Clinicopathological Significance of SALL4 and BECN1 Tissue Protein Expression in Serous Ovarian Carcinoma (SOC); An Immunohistochemical Study.
Received: 25-Jun-2018 / Accepted Date: 03-Sep-2018 / Published Date: 11-Sep-2018 DOI: 10.4172/2161-0681.1000349
Keywords: Serous ovarian carcinoma; SALL4; BECN-1; Grade; Stage; Immunohistochemistry
Introduction
Epithelial ovarian carcinoma (EOC) is considered the fourth commonest female cancer malignancy [1], and it is considered the commonest cause of postmenopausal cancer related mortality in females worldwide, serous ovarian carcinoma (SOC) is considered the commonest subtype of EOC (Jayson et al.). Despite improvement in the management modalities including surgery, chemotherapy and molecular targeted therapy of SOC it still has a dismal prognosis due to its spread to adjacent organs and through the peritoneal fluid to the peritoneal cavity which leads to late diagnosis at advanced stages and resistance to chemotherapy [2,3]. So discovering novel therapeutic targets will be beneficial to improve its prognosis, decrease rate of its recurrence, decrease the resistance to the currently used chemotherapeutic drugs and could specifically target SOC cells which are main goals of recent clinical cancer studies [4].
Spalt-like transcription factor 4 (SALL4) is the zinc finger transcriptional factor which controls several genes that are involved in normal development, in maintaining embryonic stem cells pluripotency and self-renewal [5], moreover recent studies proved that it has an initiating and promoting roles leukemogenesis and it is found to be over expressed in acute myeloid leukemia cells [6], but its detailed oncogenic roles in several other cancers is not studied yet [7]. Autophagy is considered the homeostatic process that is responsible for recycling of intracellular organelles and foreign substances and it is described as a non-apoptotic process of programmed cells death; autophagy is needed for normal cellular survival and viability [8]. The roles of autophagy in cancer initiation and the carcinogenesis process have been extensively studied [9]. Previous studies stated that autophagy might have a role controlling cancer growth and resistance to chemotherapy. But final proved results of its roles in the carinogenic process are not reached as its exact mechanism of action and its role in cancers is complicated [10], there are plethora of genetic pathways and proteins that are controlling autophagy e.g. Beclin 1 (BECN1) which have been found to have an important inducing role for such process. BECN1 has variable expression levels during cancer progression, which points to the presence of a relation between its tissue protein expression in cancer and the carcinogenic process is considered the human counterpart of yeast Atg6/Vps30, is mapped to the tumor susceptibility locus 150 kb beside BRCA1 gene on chromosome 17q21 [11]. It was originally proved to have a tumor suppressor role in cancer [12]. It was found that BECN1 lower expression could decrease the autophagy activity in malignant cells which has variable effects on cancer progression, invasion and spread according to cancer type [13]. The detailed clinicopathological role of SALL-4 and BECN1 in SOC is not well clarified yet.
Aim of the Study
To assess the clinic-pathological significance of SALL-4 and BECN1 protein expression in Serous Ovarian Carcinoma tissues, by comparing their expression with standard clinico-pathological prognostic parameters as tumor grade, stage, presence of lymph node and distant metastases.
Patients and Methods
We have included sixty archival paraffin embedded blocks of SOC which were collected from Pathology Department, Faculty of Medicine, Zagazig University. Cases were previously admitted to Gynaecology and Obstetrics department, faculty of medicine, Zagazig university, cases were managed according to tumor subtype and stage by radical excision of the tumor, we have re-evaluated them, used the International Federation of Gynaecology and Obstetrics (FIGO) staging system for its staging [14], and the WHO grading system for grading sections from all blocks [15]. We have collected all demographic data of patients e.g. age, cancer size, grade, stage, state of lymph nodes, peritoneal fluid, presence of malignant ascites and distant metastasis by examination of patient’s and slides files retrospectively in Pathology department.
Immunohistochemical technique and evaluation of both SALL4 and BECN1 expression
Streptavidin-biotin technique were used for immunohistochemistry [16], we have incubated sections with primary Mouse monoclonal anti-Sall4 antibody (ab57577) diluted 1/100 and with primary rabbit monoclonal anti BECN1 Ab1733Y antibody diluted 1/100 at 4°C overnight (Abcam, Cambridge, UK), then with secondary antibody. Last step is counterstaining sections with Mayer’s hematoxylin.
Sections from yolk sac tumor were used as a positive control for SALL4 [17] and sections of normal breast tissue were used as positive control for BECN1 respectively, negative control by replacing the primary antibody with usual phosphate-buffered saline (PBS). Degree of immunoreactivity of SALL4 and BECN1 was assessed by 2 senior pathologists from Pathology Department, Faculty of Medicine, Zagazig University.
Brown stain in the nuclei and cytoplasm of SOC cells was considered positive for SALL4 and BECN1 respectively we have given the intensity and extent scores from 0-3 according to the degree of stained cells.
