Clustrin and Resistin as Biomarkers in Metabolic Syndrome and Diabetes Mellitus in Egyptian Females
Received: 03-Aug-2021 / Accepted Date: 03-Sep-2021 / Published Date: 10-Sep-2021 DOI: 10.4172/1165-158X.1000204
Abstract
Background: In obesity, elevated fat mass is associated with changes in adipokines secretions. Adipokines are bioactive molecules have a role in the development of insulin resistance may link obesity and inflammation to the occurrence of metabolic syndrome and type 2diabetes as resistin and clustrin.
Objective: To evaluate Resistin and Clusterin level with their m RNA level in Egyptian females with DM and MS and to find its correlation with other MS parameters.
Subjects and Methods: Three groups of Egyptian females classified into group 1 (30 DM patients), group 2 (30 MS patients) and group 3 (30 healthy volunteers). All participants were subjected to the full clinical assessment including plasma cholesterol, LDL, HDL, triglycerides, HOMA-IR, hs CRP, Resistin and Clusterin besides Gene expression of Resistin and Clusterin.
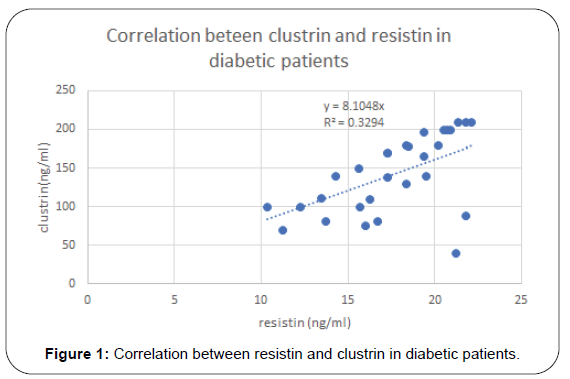
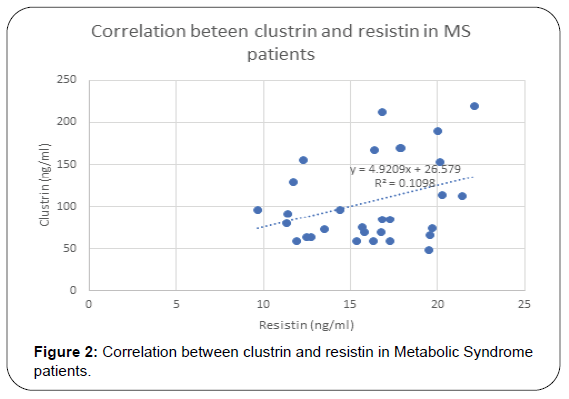
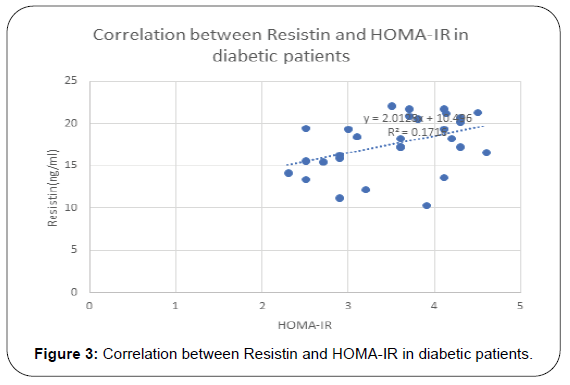
Results: Serum level of Clusterin was elevated in MS and DM patients with statistical significance. Also, it was found to has positive correlation with lipid molecules, TG (p=0.001), cholesterol (p=0.004) and LDL (p=0.03), BMI (p=0.008), HOMA-IR (p=0.002) and hs-CRP (p=0.01). Resistin was elevated in diabetic and MS patients and positively correlated TG (r= 0.24, p= 0.06), total cholesterol (r=0.23, p=0.02), and hs-CRP (r= 0.33, p=0.01), HOMA-IR(r=0.38, p=0.002) and Clustrin (r=0.38, p=0.002), Resistin and Clustrin mRNA expression showed significantly higher levels of in diabetec patients compared to controls.
Conclusion: plasma Resistin and Clusterin level could be a relevant and convenient biomarker for DM and MS and it may predict the risk of MS sequelae and could be efficient biomarker in both diseases.
Keywords: Diabetes mellitus; Metabolic syndrome; Resistin; Clusterin; HOMA-IR; hs-CRP
Introduction
Type 2 diabetes, characterized by insulin resistance (IR), is a complex chronic disorder of which the prevalence increased markedly in recent years [1]. The total number of people with diabetes is projected to rise from 171 million in 2000 to 366 million in 2030, more than 90% of whom had type 2 diabetes [2]. Obesity currently affects more than 600 million people worldwide by recent estimates and it was not only characterized by defects of insulin action but also insulin resistance (IR) which is defined as a state of cells failure to respond to insulin, resulting in the development of hyperglycemia [3,4].
Metabolic syndrome is a syndrome characterised by presence of many components as obesity, insulin resistance, high blood pressure and dyslipidemia but, with the upsurge of new findings, the list keeps increasing. The components now include hyperinsulinemia, insulin resistance, high blood pressure, central obesity and atherogenic dyslipidemia (increase in LDL-C, plasma triglycerides and decrease in HDL-C), endothelial dysfunction, genetic susceptibility, prothrombotic state, pro-inflammatory state and chronic stress, but, the diagnosis and recognition of metabolic syndrome depends on the particular criterion used. Metabolic risk factors that directly increase the risk of coronary heart disease, other forms of cardiovascular, atherosclerotic diseases and type 2 diabetes mellitus [5].
