratory rat. Academic Press 463-479.
- Boivin GP, Hickman DL, Creamer-Hente MA, Pritchett-Corning KR, Bratcher NA (2017) Review of CO₂ as a Euthanasia Agent for Laboratory Rats and Mice. J Am Assoc Lab Anim Sci 56:491-499.
- Cartner SC, Barlow SC, Ness TJ (2007) Loss of cortical function in mice after decapitation, cervical dislocation, potassium chloride injection, and CO2 inhalation. Comp Med 57:570-3.
- Damasceno DC, Silva HP, Vaz GF, Vasques-Silva FA, Calderon IM, et al. (2013) Diabetic rats exercised prior to and during pregnancy: Maternal reproductive outcome, biochemical profile, and frequency of fetal anomalies. Reprod Sci 20:730-8.
- Soulimane-Mokhtari NA, Guermouche B, Yessoufou A, Saker M, Moutairou K, et al. (2005) Modulation of lipid metabolism by n-3 polyunsaturated fatty acids in gestational diabetic rats and their macrosomic offspring. Clin Sci (Lond) 109:287-95.
- Wilson JC (1965) Methods for administering agents and detecting malformations in experimental animal. University of Chicago Press pp. 262-277.
- Barrow MV, Taylor WJ (1969) A rapid method for detecting malformations in rat fetuses. J Morphol 127:291-305.
- Dawson AB (1926) A note on the staining of the skeleton of cleared specimens with alizarin red S. Stain Technol 1: 123-4.
- Staples RE, Schnell VL (1964) Refinements in rapid clearing technic in the KOH-alizarin red S method for fetal bone. Stain Technol 39:61-3.
- Tyl RW, Marr MC (2012) Developmental toxicity testing-Methodology. CRC Press pp. 139-83.
- Damasceno DC, Kempinas WG, Volpato GT, Consoni M, Rudge MVC, et al. (2008) Anomalias Congênitas: Estudos Experimentais. Belo Horizonte: Coopmed
- Cassina M, Dilaghi A, Di Gianantonio E, Cesari E, De Santis M, et al. (2013) Pregnancy outcome in women exposed to antiepileptic drugs: Teratogenic role of maternal epilepsy and its pharmacologic treatment. Reprod Toxicol 39:50-7.
- Gedzelman E, Meador KJ (2012) Antiepileptic drugs in women with epilepsy during pregnancy. Ther Adv Drug Saf 3:71-87.
- Kaushik G, Huber DP, Aho K, Finney B, Bearden S, et al. (2016) Maternal exposure to carbamazepine at environmental concentrations can cross intestinal and placental barriers. Biochem Biophys Res Commun 474:291-295.
- Li W, Hao N, Xiao Y, Zhou D (2019) Clinical characteristics and pregnancy outcomes of new onset epilepsy during pregnancy. Medicine (Baltimore) 98:e16156.
- Liguori A, Cianfarani S (2009) Postnatal onset of severe growth retardation after in utero exposure to carbamazepine and phenobarbital: A case report. J Med Case Rep 3:7300.
- Thomas SV, Jose M, Divakaran S, Sankara Sarma P (2017) Malformation risk of antiepileptic drug exposure during pregnancy in women with epilepsy: Results from a pregnancy registry in South India. Epilepsia 58:274-281.
- Veiby G, Daltveit AK, Engelsen BA, Gilhus NE (2009) Pregnancy, delivery, and outcome for the child in maternal epilepsy. Epilepsia 50:2130-9.
- Wen X, Hartzema A, Delaney JA, Brumback B, Liu X, et al. (2017) Combining adverse pregnancy and perinatal outcomes for women exposed to antiepileptic drugs during pregnancy, using a latent trait model. BMC Pregnancy Childbirth 17:10.
- Pennell PB (2003) Antiepileptic drug pharmacokinetics during pregnancy and lactation. Neurology 61:S35-42.
- Perucca E (1987) Drug metabolism in pregnancy, infancy and childhood. Pharmacol Ther 34:129-43.
- Pschirrer ER (2004) Seizure disorders in pregnancy. Obstet Gynecol Clin North Am 31:373-84.
- Tomson T (2005) Gender aspects of pharmacokinetics of new and old AEDs: Pregnancy and breast-feeding. Ther Drug Monit 27:718-21.
- Hirama SC, Dias BCS, Matsudo ET, Gandolfo CG, Ferreira BCG, et al. (2008) Treating women with epilepsy during pregnancy–the role of traditional and new antiepileptic drugs. J Epilepsy Clin Neurophysiol 14:184-192.