Regarding stain intensity it was graded as 0-negative, 1-weak, 2- moderate and 3-strong stains. Regarding stain extent it was graded as 0 when stained tumor cells were <10%, 1 when stained tumor cells were 10%-20%, 2 when stained tumor cells were 21%-50% and 3 when stained tumor cells were ≥ 50%. We have summated values of both intensity and extent to reach the final scores of 0-6 and we have considered 3 as a cut point for easy statistical analysis; above 3 high expression of both markers and below 3 low expression of both markers [3,18].
Statistical analysis
All statistics were performed using SPSS 22.0 for windows (SPSS Inc., Chicago, IL, USA). The continuous parameters were presented as mean ± SD, and the categorical parameters were presented as number and percentage using the Chi-square test. The relationship strength between SALL4, BECN1 and clinicopathological parameters of SOC was done by calculating spearman's correlation coefficient.
p-value <0.05 was considered significant statistically.
Results
Patient demographic data were detailed in Table 1.
| Characteristics | All patients | |
|---|---|---|
| (N=60) | ||
| Age (years) | ||
| Mean ± SD | 58.53 | ± 10.53 |
| Median (Range) | 59 | (28-78) |
| <40 years | 5 | (8.7%) |
| 41-59 years | 35 | (58.7%) |
| = 60 years | 20 | (38.7%) |
| Histopathology | ||
| Positive cytology | ||
| Absent | 39 | (65%) |
| Present | 21 | (35%) |
| CA125 | ||
| =35 U/ml | 20 | (35%) |
| >35 U/ml | 40 | (65%) |
| Bilaterality | ||
| Unilateral | 44 | (73.3%) |
| Bilateral | 16 | (26.7%) |
| Implants | ||
| Absent | 37 | (63.3%) |
| Present | 23 | (36.7%) |
| Ascites | ||
| Absent | 37 | (63.3%) |
| Present | 23 | (36.7%) |
| Grade | ||
| Low | 24 | (38.3%) |
| High | 36 | (61.7%) |
| LN | ||
| Node negative | 23 | (35%) |
| Node positive | 37 | (65%) |
| M | ||
| M0 (non-metastatic) | 45 | (76.7%) |
| M1 (metastatic) | 15 | (23.3%) |
| FIGO Stage | ||
| Stage IA | 2 | (3.3%) |
| Stage IB | 3 | (1.7%) |
| Stage IC | 3 | (3.3%) |
| Stage IIA | 3 | (5%) |
| Stage IIB | 7 | (11.7%) |
| Stage IIC | 6 | (10%) |
| Stage IIIA | 8 | (15%) |
| Stage IIIB | 11 | (20%) |
| Stage IIIC | 4 | (6.7%) |
| Stage IV | 13 | (23.3%) |
| SALL4 | ||
| Low | 25 | (46%) |
| High | 35 | (64%) |
| Beclin-1 | ||
| Low | 38 | (65%) |
| High | 22 | (35%) |
Table 1: Demographic and immunohistochemical data
We have included 60 cases of SOC of variable grades and stages. Age of our patients ranged from (28-78) years and the age is: 58.53 ± 10.53years. 36 (61.7%) cases are diagnosed with high grade tumors while 24 (38.3%) cases are diagnosed with low grade SOC, lymph node metastases are present in 37 (65%) cases and distant metastases are present in 15 (23.3%) of our cases.
SALL 4 expression in SOC and its correlation with clinicopathological parameters
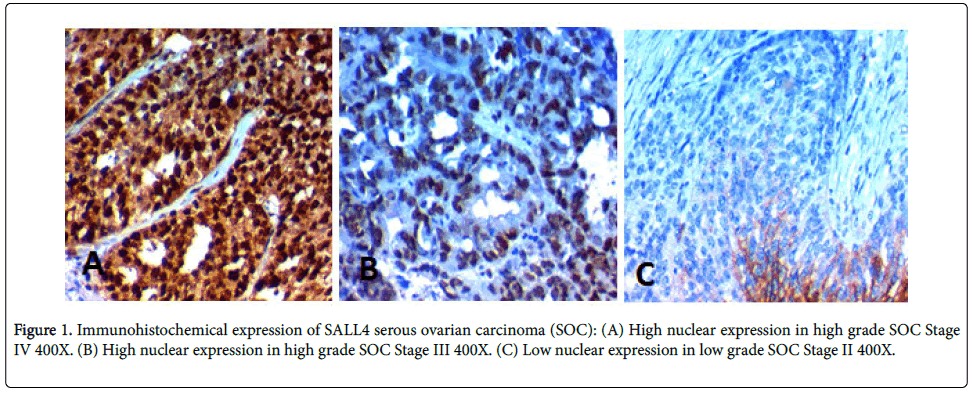
High SALL4 expression was found in 35 (64%) of cases and its high expression was significantly positively correlated with advanced age of the patients (p=0.003), higher grade of the tumor, advanced stage of the tumor (p<0.001), presence of distant metastases, positive peritoneal cytology (p=0.006), peritoneal implants (p=0.002), L.N metastases (p=0.004), and presence of malignant ascites (p=0.024), no statistically detected difference between its expression and bilaterality of the tumor (Table 2, Figure 1).