Resistin, also known as FIZZ3 or adipose tissue-specific secretory factor (ADSF), with a molecular weight of 12.5 kDa, encoded by the RETN gene [6]. Resistin, which was named after its insulin resistance ability by Steppan et al, in 2001, when they found antidiabetic drugs called thiazolidinedions [7]. The immunoneutralization of endogenous resistin improved blood glucose and insulin action in this model of type 2 diabetes. The treatment of normal mice with recombinant Resistin impaired glucose tolerance and insulin action [8-10]. From these findings the association between elevated circulating Resistin and insulin resistance in patients with type 2 diabetes has also been revealed. It is worth mentioning that peripheral blood mononuclear cells (PBMCs) are key producers of resistin in humans [11,12]. Its role in proinflammatory processes has been demonstrated in several studies [13,14].
Clusterin (CLU) is a predominantly secreted glycoprotein consisting of two chains–α -clusterin (α -Clu) and β -clusterin (β -Clu) that are linked by 5 disulphide bonds [15]. It is present in plasma as a soluble protein or as a component of a lipid-poor subclass of highdensity lipoproteins (HDLs) recent proteomic analyses revealed that CLU is also bound to LDL even its role in these lipoproteins is unknown [16]. Several roles have been ascribed to Clusterin such as complement inhibition [17,18], regulation of inflammation [19], lipid transport [20], apoptosis [21], cell differentiation [22], appetite regulation [23] and protein quality control in the extracellular space [24]. Clusterin has been shown to exhibit chaperone-like activity as well as amyloid aggregation of proteins in vitro [25,26]. Although its exact role in many conditions is not very clear, it is implicated in neurodegenerative disorders such as Alzheimer’s, several cancers, autoimmune disorders and chronic inflammatory disorders [27].
Clusterin has been shown to bind to promoter regions of Sterol Regulatory Element Binding Protein-1C (SREBP-1C), a master regulator of several lipid metabolic pathways, regulating its expression [28] and inhibiting hepatic lipid accumulation [29]. Many studies indicate a protective role for clusterin in metabolic disorders and are positively correlated with the risk of metabolic syndrome and type-II diabetes (T2DM) [30]. However, there are unclear reports on the correlation between serum Clusterin concentration and T2DM [31,32]. Clustrin has been found as one of the protein composition of HDL and it has been found that strong positive correlations between Clusterin levels in HDL and insulin sensitivity and it is possible that Clusterin depletion indicates the presence of dysfunctional HDL [33]. The role of resistin in insulin resistance remains controversial in humans. Several studies found positive correlations between resistin and insulin resistance in T2DM, obese and healthy individuals, this discovery appears to be supported by studies reporting significantly higher resistin levels in populations with T2DM [34,35]. Nevertheless, a substantial number of studies have failed to find correlations between resistin and insulin resistance [36]. The same problem face clustrin in metabolic syndrome and DM2 there is controversial results in many studies regarding its role in DM and metabolic syndrome. Moreover, up to our knowledge no other research covered the relation between clustrin and resistin in metabolic syndrome and DM2 in Egyptian female.
So, the aim of our study to find the level and relation between Clustrin and Resistin in metabolic syndrome and DM2 among Egyptian female.
Subjects and Methods
Study participants
This study is case-control prospective study was conducted on Egyptian females (90 participants) divided into 3 groups: group 1(30 DM type 2 patients), group 2 (30 Metabolic Syndrome patients) and group 3 (30 healthy volunteers). All study participants were recruited from Al-Azhar University Hospitals. Informed consent was obtained from all participants in the study prior to enrolment.
Inclusion criteria
For Group 1:
Female patients with type 2 DM with disease duration 5 years or more. For Group 2: Female patients with metabolic syndrome (Diagnosed according to the criteria of the National Cholesterol Education Program (NCEP) Adult Treatment Panel III).
The exclusion criteria
Pregnancy, chronic liver disease, chronic renal disease, thyroid diseases, acute and chronic inflammatory diseases, cardiovascular diseases, autoimmune diseases and DM Type I.
Patient assessment
Full history of the patents stressing in age, duration of DM then thorough clinical examination. Anthropometric measurements including the heights, weights, Waist circumference (WC) and Hip circumference (HC) then the body mass index (BMI) was calculated as the Weight/height2 (kg/m2) and the waist-hip ratio(WHR) was calculated as waist measurement divided by hip measurement.
Blood pressure was measured from the right arm after a minimal resting time of 25 minutes.
Sample collection
A volume of 8 ml of venous blood was drawn from each subject after overnight fasting (8 -12 Hrs) in 2 tubes one containing EDTA or sodium fluoride and oxalate for Complete Blood count, ESR, RNA extraction and blood glucose the other was red vacutainer tube (plain). The later was left to be clotted then centrifuged (at 1500 g for 15 minutes) was used for assay of lipid profile and fasting insulin, hs-CRP, Clustrin and Resistin.
Analytical methods: Lipid profile and fasting blood sugar were assayed using fully automated chemistry analyzerBiolis 50i Superior supplied by Life Trade Egypt,ESR was assayed by Westergreen method, Fasting Insulin was assayed by human insulin ELISA kit (Thermo fisher, Catalog KAQ1251). Calculation of Insulin resistance index (HOMAIR) according to the equation: Fasting insulin (mU/L) x fasting glucose (mg/dL)/405.