- Tanaka (1999) Gender-related differences in pharmacokinetics and their clinical significance. J Clin Pharm Ther 24:339-46.
- Czeizel AE, Dudás I, Bánhidy F (2011) Interpretation of controversial teratogenic findings of drugs such as phenobarbital. ISRN Obstet Gynecol 2011:719675.
- Weston J, Bromley R, Jackson CF, Adab N, Clayton-Smith J, et al. (2016) Monotherapy treatment of epilepsy in pregnancy: Congenital malformation outcomes in the child. Cochrane Database Syst Rev 11:CD010224.
- Brosh K, Matok I, Sheiner E, Koren G, Wiznitzer A, et al. (2011) Teratogenic determinants of first-trimester exposure to antiepileptic medications. J Popul Ther Clin Pharmacol 18:e89-98.
- Hernandez-Diaz S, Smith CR, Wyszynski DF, Holmes LB (2007) Risk of major malformations among infants exposed to carbamazepine during pregnancy. Birth Def Res Part A 79: 357.
- Jentink J, Loane MA, Dolk H, Barisic I, Garne E, et al. (2010) Valproic acid monotherapy in pregnancy and major congenital malformations. N Engl J Med 362:2185-93.
- Johnson EL, Stowe ZN, Ritchie JC, Newport DJ, Newman ML, et al. (2014) Carbamazepine clearance and seizure stability during pregnancy. Epilepsy Behav 33:49-53.
- Vajda FJ, O'Brien TJ, Graham J, Lander CM, Eadie MJ (2013) Associations between particular types of fetal malformation and antiepileptic drug exposure in utero. Acta Neurol Scand 128:228-34.
- Lemonica IP, Damasceno DC, di-Stasi LC (1996) Study of the embryotoxic effects of an extract of rosemary (Rosmarinus officinalis L.). Braz J Med Biol Res 29:223-7.
- Ortiz ME, Villalón M, Croxatto HB (1979) Ovum transport and fertility following postovulatory treatment with estradiol in rats. Biol Reprod 21:1163-7.
- Waynforth HB (1971) Changes in the volume of rat coprus luteum during pregnancy and after surgical interference with the uterus and placenta. Acta Endocrinol (Copenh) 66:296-302.
- Chahoud I, Ligensa A, Dietzel L, Faqi AS (1999) Correlation between maternal toxicity and embryo/fetal effects. Reprod Toxicol 13:375-81.
- Chernoff N, Rogers EH, Gage MI, Francis BM (2008) The relationship of maternal and fetal toxicity in developmental toxicology bioassays with notes on the biological significance of the "no observed adverse effect level". Reprod Toxicol 25:192-202.
- Chung MK, Kim CY, Kim JC (2007) Reproductive toxicity evaluation of a new camptothecin anticancer agent, CKD-602, in pregnant/lactating female rats and their offspring. Cancer Chemother Pharmacol 59:383-95.
- Kim SH, Lee IC, Lim JH, Shin IS, Moon C, et al. (2011) Effects of melamine on pregnant dams and embryo-fetal development in rats. J Appl Toxicol 31:506-14.
- Francis EZ, Farland WH (1987) Application of the preliminary developmental toxicity screen for chemical hazard identification under the Toxic Substances Control Act. Teratog Carcinog Mutagen 7:107-17.
- Hood RD, Rogers JM (2011) Maternally-mediated effects on development. CRC Press pp. 1:60-74.
- Parker RM(2012) Reproductive toxicity testing-Methodology. CRC Press pp. 184-228.
- Roblero LS, Fernández O, Croxatto HB (1987) The effect of RU486 on transport, development and implantation of mouse embryos. Contraception 36:549-55.
- Almeida SA, Kempinas WG, Lamano Carvalho TL (2000) Sexual behavior and fertility of male rats submitted to prolonged immobilization-induced stress. Braz J Med Biol Res 33:1105-9.
- Bath KG, Scharfman HE (2013) Impact of early life exposure to antiepileptic drugs on neurobehavioral outcomes based on laboratory animal and clinical research. Epilepsy Behav 26:427-39.
- Luef G (2009) Female issues in epilepsy: A critical review. Epilepsy Behav 15:78-82.
- Nie Q, Su B, Wei J (2016) Neurological teratogenic effects of antiepileptic drugs during pregnancy. Exp Ther Med 12:2400-2404.
- Afshar M, Moallem SA, Houshang Mohammadpour A, Shiravi A, Majid Jalalian S, et al. (2010) Teratogenic effects of carbamazepine on embryonic eye development in pregnant mice. Cutan Ocul Toxicol 29:10-5.