| Characteristics | All | SALL4 | p-value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Low (N=25) |
High (N=35) |
||||||||
| (60 cases) | |||||||||
| No. | (%) | No. | (%) | No. | (%) | ||||
| Age (years) | |||||||||
| Mean ± SD | 58.53 | ± 10.53 | 47.54 | ± 10.10 | 60.15 | ± 7.68 | <0.001* | ||
| Median (Range) | 59 | (28-78) | 45 | (25-65) | 59 | (46-75) | |||
| <40 years | 5 | (8.7%) | 4 | (100%) | 0 | (0%) | 0.003‡ | ||
| 41-59 years | 35 | (58.7%) | 16 | (44.1%) | 18 | (55.9%) | |||
| ≥ 60 years | 20 | (33.7%) | 5 | (13.6%) | 17 | (86.4%) | |||
| Positive cytology | |||||||||
| Absent | 36 | (65%) | 21 | (53.8%) | 15 | (46.2%) | 0.006‡ | ||
| Present | 24 | (35%) | 4 | (4.8%) | 20 | (95.2%) | |||
| CA125 | |||||||||
| ≤35 U/ml | 20 | (35%) | 17 | (81%) | 3 | (19%) | <0.001‡ | ||
| >35 U/ml | 40 | (65%) | 8 | (12.8%) | 32 | (87.2%) | |||
| Bilaterality | |||||||||
| Unilateral | 44 | (73.3%) | 19 | (40.9%) | 25 | (59.1%) | 0.258‡ | ||
| Bilateral | 16 | (26.7%) | 6 | (25%) | 10 | (75%) | |||
| Implants | |||||||||
| Absent | 37 | (63.3%) | 21 | (52.6%) | 16 | (47.4%) | 0.002‡ | ||
| Present | 23 | (36.7%) | 4 | (9.1%) | 19 | (90.9%) | |||
| Ascites | |||||||||
| Absent | 37 | (63.3%) | 19 | (47.4%) | 18 | (52.6%) | 0.024‡ | ||
| Present | 23 | (36.7%) | 6 | (18.2%) | 17 | (81.8%) | |||
| Grade | |||||||||
| Low | 24 | (38.3%) | 21 | (78.3%) | 3 | (21.7%) | <0.001‡ | ||
| High | 36 | (61.7%) | 4 | (10.8%) | 32 | (89.2%) | |||
| LN | |||||||||
| Node negative | 23 | (35%) | 20 | (81%) | 3 | (19%) | 0.004‡ | ||
| Node positive | 37 | (65%) | 5 | (12.8%) | 32 | (87.2%) | |||
| M | |||||||||
| M0 (non-metastatic) | 45 | (76.7%) | 21 | (45.7%) | 23 | (54.3%) | 0.006‡ | ||
| M1 (metastatic) | 15 | (23.3%) | 4 | (7.1%) | 12 | (92.9%) | |||
| FIGO Stage | |||||||||
| Stage IA | 2 | (3.3%) | 2 | (100%) | 0 | (0%) | <0.001§ | ||
| Stage IB | 3 | (1.7%) | 3 | (100%) | 0 | (0%) | |||
| Stage IC | 3 | (3.3%) | 3 | (100%) | 0 | (0%) | |||
| Stage IIA | 3 | (5%) | 3 | (100%) | 0 | (0%) | |||
| Stage IIB | 7 | (11.7%) | 5 | (71.4%) | 2 | (28.6%) | |||
| Stage IIC | 6 | (10%) | 4 | (66.7%) | 2 | (33.3%) | |||
| Stage IIIA | 8 | (15%) | 1 | (11.1%) | 7 | (88.9%) | |||
| Stage IIIB | 11 | (20%) | 2 | (16.7%) | 9 | (83.3%) | |||
| Stage IIIC | 4 | (6.7%) | 1 | (25%) | 3 | (75%) | |||
| Stage IV | 13 | (23.3%) | 1 | (7.1%) | 12 | (92.9%) | |||
| Beclin-1 | |||||||||
| Low | 38 | (58.3%) | 5 | (5.7%) | 33 | (94.3%) | <0.001‡ | ||
| High | 22 | (41.7%) | 18 | (80%) | 3 | (20%) | |||
Table 2: Correlation between clinicopathological features and immunohistochemical expression of SALL-4 in our cases (*Independent samples Student's test; ? Mann Whitney U test; ‡Chi-square test; §Chi-square test for trend; p
BECN1 expression in SOC and its correlation with clinicopathological parameters
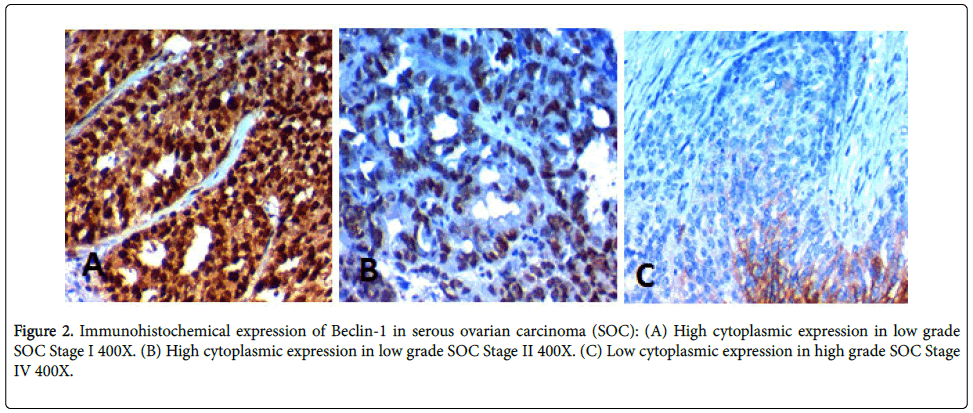
High BECN1 expression was found in 38 (65%) of cases and its high expression was significantly negatively correlated with advanced age of the patients (p=0.003), higher grade of the tumor, advanced stage of the tumor, L.N metastases, presence of distant metastases, peritoneal implants (p<0.001), positive peritoneal cytology (p=0.004), bilaterality of the tumor (p=0.020) and presence of malignant ascites (p=.006) (Table 3, Figure 2).