Assay of serum Clustrin and resistin: Clustrin and resistin concentrations were measured using commercially available enzyme –linked immunosorbent assay (ELISA) kit supplied by Bioassay Technology Laboratory and Human Resistin ELISA Kit (ab183364) supplied by Abacam.
Assay of hs–CRP: by commercially available ELISA Kit supplied by DRG International Inc.
Gene expression analysis (clustrin and resistin m RNA measurement)
Sample preparation and RNA isolation: Total RNA was isolated according to RNA isolation kit (Gentra, Minneapolis, MN 55441 USA) following the manufacturer’s protocol. The purity and integrity of total RNA were monitored by the absorbance of ultraviolet light spectrophotometrically at 260/280 nm.
Reverse transcription and complementary DNA (cDNA) synthesis: For the synthesis of complementary DNA (cDNA), the extracted RNA was reverse transcribed by Quanti Tect SYBR Green reverse transcription (RT–PCR) kit (Qiagen; catalog no.204243) as recommended by the manufacturer. 2 μg of RNA was reverse transcribed in a final volume of 40 μL. The reverse transcription reaction was completed at 25°C for 10 min, 42°C for 15 min and 99°C for 5 min using (Perkin Elmer Gene Amp PCR System 2400). The cDNA was stored at −20°C until time of analysis.
Measurement of clustrin and resistin mRNA expression by real time polymerase chain reaction: Expression levels of clustrin and resistin mRNA were determined by real time polymerase chain reaction using Step One TM System (Applied Biosystems). Glyceraldehyde- 3- phosphate dehydrogenase gene (G3PDH) was used as an internal control. Primer’s sequence of Clustrin is 3′-TGT TGG TCG AAC AGT CCA CAG and 5′-GCC AGT GTG AGA AGT GCC AAG).
Resistin is forward (CTGTTGGTGTCTAGCAAGACC) 104 Product length (bp) Resistin reverse (CCAATGCTGCTTATTGCCCTAAA).
Calculation of relative gene expression by usage of comparative CT method (ΔΔCT; 19).
Statistical methods
The program used in statistical methods was SPSS (Computer software package, version 25.0 (Chicago IL, USA) continuous data were represented as mean ± standard deviation (parametric data) while nonparametric data were represented as median and (range). For Comparisons studies between parametric data One-way ANOVA was applied while for non-parametric data Kruskal-Wallis one-way analysis of variance. Turkish HSD (post hoc test) for comparison of 2 different groups. Probability value (p value) less than 0.05 was considered significant. In correlation studies Pearson correlation coefficient was applied for parametric data while Spearman’s correlation coefficient was applied for nonparametric data.
Results
Results of this study are shown in Tables 1-4 and Figures 1-3. Descriptive statistics of different studied parameters in all studied groups as mean ±SD. One way ANOVA between studied groups regarding weight, height, BMI, WHR, TG, cholesterol, LDL, HDL, HOMA-IR, hs-CRP, resistin and clustrin was represented in Table 1. There are statistically significant differences in all parameters with p value < 0.05, while no statistically significant difference regarding age between all studied groups (Table 1).
| Groups | (I) | (II) | III | P-value |
|---|---|---|---|---|
| Parameters | DM (Diabetes mellitus) | MS (Metabolic syndrome) | Control | (One way ANOVA) |
| Age (year) | 36. ± 6.4 | 33.2 ± 5.5 | 33.6 ± 5.2 | NS |
| Weight (kg) | 81.9 ± 9.1 | 82.33 ± 12.7 | 71.9 ± 4.8 | 0.000* |
| Height (metre) | 1.6 ± 0.03 | 1.64 ± 0.03 | 1.63 ± 0.04 | 0.ooo* |
| BMI | 30.9 ± 3.8 | 31.8 ± 3.6 | 28.9 ± 1.8 | 0.001* |
| Weight circumference (Wc) | 104 ± 5.5 | 102.6 ± 5.8 | 90.3 ± 4.2 | 0.000* |
| Waist/hip ratio (WHR) | 0.80 ± 0.04 | 0.90 ± 0.03 | 0.87 ± 0.01 | 0.000* |
| Total Cholesterol (mg/dL) | 214 ± 21.07 | 224 ± 34 | 171.1 ± 15 | 0.000* |
| TG (mg/dl) | 162.5 ± 25.04 | 168.2 ± 27.6 | 103.4 ± 15.9 | 0.000* |
| HDL – Cholesterol(mg/dL) | 44.3 ± 4.8 | 43.5 ± 4.7 | 56.2 ± 6.9 | 0.000* |
| LDL (mg/dL) | 139 ± 22.5 | 143.5 ± 27.6 | 94.6 ± 14 | 0.000* |
| hs-CRP *(mg/L) | 3.4 ± 1.3 | 3.6 ± 1.5 | 1.3 ± 0.5 | 0.000* |
| HOMA-IR | 3.5 ± 0.6 | 3.4 ± 0.9 | 1.1 ± 0.2 | 0.000* |
| Clustrin (ng/L) | 99.5 ± 45.8 | 95.4 ± 41.9 | 43.7 ± 14.5 | 0.001* |
| Resistin (ng/mL) | 17.6 ± 3.3 | 16.1 ± 3.3 | 6.8 ± 2.9 | 0.000* |
The comparisons using the student one way ANOVA for parametric data and Kruskal-Wallis H test for non-parametric data, p > 0.05: No significant difference, p< 0.05*: Significant difference. NS non-significant.