- Wlodarczyk BJ, Palacios AM, George TM, Finnell RH (2012) Antiepileptic drugs and pregnancy outcomes. Am J Med Genet A 158A:2071-90.
- Chahoud I, Paumgartten FJ (2005) Relationships between fetal body weight of Wistar rats at term and the extent of skeletal ossification. Braz J Med Biol Res 38:565-75.
- Brodsky D, Christou H (2004) Current concepts in intrauterine growth restriction. J Intensive Care Med 19:307-19.
- Flenady V, Koopmans L, Middleton P, Frøen JF, Smith GC, et al. (2011) Major risk factors for stillbirth in high-income countries: a systematic review and meta-analysis. Lancet 377:1331-40.
- Frøen JF, Gardosi JO, Thurmann A, Francis A, Stray-Pedersen B (2004) Restricted fetal growth in sudden intrauterine unexplained death. Acta Obstet Gynecol Scand 83:801-7.
- Walker SK, Hartwich KM, Robinson JS (2000) Long-term effects on offspring of exposure of oocytes and embryos to chemical and physical agents. Hum Reprod Update 6:564-77.
- Diav-Citrin O, Shechtman S, Arnon J, Ornoy A (2001) Is carbamazepine teratogenic? A prospective controlled study of 210 pregnancies. Neurology 57:321-4.
- El-Gaafarawi I, Abouel-Magd M (2015) Teratogenic effect of carbamazepine administration in pregnant rats. Egypt J Hosp Med 59:244-257.
- Sucheston ME, Hayes TG, Eluma FO (1986) Relationship between ossification and body weight of the CD-1 mouse fetus exposed in utero to anticonvulsant drugs. Teratog Carcinog Mutagen 6:537-46.
- Kimmel GL, Kimmel CA, Francis EZ (1987) Implications of the consensus workshop on the evaluation of maternal and developmental toxicity. Teratog Carcinog Mutagen 7:329-38.
- Carney EW, Kimmel CA (2007) Interpretation of skeletal variations for human risk assessment: Delayed ossification and wavy ribs. Birth Defects Res B Dev Reprod Toxicol 80:473-96.
- Daston GP, Seed J (2007) Skeletal malformations and variations in developmental toxicity studies: Interpretation issues for human risk assessment. Birth Defects Res B Dev Reprod Toxicol 80:421-4.
- Arad I, Bar-Oz B, Ergaz Z, Nir A, Barak V (2010) Interleukin-6 and N-terminal pro-brain natriuretic peptide cord blood levels in premature infants: Correlations with perinatal variables. Isr Med Assoc J 12:419-23.
- Krishna U, Bhalerao S (2011) Placental insufficiency and fetal growth restriction. J Obstet Gynaecol India 61:505-11.
- Sharma D, Shastri S, Farahbakhsh N, Sharma P (2016) Intrauterine growth restriction - part 1. J Matern Fetal Neonatal Med 29:3977-87.
- Dennery PA (2007) Effects of oxidative stress on embryonic development. Birth Defects Res C Embryo Today 81:155-62.
- Barbosa KBF, Costa NMB, Alfenas RG, De Paula SO, Minim VPR, et al. (2010) Estresse oxidativo: Conceito, implicações e fatores modulatórios. Rev Nutr 23:629-43.
- Calabrese V, Cornelius C, Dinkova-Kostova AT, Calabrese EJ, Mattson MP (2010) Cellular stress responses, the hormesis paradigm, and vitagenes: Novel targets for therapeutic intervention in neurodegenerative disorders. Antioxid Redox Signal 13:1763-811.
- Gracy X. Rosario RA, Toshihiro K, Michael JS (2009) Intrauterine fate of invasive trophoblast cells. Placenta 30:457-63.
- Lamb SE, Hansen Z, Lall R, Castelnuovo E, Withers EJ, et al. (2010) Group cognitive behavioural treatment for low-back pain in primary care: A randomised controlled trial and cost-effectiveness analysis. Lancet 375:916-23.
- Wells PG, Bhuller Y, Chen CS, Jeng W, Kasapinovic S, et al. (2005) Molecular and biochemical mechanisms in teratogenesis involving reactive oxygen species. Toxicol Appl Pharmacol 207:354-66.
- Finsterer J, Scorza FA (2017) Effects of antiepileptic drugs on mitochondrial functions, morphology, kinetics, biogenesis, and survival. Epilepsy Res 136:5-11.
- Pérez Martín JM, Fernández Freire P, Labrador V, Hazen MJ (2008) Carbamazepine induces mitotic arrest in mammalian Vero cells. Mutat Res 637:124-33.