| Characteristics | All | BECN1 | p-value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Low (N=38) |
High (N=22) |
||||||||
| (N=60) | |||||||||
| No. | (%) | No. | (%) | No. | (%) | ||||
| Age (years) | |||||||||
| Mean ± SD | 55.53 | ± 10.53 | 61.20 | ± 7.23 | 47.60 | ± 9.28 | <0.001• | ||
| Median (Range) | 57 | (25-75) | 60 | (48-75) | 45 | (25-60) | |||
| <40 years | 4 | (6.7%) | 0 | (0%) | 4 | (100%) | 0.003‡ | ||
| 41-59 years | 34 | (56.7%) | 18 | (47.1%) | 16 | (52.9%) | |||
| ≥ 60 years | 22 | (36.7%) | 22 | (86.4%) | 2 | (13.6%) | |||
| Positive cytology | |||||||||
| Absent | 36 | (65%) | 14 | (35.9%) | 22 | (100%) | 0.004‡ | ||
| Present | 24 | (35%) | 24 | (100%) | 0 | (0%) | |||
| CA125 | |||||||||
| ≤ 35 U/ml | 20 | (35%) | 3 | (14.3%) | 17 | (85.7%) | <0.001‡ | ||
| >35 U/ml | 40 | (65%) | 35 | (82.1%) | 5 | (17.9%) | |||
| Bilaterality | |||||||||
| Unilateral | 44 | (73.3%) | 24 | (50%) | 20 | (50%) | 0.020‡ | ||
| Bilateral | 16 | (26.7%) | 14 | (81.3%) | 2 | (18.8%) | |||
| Implants | |||||||||
| Absent | 37 | (63.3%) | 16 | (36.8%) | 21 | (63.2%) | <0.001‡ | ||
| Present | 23 | (36.7%) | 22 | (95.5%) | 1 | (4.5%) | |||
| Ascites | |||||||||
| Absent | 37 | (63.3%) | 17 | (44.7%) | 20 | (55.3%) | 0.006‡ | ||
| Present | 23 | (36.7%) | 21 | (81.8%) | 2 | (18.2%) | |||
| Grade | |||||||||
| Low | 24 | (38.3%) | 6 | (13%) | 18 | (87%) | <0.001‡ | ||
| High | 36 | (61.7%) | 32 | (86.5%) | 4 | (13.5%) | |||
| LN | |||||||||
| Node negative | 23 | (35%) | 8 | (14.3%) | 15 | (85.7%) | <0.001‡ | ||
| Node positive | 37 | (65%) | 30 | (82.1%) | 7 | (17.9%) | |||
| M | |||||||||
| M0 (non-metastatic) | 45 | (76.7%) | 23 | (45.7%) | 22 | (54.3%) | <0.001‡ | ||
| M1 (metastatic) | 15 | (23.3%) | 15 | (100%) | 0 | (0%) | |||
| FIGO Stage | |||||||||
| Stage IA | 2 | (3.3%) | 0 | (0%) | 2 | (100%) | <0.001§ | ||
| Stage IB | 3 | (1.7%) | 2 | (3.3%) | 1 | (100%) | |||
| Stage IC | 3 | (3.3%) | 1 | (1.6%) | 2 | (100%) | |||
| Stage IIA | 3 | (5%) | 0 | (0%) | 3 | (100%) | |||
| Stage IIB | 7 | (11.7%) | 0 | (0%) | 7 | (100%) | |||
| Stage IIC | 6 | (10%) | 4 | (60%) | 2 | (50%) | |||
| Stage IIIA | 8 | (15%) | 6 | (66.7%) | 2 | (33.3%) | |||
| Stage IIIB | 11 | (20%) | 9 | (75%) | 2 | (25%) | |||
| Stage IIIC | 4 | (6.7%) | 3 | (75%) | 1 | (25%) | |||
| Stage IV | 13 | (23.3%) | 13 | (100%) | 0 | (0%) | |||
| SALL-4 | |||||||||
| Low | 22 | (36.7%) | 2 | (9.1%) | 20 | (90.9%) | <0.001‡ | ||
| High | 38 | (63.3%) | 33 | (86.8%) | 5 | (13.2%) | |||
Table 3: Correlation between clinicopathological features and immunohistochemical expression of BECN1 in our cases (*Independent samples Student's test; •Mann Whitney U test; ‡ Chisquare test; § Chi-square test for trend; p
We found an inverse relationship between SALL4 and BECN1 (Spearman’s r=-0.198) (p=0.031) (Table 4).