Table 1: Baseline clinical and laboratory characteristics of the studied groups (control, diabetic and metabolic syndrome)-one way ANOVA amongst studied groups.
| Groups | (I) & (II) DM & MS | (II) & (III) MS & Control | I & III DM & Control |
|---|---|---|---|
| Parameters | P value | P value | P value |
| Weight (kg) | NS | 0.0001* | 0.003* |
| Height (metre) | 0.000* | NS | 0.0025* |
| BMI | NS | 0.001* | 0.04* |
| Weight circumference (Wc) | NS | 0.000* | 0.000* |
| Waist/hip ratio (WHR) | 0.000* | 0.005* | 0.000* |
| Total Cholesterol (mg/dl) | NS | 0.000* | 0.000* |
| TG (mg/dL) | NS | 0.000* | 0.000* |
| HDL (mg/dL) | NS | 0.000* | 0.000* |
| LDL (mg/dL) | NS | 0.000* | 0.000* |
| hs-CRP (mg/L) | NS | 0.000* | 0.000* |
| HOMA-IR | NS | 0.000* | 0.000* |
| clustrin (ng/L) | NS | 0.0007* | 0.0003* |
| Resistin(ng/mL) | NS | 0.000* | 0.000* |
Table 2: Post-hoc test -Tukey HSD (Honestly significant difference amongst all studied groups).
| Parameter | Resistin (ng/mL) | Resistin (ng/mL) | ||
|---|---|---|---|---|
| Group 1 (Diabetes mellitus) | Group 11 (Metabolic syndrome) | |||
| R | P value | R | P value | |
| Age (year) | -0.16 | NS | - | - |
| Weight(kg) | 0.65 | <0.001 | 0.31 | 0.01 |
| Body Mass Index (BMI)(KG/M2) | 0.64 | <0.001 | - | - |
| Weight circumference (Wc) | 0.06 | NS | - | - |
| Waist/hip ratio (WHR) | 0.13 | NS | - | - |
| Total Cholesterol (mg/dl) | 0.33 | 0.01 | 0.33 | 0.02 |
| TG (mg/dl) | 0.24 | NS | 0.24 | NS |
| HDL(mg/dl) | 0.22 | NS | 0.14 | NS |
| hs-CRP (mg/L) | 0.57 | <0.001 | 0.33 | 0.01 |
| HOMA-IR | 0.42 | <0.001 | 0.38 | 0.002 |
| Clustrin(ng/L) | 0.52 | <0.001 | 0.33 | 0.007 |
Table 3: Correlation studies between serum resistin and biochemical variables in DM and metabolic syndrome patients.
| Parameter | Clustrin (ng/l) | Clustrin (ng/l) | ||
|---|---|---|---|---|
| Metabolic syndrome 11 | Diabetes mellitus 1 | |||
| R | P | R | P | |
| Age (year) | -0.34 | NS | -0.2 | NS |
| Weight (kg) | 0.51 | 0.003 | 0.17 | NS |
| Body Mass Index (BMI) | 0.47 | 0.008 | 0.18 | NS |
| Weight circumference (Wc) | 0.46 | 0.01 | - | - |
| Waist/hip ratio (WHR) | 0.2 | NS | - | - |
| Total Cholesterol (mg/dl) | 0.5 | 0.004 | 0.12 | NS |
| TG (mg/dl) | 0.55 | 0.001 | 0.2 | NS |
| LDL (mg/dl) | 0.38 | 0.03 | -0.2 | NS |
| HDL (mg/dl) | 0.2 | NS | 0.19 | NS |
| hs-CRP (mg/L) | 0.46 | 0.01 | 0.6 | <0.001* |
| HOMA-IR | 0.54 | 0.002 | 0.24 | 0.05* |
Table 4: Correlation studies between serum Clustrin and biochemical variable in metabolic syndrome and diabetic patients.
Evaluation the difference between all studied groups by post -hoc test (Tukey HSD) to evaluate the difference between each 2 groups reveals statistically significant differences between diabetic patients and control. The same finding regarding differences between metabolic syndrome patients and controls. While no statistical differences between metabolic syndrome and diabetes mellitus in most of studied parameters (Table 2).
Positive correlation between Resistin and most studied parameters in Diabetic patients. While there was no- significant correlation between Resistin and age, WC, WHR, TG and HDL in studied groups. Regarding patients with metabolic syndrome there are positive correlation between resistin and clustrin, weight, clustrin, hs-CRPand HOMA -IR while there are non-significant correlation between resistin and (TG and HDL) (Table 3).
For clustrin and different studied parameters in metabolic syndrome patients, there are positive correlation between Clustrin and Wt, BMI, Wc, serum cholesterol, TG, LDL, hs-CRP and HOMA-IR in Metabolic syndrome patient group. While there was no significant correlation between clusrtin and age and HDL in Metabolic Syndrome groups. Regarding diabetic patients positive correlation is found between serum clustrin and resistin, hs- CRP, HOMA-IR in Diabetes Mellitus patient group as shown in (Table 4).
In addition, the results of CLU mRNA and resistin m RNA expression showed significantly higher levels of CLU and resistin mRNA in patients with diabetes compared to controls.
Discussion
Resistin an adipokine first discovered by Steppan in 2001 has been shown to induce insulin resistance in rodents. Increased resistin levels were also found in diet-induced or genetically obese mice, in human studies, individuals with severe insulin resistance had higher resistin levels than individuals with normal insulin action [37]. Therefore, they hypothesized that resistin might also play a role in insulin resistance, possibly providing new therapeutic targets for the treatment and prevention of diabetes
There are many theories supposed to explain the role of resistin in insulin resistance as that resistin playsa role in pro-inflammatory processes. In vitro studies suggested that expression of resistin have been the result of the production of the pro-inflammatory cytokines, such as tumor necrosis factor-a (TNF-a) and interleukin-6 (IL-6). t TNF-a signaling pathway activates intracellular kinases that inhibit insulin receptor signaling via serine phosphorylation of insulin receptor substrate 1 (IRS-1), IL- 6 and adiponectin also regulate insulin sensitivity [38].