- Celik A (2006) The assessment of genotoxicity of carbamazepine using cytokinesis-block (CB) micronucleus assay in cultured human blood lymphocytes. Drug Chem Toxicol 29:227-36.
- Adab N, Tudur Smith C, Vinten J, Williamson PR, Winterbottom JB, et al. (2015) WITHDRAWN: Common antiepileptic drugs in pregnancy in women with epilepsy. Cochrane Database Syst Rev (1):CD004848.
- El-Sayed YY (1998) Obstetric and gynecologic care of women with epilepsy. Epilepsia 8:S17-25.
- El-Sayed MG, Aly AE, Kadri M, Moustafa AM (1983) Comparative study on the teratogenicity of some antiepileptics in the rat. East Afr Med J 60:407-15.
- Hernández-Díaz S, Smith CR, Shen A, Mittendorf R, Hauser WA, et al. (2012) Comparative safety of antiepileptic drugs during pregnancy. Neurology 78:1692-9.
- Jones KL, Lacro RV, Johnson KA, Adams J (1989) Pattern of malformations in the children of women treated with carbamazepine during pregnancy. N Engl J Med 320:1661-6.
- Martinez Ferri M, Peña Mayor P, Perez López-Fraile I, Escartin Siquier A, Martin Moro M, et al. (2018) Comparative study of antiepileptic drug use during pregnancy over a period of 12 years in Spain. Efficacy of the newer antiepileptic drugs lamotrigine, levetiracetam, and oxcarbazepine. Neurologia (Engl Ed) 33:78-84.
- Ornoy A (2006) Neuroteratogens in man: An overview with special emphasis on the teratogenicity of antiepileptic drugs in pregnancy. Reprod Toxicol 22:214-26.
- Pennell PB (2008) Antiepileptic drugs during pregnancy: What is known and which AEDs seem to be safest? Epilepsia 49 Suppl 9:43-55.
- Tomson T, Battino D, Bonizzoni E, Craig J, Lindhout D, et al. (2018) Comparative risk of major congenital malformations with eight different antiepileptic drugs: A prospective cohort study of the EURAP registry. Lancet Neurol 17:530-538.
- Amore BM, Kalhorn TF, Skiles GL, Hunter AP, Bennett GD, et al. (1997) Characterization of carbamazepine metabolism in a mouse model of carbamazepine teratogenicity. Drug Metab Dispos 25:953-62.
- Fex G, Larsson K, Andersson A, Berggren-Söderlund M (1995) Low serum concentration of all-trans and 13-cis retinoic acids in patients treated with phenytoin, carbamazepine and valproate. Possible relation to teratogenicity. Arch Toxicol 69:572-4.
- Raymond GV, Buehler BA, Finnell RH, Holmes LB (1995) Anticonvulsant teratogenesis: 3. Possible metabolic basis. Teratology 51:55-6.
- Vorhees CV, Acuff KD, Weisenburger WP, Minck DR (1990) Teratogenicity of carbamazepine in rats. Teratology 41:311-7.
- Fahnehjelm KT, Wide K, Ygge J, Hellström A, Tomson T, et al. (1999) Visual and ocular outcome in children after prenatal exposure to antiepileptic drugs. Acta Ophthalmol Scand 77:530-5.
- Kroes HY, Reefhuis J, Cornel MC (2002) Is there an association between maternal carbamazepine use during pregnancy and eye malformations in the child? Epilepsia 43:929-31.
- Sutcliffe AG, Jones RB, Woodruff G (1998) Eye malformations associated with treatment with carbamazepine during pregnancy. Ophthalmic Genet 19:59-62.
- Gladstone DJ, Bologa M, Maguire C, Pastuszak A, Koren G (1992) Course of pregnancy and fetal outcome following maternal exposure to carbamazepine and phenytoin: A prospective study. Reprod Toxicol 6:257-61.
- Fagundes DJ, Taha MO (2004) Modelo animal de doença: Critérios de escolha e espécies de animais de uso corrente. Acta Cir Bras 19:59-65.
- Schanaider A, Silva PC (2004) Uso de animais em cirurgia experimental. Uso de animais em cirurgia experimental. Acta Cir Bras 19:441-7.
Citation: Nunes M, de Sousa FDC, Andretta RR, Miraglia SM, Oliva SU, et al. (2021) Carbamazepine Causes Fetal Intrauterine Growth Restriction (IUGR) in Rats. J Clin Exp Neuroimmuno l 6: 129.
Copyright: © 2021 Nunes M, et al. This is an open-access article distrubuted under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 2517
- [From(publication date): 0-2021 - Dec 20, 2025]
- Breakdown by view type
- HTML page views: 1793
- PDF downloads: 724