| SALL4 | BECN1 | SALL4/BECN1 | ||||
|---|---|---|---|---|---|---|
| (Low, high) | (Low, high) | (Low/low……..high/high) | ||||
| r | p-value | r | p-value | r | p-value | |
| Age (years) | 0.602 | <0.001 | -0.501 | <0.001 | -0.718 | <0.001 |
| FIGO stage (IA, ………,IV) | 0.873 | <0.001 | -0.588 | <0.001 | -0.701 | <0.001 |
| Grade (low, high) | 0.667 | 0.001 | -0.528 | <0.001 | -0.544 | <0.001 |
| LN (negative, positive) | 0.64 | <0.001 | -0.446 | <0.001 | -0.563 | <0.001 |
| DM (negative, positive) | 0.485 | 0.006 | -0.578 | 0.001 | -0.653 | <0.001 |
| SALL4 (low, high) | --- | --- | -0.198 | 0.161 | --- | --- |
| BECN1 (low, high) | -0.198 | 0.161 | --- | --- | --- | --- |
Table 4: Association and correlation between clinicopathological parameters, SALL4 and BECN1 in our cases (r correlation coefficient; p
Discussion
We found that high SALL4 expression was found in 35 (64%) of cases and its high expression was significantly positively correlated with advanced age of the patients (p=0.003), higher grade of the tumor, advanced stage of the tumor (p<0.001), presence of distant metastases, positive peritoneal cytology (p=0.006), peritoneal implants (p=0.002), L.N metastases (p=0.004), and ascites (p=.0.024). Yang et al. [19] have found similar results in SOC, similarly, results of previous studies regarding the association between SALL4 and poor prognosis of multiple solid tumors [20-23].
So, our results proved that increased tissue protein expression of SALL4 could increase migration, proliferation, invasion and metastatic potential of SOC cells which pointed to that SALL4 is a cancer promoting agent which might be used as novel therapeutic molecular target for improving management modalities of SOC.
The explanation of our results is provided by Zeng et al. [24] who stated that SALL4 has an essential role in regulation of cancer stem cells proliferation which is responsible for high proliferation rate, invasion and metastases of cancer, additionally it was found that inhibition of SALL4 could result in down-regulation of stem cell markers, in addition to decreasing the invasion potential of cancer cells [17], SALL4 has many other roles in tumor promotion and progression as it could interact with b-catenin which results in aberrant activation of Wnt/b-catenin signaling pathway that have major roles in increasing cancer cells invasion, lymph node and distant metastasis [17]. Moreover SALL4 is found to be involved in c-Myc, HOXA9, and PTEN-AKT pathways, which are responsible for activation of the epithelial-mesenchymal transition (EMT) [21,25]. The process of EMT has an important role in increasing spread of SOC [26,27]. Our results are similar to results of Liu et al. [28] who proved that increased SALL4 expression is related to poor prognosis of endometrial carcinoma and explained his results by the association of SALL4 expression and chemoresistance to the currently used chemotherapeutic drugs in endometrial carcinoma, similarly other studies have proved that SALL4 could induce chemoresistance and poor prognosis in many cancers [29]. As chemotherapy has major role in management of SOC the induction of chemoresistance markedly worsen its prognosis. The role of SALL4 in drug resistance is explained by that drug resistance in cancer is associated with ATP-binding cassette (ABC) which is multidrug transporters, SALL4 could induce chemotherapeutic resistance by regulation of ABCB1 in cancer cells. ABC are closely related to drug resistance and ABCB1 which is the prototype of this family of genes, and its dis-regulation is related to drug resistance in cancers of several organs [28]. Another explanation for the association between SALL4 and poor prognosis in cancers is due to its ability to promote c-Myc transcriptional activity which has many roles cancer drug resistance, invasion and metastasis [25].
Our results are similar to results of Du et al., who proved the upregulation of SALL4 by EGFR activation that could be able to regulate the stemness of CD44-positive cells in lung cancer [30]. In addition to its role in cancer progression several other functions e.g. Yang has found that SALL4 is a transcriptional and epigenetic regulator in normal and leukemic hematopoiesis [31].
Our study proved that anti- SALL4 drugs could specifically target SOC cells and could decrease its invasion, spread and improving its prognosis.
To strengthen our results we have tried to find the association between SALL4 and BECN1 which is an autophagy marker and find the effect of their combined expression on the prognosis of SOC. In this study we have found that increased tissue BECN1 expression in SOC was associated with favorable clinic-pathological parameters. High BECN1 expression was found in 38 (65%) of cases and its high expression was significantly negatively correlated with advanced age of the patients (p=0.003), higher grade of the tumor, advanced stage of the tumor, L.N metastases, presence of distant metastases, peritoneal implants (p<0.001), positive peritoneal cytology (p=0.004), bilaterality of the tumor (p=0.020) and presence of malignant ascites (p=0.006).