Our results showed increase in resist in level in DM and metabolic syndrome. Where resistin level in diabetic patients is (17.3 ± 3.3), in MT patient (16.1 ± 3.3) and control (6.8 ± 2.9)
These results are hand by hand with meta-analysis done by Kaizhen Su et al. who concluded that resist in levels are correlated with insulin resistance in obese and T2DM patients, and this correlation was associated with elevated resistin levels [39].
In addition, the same results were obtained by Al-Harithy et al. (2005) who reported a positive correlation between resistin levels and insulin resistance in diabetic women and obese or overweight nondiabetic women [40].
A close study was done in Egypt by Mabrouk et al. and they found a positive correlation only in obese diabetic Egyptian subjects, but not in obese non-diabetic groups [41].
However, some studies failed to find a significant correlation between resistin levels and insulin resistance in diabetic groups. Bu et al. found resistin levels had no relationship to IR in both T2DM group and normoglycemic group [42]. Park et al. also observed these results in a cross-sectional study [43].
It has been suggested that resistin causes insulin resistance when insulin reaches a certain critical level. There are also several other hypotheses regarding the controversial correlation between resistin and insulin resistance. Several single nucleotide polymorphisms (SNPs) of the resistin RETN gene have been reported to be associated with resistin concentration, while this association remains controversial in various ethnicities, possibly explaining the conflicting results of the correlation [44].
Moreover, in meta-analysis conducted by Shi-Min Hu, et al. they found higher maternal serum resistin level is related to GDM risk and suggested that the serum level of resistin may be related to the severity of GDM [45].
A number of treatments targeting adipokines have recently emerged. For example, monoclonal antibody infliximab, which neutralizes TNF-a, is a new approach aimed at inflammation associated insulin resistance [46]. That study indicated that resistin antagonisation might be a therapy targeting insulin resistance in the patients with hyper resistinemia. So, we hope a new therapy to reduce resistin levels may alleviate insulin resistance in the future.
In diabetic patients our results showed that resistin has positive correlation with weight (r= 0.56, p= 0.001), BMI (r=0.64, p=<0.001) total cholesterol (r=0.33, p=0.01), and hs-CRP (r= 0.57, p=0.01), HOMA-IR (r=0.42, p=<0.001) and clustrin (r=0.52, p=<0.001) and not correlated with HDL.
In MS patients our results showed that resistin has positive correlation with Weght (r=0.31, p=0.01),TG in (r= 0.24, p= 0.06), total cholesterol (r=0.33, p=0.02), and hs-CRP (r=0.33, p=0.01), HOMA-IR (r=0.38, p=0.002) and Clustrin (r=0.33, p=0.007) and not correlated with HDL.
Our results go hand by hand by a large population-based study that found circulating resistin levels have a strong positive correlation with fasting levels of plasma TG [47]. In Indian men, serum resistin levels are positively correlated with TG and very low-density lipoprotein (VLDL) [48].
Osawa et al. showed a positive correlation between serum resistin and TG levels in levels in Japanese T2DM patients [49]. Luis et al. showed a significant correlation among resistin levels and TG in patients with morbid obesity [50]. Furthermore, a study on general population (6637 normal subjects) in Spain, between 2000 and 2005, showed a strong positive correlation between serum levels of resistin and triglyceride in diabetic and MS patients [51].
However, in Taiwan study, no correlation was found between plasma levels of resistin and triglycerides [52]. Moreover, among Iranian population, also no significant correlation between resistin serum levels and TG was observed [53].
Regarding cholesterol correlation our results are in agreement with a study, which was conducted in China, another one among Iranian population, serum levels of resistin were not found to be correlated with levels of HDL-C. However, Luis et al. have shown that there is no significant relationship between serum levels of resistin and cholesterol in obese female patients. Also, Hsu BG have found a positive correlation between serum levels of resistin and HDL.
Regarding correlation of resistin and WC and WHR our results agreed a study on north Indians indicated that plasma resistin levels were well correlated with waist circumference and waist/hip ratio (WHR) [54].
As regards correlation between resitin and BMI the same results were obtained by Chanchay Swho found a significant correlation of plasma resistin with body mass index (BMI), waist circumference, and WHR in nondiabetic and diabetic overweight/obese [55]. Furthermore, the serum resistin concentration was positively correlated with BMI, percent of body fat (BF%), and WHR in obese children [56]. Also, another study showed that in type 2 diabetic patients, plasma levels of resistin had a significant positive correlation with BMI [57]. A study on Saudi women with type 2 diabetes mellitus showed that resistin correlated significantly and positively with hip circumferences in diabetic women, but no correlation was observed with hip, waist, and WHR in overweight and obese nondiabetic subjects [58].
A significant positive correlation has been reported between blood resistin concentration against waist and hip circumferences in patients with myocardial infarction [59].
study conducted on Spanish female obese patients showed that serum resistin was correlated with weight, fat mass, waist circumference of obese subjects, although, serum resistin was not associated with the presence of metabolic syndrome [60]. These discrepancies in results can result from different sample size or genetic groups, gender, and also the studied disease in experiments.
In MS patients Also we found that plasma Clu levels were correlated with weight ( r=0.51, p = 0.003), BMI (r=0.47, p=0.008), wc (r=0.46, p=0.01), total cholesterol=(r=0.05, p=0, 004)TG=(r-0.55, p=0.001) LDL (r=0.38, p=0.03), hs CRP (r=0.46, p=0.01) and HOMA-IR (r=0.54, p=0.002). These results are hand by hand with previous study showed Clu levels in MS Mexican children However, another previous study reported that, plasma Clu levels reported no difference between obese and lean adolescents [61].
In diabetic patients plasma Clu levels were correlated with hs CRP (r=0.4, p=0.001) and HOMA-IR (r=-0.24, p=0.05) and resistin (r=0.6, p=<0.001).
Our results are in agreement with Andrew N. Hoofnagle, 2010 who found strong negative.
Correlation between the concentration of clusterin in HDL and both insulin sensitivity and BMI in two different populations of male subjects.
Surprisingly, we noticed conversion of the correlation between serum clusterin and each of BMI , TG , LDL and cholesterol from positive in MS patients to negative in diabetic patients, unlike what was noticed in case of resistin correlation with each of these parameters. This may point to the different nature of the role of elevation of these two adipokines. Clusterin plays an active protective role against LDL aggregation. So high expression of Clu in atherosclerotic lesions could be a response of arterial wall to LDL aggregation.
Clusterin might play a protective role in MS through its chaperone functions that assists the clearance of cell debris from ECM via endocytosis and lysosomal degradation, these protective roles may be lost with the development of type 2 diabetes.
Our results found that m RNA of clustrin is higher in diabetic patients than control which goes hand by hand with He J who found the same results.
In addition, our results found that clustrin gene expression is increased in diabetic paients than control which is supported by Michael W. Rajala.
Conclusion
Resistin and clustrin have a potential to be used as a biomarker for the diagnosis of DM and Metabolic Syndrome patients in Egyptian female patients and could be used as biomarker for diagnosis of diabetes and MS. It is imperative to suggest that an extensive study is warranted to further establish the association between serum resistin and clustrin with the development of DM and MS and its risk factors in this particular group.
Limitation of Study
Nevertheless, potential limitations of the present study have to be addressed. We realize that being a case -control study, the predictive power to explore the possible relationship between serum clustrin and resistin with the development of DM and MS in this subtribe cannot be ascertained.
Consent to Participate
Informed consent was obtained from all individual participants included in the study.
Consent to Publish
In the present study, all the testing procedures were performed using non-invasive techniques and adhering to the conditions of the ethical approval committee of the institute. Agreement with written knowledgeable consent was gained from the participant.
Conflict of Interests
The authors have no conflict of interests to report.
Data Availability Statements
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Author Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by [Noura Mostafa Mohamed], [Ahmed Fathy Abd El-Aziz] and [Youssef Nassar]. The first draft of the manuscript was written by [Noura Mostafa Mohamed] and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
References
- De Fronzo, R. A., Ferrannini, E., Groop, L., Henry, R. R., Herman, W.nH., Holst, J. J., et al.nType 2 diabetes mellitus.nNat Rev Dis Primers., 2015;1: 15019.
- GBD Disease and Injury Incidence and Prevalence Collaborators.nGlobal, regional, and national incidence, prevalence, and years lived withndisability for 310 diseases and injuries, 1990–2015: a systematic analysis fornthe Global Burden of Disease Study 2015.nLancet., 2016;388: 1545-1602.
- Lois, K., & Kumar, S.nObesity and diabetes.nEndocrinol Nutr., 2009;56: 38-42.
- Sah, S. P., Singh, B., Choudhary, S., & Kumar, A.nAnimal models of insulin resistance: a review.nPharmacol Rep., 2016;68(6): 1165-1177.
- Akpan, U. & Bassey, I. E.nBiomarkers of metabolic syndrome in male cigarette smokers in Calabar,nSouthern.nNigeriaN Z J Med Lab Sci., 2019;73: 119-125.
- Wang, H., Chu, W. S., Hemphill, C., & Elbein, S. C.nHuman resistin gene: molecular scanning and evaluation of association withninsulin sensitivity and type 2 diabetes in Caucasians.nJ Clin Endocrinol Metab., 2002;87(6): 2520-2524. doi: 10.1210/jcem.87.6.8528
- Steppan, C. M., Bailey, S. T., Bhat, S., Brown, E. J., Banerjee, R. R., Wright, C.nM., et al.nThe hormone resistin links obesity to diabetes.nNature., 2001;409(6818): 307-312.
- Muse, E. D., Obici, S., Bhanot, S., Monia, B. P., McKay, R. A., Rajala, M. W.,net al.nRole of resistin in diet-induced hepatic insulin resistance.nJ Clin Invest., 2004;114(2): 232-239.
- Dietze, D., Koenen, M., Rohrig, K., Horikoshi, H., Hauner, H., & Eckel, J.nImpairment of insulin signaling in human skeletal muscle cells by co-culturenwith human adipocytes. Diabetes., 2002;51(8): 2369-2376.
- Minn, A. H., Patterson, N. B., Pack, S., Hoffmann, S. C., Gavrilova, O., Vinson,nC., et al.nResistin is expressed in pancreatic islets.nBiochem Biophys Res Commun., 2003;310(2): 641-645.
- Patel, L., Buckels, A. C., Kinghorn, I. J.,Murdock, P. R., Holbrook, J. D.,nPlumpton, C., et al.nResistin is expressed in human macrophages and directly regulated by PPARngamma activators.nBiochem Biophys Res Commun., 2003;300(2): 472-476.