BECN1 role in oncogenesis has taken attention it is found to be overexpressed in many cancers while down regulated in other cancers [32], so BECN1 might have variable roles in cancer according to cancer type. Autophagy has variable roles in cancer initiation, promotion and progression which is different according to type of cancer as it may have an inhibitory effect on cancer by maintaining genomic stability or a stimulatory effect by acting as a pro-survival signal which protects malignant cells from any cellular stress [32]. Recently BECN1 which is an autophagy related regulatory protein has been found to be down regulated in many cancers and this is considered recent evidence which linked autophagy to malignant suppression [33].
Similarly, Cai et al. [33] found that increased BECN1 expression is associated with high degree of ovarian cancer differentiation suggesting that BECN1 might behave as a protective factor in cancer ovary. Additionally, Osman et al. [34] have proved the association between BECN1 expression and high degree of differentiation in HCC, and its expression was negatively associated with unfavorable clinicopathological criteria and poor prognosis in HCC, additionally, Wu et al. [35] have proved similar results in cancers of many organs. Results of our study in addition to results of previous researchers declared that BECN1 acts as a tumor suppressor gene that decreased aggressively of cancer.
Moreover, Ying et al. [3] assessed the relations between BECN1 expression and response to chemotherapy; they have found that its expression was higher in drug sensitive ovarian cancer patients than in drug resistant patients, which proved that decreased BECN1 expression is associated with chemoresistance resistance and dismal outcome in ovarian carcinoma patients.
We proved that BECN1 high expression was found in early stage SOC than the late stages; similarly, Fei et al. [36] found increased BECN1 expression in early stages of cancer stomach than in late stages.
Such results are explained by that tissue protein expression levels of BECN1 have decreased with cancer progression as cancer cells lose the autophagy capacity during cancer progression, escape cell death and become more liable for invasion, spread and more aggressive behavior [36]. Miracco et al. [37] assessed BECN1 protein expression in atypical meningioma and ependymal high-grade neoplasms and they have proved decreased its expression in high grade than in low grade tumors, that points to that decreased BECN1 expression is associated with more aggressive brain tumors, which was similar to our results in SOC.
Results about role of BECN1 in cancer and its association with good prognosis in SOC is unproved yet, as different results were found by Zhao et al. [38] who concluded that BECN1 has no relation to ovarian carcinoma prognosis these results might be explained by different number of cases and different clone of the primary anti-BECN1 antibody that was used in the study.
Other studies which declared similar results; Cai et al. [33] found that high increased tissue protein BECN1 expression is associated with favorable outcome in SOC patients. Many theories were put to explain their results (1) BECN1 increased autophagy in ovarian carcinoma cells that are lacking apoptotic ability leading to their death and decreasing their survival; (2) BECN1 decreased the occurrence of gene mutations by stabilization of the mitochondrial structure so decreased emergence of aggressive, invasive and metastasizing clones (3) Increased BECN1 expression stops the completion of cell cycle, so inhibits cancer cell proliferation, stimulates autophagy and apoptosis (4) Moreover BECN1 delays cell cycle progression [33]. Moreover, high BECN1 expression leads to increased phosphorylation of p53 and Bcl-2 [39], and caspase-9 activity [40], which enhanced autophagy related cell death Dong et al. [41] proved the protective roles of BECN1 in many cancers, which was similar to our work.
Other studies have proved different results regarding the association between BECN1 expression and cancer prognosis. Contrast to our results, Wan et al. [42] and Tang et al. [43] proved the association between high BECN1 expression and aggressive tumor behavior in nasopharyngeal carcinoma a and in squamous cell carcinoma, which is explained by differences in the intrinsic properties of each cancer.
Shi et al. [44] have stated that BECN1 down regulation in hepatocellular cancer lead to increased antiapoptotic Bcl-XL expression, which allows increase hepatocellular carcinoma cells survival. The interaction between BECN1 and Bcl-XL inhibits autophagy and promotes the cancer progression.
Also more recent study by Zhao et al. have proved the role of BECN1 and LC3 as predictive biomarkers for metastatic colorectal carcinoma which was similar to our results [45].
We have found an inverse relationship between SALL 4and BECN1 (Spearman’s r=-0.198) (p=0.030).
Conclusion
We have proved negative correlations between SALL4 and BECN1 expression as SALL4 increased SOC progression, invasion and spread by inducing EMT and activation of Wnt/b-catenin signaling pathway, while BECN1 expression is related to favorable outcome due to increased autophagy and decreased emergence of aggressive subclones, moreover SALL4 expression was related to resistance to chemotherapy which leads to SOC progression, so therapeutic targets against both SALL4 and BECN1 can be beneficial recent management modalities for SOC.
Recommendations
It is recommended to do a prospective large scale study on larger number of patients to clarify detailed molecular roles of SALL4 and BECN1 in progression and spread of SOC.
References
- Konstantinopoulos PA, Matulonis UA (2013) CurÂrent status and evolution of preclinical drug development models of epithelial ovarian canÂcer. Front Oncol 3: 296.
- Jayson GC, Kohn EC, Kitchener HC, LederÂmann JA (2014) Ovarian cancer. Lancet 384: 1376-1388.
- Ying H, Qu D, Liu C, Ying T, LV J, et al. (2015) Chemoresistance is associated with Beclin-1 and PTEN expression in epithelial ovarian cancers Oncology Letters 9: 1759-1763.
- Takebe N, Miele L, Harris PJ, Jeong W, Bando H, et al. (2015) Targeting Notch, Hedgehog, and Wnt pathways in cancer stem cells: clinical update. Nat Rev Clin Oncol 12: 445-464.
- Sakaki-Yumoto M, Kobayashi C, Sato A, Fujimura S, Matsumoto Y, et al. (2006) The murine homolog of SALL4, a causative gene in Okihiro syndrome, is essential for embryonic stem cell proliferation, and cooperates with SALL1 in anorectal, heart, brain and kidney development. Development 133: 3005–3013.
- Ma Y, Cui W, Yang J, Qu J, Di C, et al. (2006) SALL4, a novel oncogene, is constitutively expressed in human acute myeloid leukemia (AML) and induces AML in transgenic mice. Blood 108: 2726-2735.
- Zhang X, Yuan X, Zhu W, Qian H, Xu W, et al. (2015) SALL4: an emerging cancer biomarker and target. Cancer Lett 357: 55-62.
- Kundu M, Thompson CB (2008) Autophagy: basic principles and relevance to disease. Annu Rev Pathol 3: 427–455.
- Koren I, Kimchi A (2012) Cell biology. Promoting tumorigenesis by suppressing autophagy. Sci 338: 889-890.
- Baspinar S, Bircan S, Orhan H, Kapucuoglu N, Bozkurt KK (2014) The relation of beclin 1 and bcl‑2 expressions in high grade prostatic intraepithelial neoplasia and prostate adenocarÂcinoma: A tissue microarray study. Pathol Res Pract 210: 412‑418.
- Sun Q, Fan W, Zhong Q (2009) Regulation of Beclin 1 in autophagy. Autophagy 5: 713–716.
- Edinger AL, Thompson CB (2003) Defective autophagy leads to cancer. Cancer Cell 4: 422–424.
- Kang KF, Wang XW, Chen XW, Kang ZJ, Zhang X, et al. (2013) Beclin 1 and nuclear factor-κBp65 are up-regulated in hepatocellular carcinoma. Oncol Lett 5: 1813–1818.
- Prat J, FIGO Committee on Gynecologic Oncology (2014) Staging classification for cancer of the ovary, fallopian tube, and peritoneum. Int J Gynaecol Obstet 124: 1-5.Â
- Kurman RJ, Carcangiu ML, Herrington CS, Young RH (2014) WHO Classification of Tumours of Female Reproductive Organs. 4th ed.Lyon: International Agency for Research on Cancer.
- Hsu SM, Raine L, Fanger H (1981) Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem 29: 577-580.
- Hao L, Zhao Y, Wang Z, Yin H, Zhang X, et al. (2016) Expression and clinical significance of SALL4 and b-catenin in colorectal cancer. J Mol Hist  47: 117-128.
- Yong KJ, Chai L, Tenen DG (2013) Oncofetal gene SALL4 in aggressive hepatocellular carcinoma. N Engl J Med 369: 1170-1172.
- Yang M, Xie X, Ding Y (2016) SALL4 is a marker of poor prognosis in serous ovarian carcinoma promoting invasion and metastasis. Oncol Rep 35: 1796-1806.
- Ardalan Khales S, Abbaszadegan MR, Abdollahi A Raeisossadati R, Tousi MF, et al. (2015) SALL4 as a new biomarker for early colorectal cancers. J Cancer Res Clin Oncol 141: 229-235.
- Li A, Jiao Y, Yong KJ, Wang F, Gao C, et al. (2015) SALL4 is a new target in endometrial cancer. Oncogene 34: 63-72.
- Zhang L, Xu Z, Xu X, Zhang B, Wu H, et al. (2014) SALL4, a novel marker for human gastric carcinogenesis and metastasis. Oncogene 33: 5491-5500.
- Forghanifard MM, Khales SA, Javdani-Mallak A, Rad A, Farshchian M, et al. (2014) Stemness state regulators SALL4 and SOX2 are involved in progression and invasiveness of esophageal squamous cell carcinoma. Med Oncol 31: 922–929.
- Zeng SS, Yamashita T, Kondo M, Nio K, Hayashi T, et al. (2014) The transcription factor SALL4 regulates stemness of EpCAM-positive hepatocellular carcinoma. J Hepatol 60: 127-134.
- Li A, Yang Y, Gao C, Lu J, Jeong HW, et al. (2013) A SALL4/MLL/HOXA9 pathway in murine and human myeloid leukemogenesis. J Clin Invest 123: 4195-4207.
- Yi BR, Kim TH, Kim YS, Choi KC (2015) Alteration of epithelial‑mesenchymal transition markers in human normal ovaries and neoplastic ovarian cancers. Int J Oncol 46: 272-280.