- Kusminski, C. M., McTernan, P. G., & Kumar, S.nRole of resistin in obesity, insulin resistance and type II diabetes.nClin Sci., 2005;109(3): 243-256.
- Kaser, S., Kaser, A., Sandhofer, A., Ebenbichler, C. F., Tilg, H., & Patsch, J. R.nResistin messenger-RNA expression is increased by proinflammatoryncytokines in vitro.nBiochem Biophys Res Commun., 2003;309(2): 286-290.
- Bokarewa, M., Nagaev, I., Dahlberg, L., Smith, U., & Tarkowski, A.nResistin, an adipokine with potent proinflammatory properties.nJ Immunol., 2005;174(9): 5789-5795.
- de Silva, H. V., Harmony, J. A., Stuart, W. D., Gil, C. M. & Robbins, J.nApolipoprotein J: structure and tissue distribution.nBiochemistry., 1990;29(22): 5380-5389.
- Baralla A, Sotgiu E, Deiana M, PasellaS,Pinna S, Mannu A, et al.nPlasma Clusterin and Lipid Profile: A Link with Aging and CardiovascularDiseasesnin a Population with a Consistent Number of Centenarians.nPLoS ONE 2015;106(6): e0128029.
- Fritz, I. B., Burdzy, K., Setchell, B. & Blaschuk, O.nRam rete testis fluid contains a protein clusterin which influences cell-cellninteractions in vitro.nBiol Reprod., 1983;28(5): 1173-1188.
- McDonald, J. F. & Nelsestuen, G. L.nPotent inhibition of terminal complement assembly by clusterin: characterizationnof its impact on C9 polymerization.nBiochemistry., 1997;36: 7464-7473.
- Falgarone, G. & Chiocchia, G.nIn Advances in Cancer Research.nElsevier Inc., 2009;104(8): 139-170.
- de Silva, H. V., Stuart, W. D., Duvic, C. R., Wetterau, J. R., Ray, M. J.,nFerguson, D. G., et al.nA 70-kDa apolipoprotein designated ApoJ is a marker for subclasses of humannplasma high density lipoproteins.nJ Biol Chem., 1990;265(22): 13240-13247.
- Kim, N., Yoo, J. C., Han, J. Y., Hwang, E. M., Kim, Y. S., Jeong, E. Y., et al.nHuman nuclear clusterin mediates apoptosis by interacting with Bcl-XL throughnC-terminal coiled coil domain.nJ Cell Physiol., 2012;227(3): 1157-1167.
- Ahuja, H. S., Tenniswood, M., Lockshin, R. & Zakeri, Z. F.nExpression of clusterin in cell differentiation and cell death.nBiochem Cell Biol., 1994;72(11-12): 523-530.
- Bajari, T. M., Strasser, V., Nimpf, J. & Schneider, W. J.nA model for modulation of leptin activity by association with clusterin.nFASEB J. 2003;17(11): 1505-1507.
- Carver, J. A., Rekas, A., Thorn, D. C. & Wilson, M. R.nSmall heat-shock proteins and clusterin: intra- and extracellular molecularnchaperones with a common mechanism of action and function?nIUBMB Life., 2003;55(12): 661-668.
- Poon, S., Treweek, T. M., Wilson, M. R., Easterbrook-Smith, S. B. & Carver, J.nA.nClusterin is an extracellular chaperone that specifically interacts with slowlynaggregating proteins on their off-folding pathway.nFEBS Lett., 2002;513(2-3): 259-266.
- Yerbury, J. J., Poon, S., Mehaan, S., Thompson, B., Kumita, J. R., Dobson, C.nM., et al.nThe extracellular chaperone clusterin influences amyloid formation and toxicitynby interacting with prefibrillar structures.nFASEB J., 2007;21(10): 2312-2322.
- Oh, G. S., Kim, G., Yoon, J., Kim, G. H. & Kim, S. W.nThe E-box-like sterol regulatory element mediates the insulin-stimulatednexpression of hepatic clusterin.nBiochem Biophys Res Commun., 2015;465(3): 501-506.
- Seo, H. Y., Kim, M. K., Jung, Y. A., Jang, B. K., Yoo, E. Y., Park, K. G., et al.nClusterin decreases hepatic SREBP-1c expression and lipid accumulation.nEndocrinology., 2013;154(5): 1722-1730.
- Daimon, M., Oizumi, T., Karasawa, S., Kaino, W., Takase, K., Tada, K., et al.nAssociation of the clusterin gene polymorphisms with type 2 diabetes mellitus.nMetabolism., 2011;60(6): 815-822.
- Liu, X. F., Yu, J. Q., Dalan, R., Liu, A. Q. & Luo, K. Q.nBiological factors in plasma from diabetes mellitus patients enhancenhyperglycaemia and pulsatile shear stress-induced endothelial cell apoptosis.nIntegr Biol Camb., 2014;6: 511-522.
- Rader, D. J.nMolecular regulation of HDL metabolism and function: implications for novelntherapies. J Clin Invest., 2006;116(12): 3090-3100.
- Hoofnagle, A. N., Wu, M., Gosmanova, A. K., Becker, J. O., Wijsman, E. M.,nBrunzell, J. D., et al.nLow Clusterin Levels in High Density Lipoprotein Associate with InsulinnResistance, Obesity, and Dyslipoproteinemia.nArteriosclerThromb Vasc Biol., 2010;30(12): 2528-2534.