- Davidson B, Holth A, Hellesylt E, Tan TZ, Huang RY, et al. (2015) The clinical role of epithelial‑mesenchymal transition and stem cell markers in advanced-stage ovarian serous carcinoma effusions. Hum Pathol 46: 1-8.
- Liu L, Zhang J, Yang X, Fang C, Xu H, et al. (2015) SALL4 as an Epithelial-Mesenchymal Transition and Drug Resistance Inducer through the Regulation of c-Myc in Endometrial Cancer. PLoS One 10.
- Hupfeld T, Chapuy B, Schrader V, Beutler M, Veltkamp C, et al. (2013) Tyrosinekinase inhibition facilitates cooperation of transcription factor SALL4 and ABC transporter A3 towards intrinsic CML cell drug resistance. Br J Haematol 161: 204–213.
- Du W, Ni L, Liu B, Ying Wei, Yubao Lv, et al. (2018) Upregulation of SALL4 by EGFR activation regulates the stemness of CD44-positive Oncogenesis 7: 36.Â
- Yang J (2018) SALL4 as a transcriptional and epigenetic regulator in normal and leukemic hematopoiesis. Biomark Res 6: 1.
- Yang ZJ, Chee CE, Huang S, Sinicrope FA (2011) The role of autophagy in cancer: therapeutic implications. Mol Cancer Ther 10: 1533–1541.
- Cai M, Zhenhua Hu, Juanjuan Liu, Gao J, Liu C, et al. (2014) Beclin 1 Expression in Ovarian Tissues and Its Effects on Ovarian Cancer Prognosis. Int. J. Mol. Sci 15: 5292-5303.
- Osman NA, Abd El-Rehim DM, Kamal IM (2015) Defective Beclin-1 and elevated hypoxia-inducible factor (HIF)-1α expression are closely linked to tumorigenesis, differentiation, and progression of hepatocellular carcinoma. Tumour Biol 36: 4293–4299.
- Wu X, Chen J, Cao Q, Dong M, Lin Q, et al. (2012) Beclin 1 activation enhances chemosensitivity and predicts a favorable outcome for primary duodenal adenocarcinoma. Tumor Biol 34: 713–722.
- Fei B, Ji F, Chen X, Liu Z, Li S, et al. (2016) Expression and clinical significance of Beclin‑1 in gastric cancer tissues of various clinical stages. Oncol Lett 11: 2271-2277.
- Miracco C, Cosci E, Oliveri G, Luzi P, Pacenti L, et al. (2007) Protein and mRNA expression of autophagy gene Beclin-1 in human brain tumours. Int J Oncol 30: 429–436.
- Zhao Y, Chen S, Gou WF, Xiao LJ, Takano Y, et al. (2014) Aberrant Beclin-1 expression is closely linked to carcinogenesis, differentiation, progression, and prognosis of ovarian epithelial carcinoma. Tumour Biol 35: 1955-1964.
- Park KJ, Lee SH, Lee CH, Jang JY, Chung J, et al. (2009) Upregulation of Beclin 1 expression and phosphorylation of Bcl-2 and p53 are involved in the JNK-mediated autophagic cell death. Biochem Biophys Res Commun 382: 726–729.
- Furuya D, Tsuji N, Yagihashi A, Watanabe N (2005) Beclin 1 augmented cis-diamminedichloroplatinum induced apoptosis via enhancing caspase-9 activity. Exp. Cell Res 307: 26–40.
- Dong L.W, Hou Y.J, Tan Y.X, Tang L, Pan Y.F, et al. (2011) Prognostic significance of Beclin 1 in intrahepatic cholangiocellular carcinoma. Autophagy 7: 1222–1229.
- Wan XB, Fan XJ, Chen MY, Xiang J, Huang PY, et al. (2010) Elevated Beclin 1 expression is correlated with HIF-1alpha in predicting poor prognosis of nasopharyngeal carcinoma. Autophagy 6: 395–404.
- Tang JY, Fang YY, Hsi E, Huang YC, Hsu NC, et al. (2013) Immunopositivity of Beclin1 and ATG5 as indicators of survival and disease recurrence in oral squamous cell carcinoma. Anticancer Res 33: 5611–5616.
- Shi YH, Ding ZB, Zhou J, Qiu SJ, Fan J (2009) Prognostic significance of Beclin 1-dependent apoptotic activity in hepatocellular carcinoma. Autophagy 5: 380–382.
- Zhao H, Yang M, Zhao B (2017) Beclin 1 and LC3 as predictive biomarkers for metastatic colorectal carcinoma. Oncotarget 8: 59058–59067.
Citation: Fouad E, Salem A, Mahdy E (2018) Clinicopathological Significance of SALL4 and BECN1 Tissue Protein Expression in Serous Ovarian Carcinoma (SOC); An Immunohistochemical Study. J Clin Exp Pathol 8: 349. DOI: 10.4172/2161-0681.1000349
Copyright: © 2018 Salem AM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 3954
- [From(publication date): 0-2018 - Dec 09, 2025]
- Breakdown by view type
- HTML page views: 3007
- PDF downloads: 947