- Su, K., Li, Y., Zhang, D., Yuan, J., Zhang, C., Liu, Y., et al.nRelation of Circulating Resistin to Insulin Resistance in Type 2Diabetes andnObesity: A Systematic Review and Meta-Analysis.nFront Physiol., 2019;10: 1399.
- Heilbronn, L. K., Rood, J., Janderova, L., Albu, J. B., Kelley, D. E., Ravussin,nE., et al.nRelationship between serum resistin concentrations and insulin resistance innnonobese, obese, and obese diabetic subjects.nJ Clin Endocrinol Metab., 2004;89(4); 1844-1848.
- Bu, J., Feng, Q., Ran, J., Li, Q., Mei, G., & Zhang, Y.nVisceral fat mass is always, but adipokines adiponectin and resistin arendiversely associated with insulin resistance in Chinese type 2 diabetic andnnormoglycemic subjects.nDiabetes Res Clin Pract., 2012;96(2): 163-169.
- Zaidi, S. I., & Shirwany, T. A.nRelationship of serum resistin with insulin resistance and obesity.nJ Ayub Med Coll., 2015;27(3): 552-555.
- Weigert, C., Hennige, A. M., Lehmann, R., Brodbeck, K., Baumgartner, F.,nSchauble, M., et al.nDirect cross-talk of interleukin-6 and insulin signal transduction via insulinnreceptor substrate-1 in skeletal muscle cells.nJ Biol Chem., 2006;281(11): 7060-7067.
- Al-Harithy, R. N., & Al-Ghamdi, S.nSerum resistin, adiposity and insulin resistance in Saudi women with type 2ndiabetes mellitus.nAnn Saudi Med., 2005;25(4): 283-287.
- Mabrouk, R., Ghareeb, H., Shehab, A., Omar, K., El-Kabarity, R. H., Soliman,nD. A., et al.nSerum visfatin, resistin and IL-18 in A group of Egyptian obese diabetic andnnon diabetic individuals. Egypt J Immunol., 2013;20(1): 1-11.
- Park, H., Hasegawa, G., Obayashi, H., Fujinami, A., Ohta, M., Hara, H., et al.nRelationship between insulin resistance and inflammatory markers andnanti-inflammatory effect of losartan in patients with type 2 diabetes andnhypertension.nClin Chim Acta., 2006;374(1-2): 129-134.
- Hivert, M. F., Manning, A. K., McAteer, J. B., Dupuis, J., Fox, C. S., Cupples,nL. A., et al.nAssociation of variants in RETN with plasma resistin levels and diabetesrelatedntraits in the Framingham Offspring Study.nDiabetes 2009;58: 750-756.
- Hu, S. M., Chen, M. S., & Tan, H. Z.nMaternal serum level of resistin is associated with risk for gestational diabetesnmellitus: A meta-analysis.nWorld J Clin Cases., 2019;7(5): 585-599.
- Ursini, F., Naty, S., & Grembiale, R. D.nInfliximab and insulin resistance.nAutoimmun Rev., 2010;9: 536-539.
- Norata, G., Ongari, M., Garlaschelli, K., Raselli, S., Grigore, L., & Catapano, A.nPlasma resistin levels correlate with determinants of the metabolic syndrome.nEur J Endocrinol., 2007;156: 279-284.
- Singh, A. K., Tiwari, S., Gupta, A., Shukla, K. K., Chhabra, K. G., Pandey, A.,net al.nAssociation of resistin with insulin resistance and factors of metabolicnsyndrome in north Indians.nIndian J Clin Biochem 2015;30: 255-262.
- Osawa, H., Ochi, M., Tabara, Y., Kato, K., Yamauchi, J., Takata, Y., et al.nSerum resistin is positively correlated with the accumulation of metabolicnsyndrome factors in type 2 diabetes.nClin Endocrinol., 2008;69: 74-80.
- De Luis, D. A., Sagrado, M. G., Conde, R., Aller, R., & Izaola, O.nResistin levels and inflammatory markers in patients with morbid obesity.nNutr Hosp., 2010;25(4): 630-634.
- de León, A. C., González, D. A., Hernández, A. G., Coello, S. D., Marrugat, J.,nSánchez, J. J. A., et al.nRelationships between serum resistin and fat intake, serum lipid concentrationsnand adiposity in the general population.nJ AtherosclerThromb., 2014;21(5): 454-462.
- Hsu, B. G., Lee, C. J., Yang, C. F., Chen, Y. C., & Wang, J. H.nHigh serum resistin levels are associated with peripheral artery disease in thenhypertensive patients. BMC Cardiovasc Disord., 2017;17: 80.
- Asgary, S., SamsamShariat, S. Z., Ghorbani, A., Keshvari, M., Sahebkar, A., &nSarrafzadegan, N.nRelationship between serum resistin concentrations with metabolic syndrome and its components in an Iranian population.nDiabetes Metab Syndr Clin Res Rev., 2015;9: 266-270.
- de Luis, D., Sagrado, M. G., Conde, R., Aller, R., Izaola, O., & Primo, D.nLack of association of serum resistin levels with metabolic syndrome criteria innobese female patients. Clin Biochem., 2011;44: 1280-1283.
- Chanchay, S., Tungtrongchitr, R., Harnroongroj, T., Phonrat, B.,nRungseesakorn, O., Paksanont, S., et al.nPlasma resistin, insulin concentration in non-diabetic and diabetic, overweight/nobese Thai.nInt J Vitamin Nutr Res., 2006;76: 125-131.
- Liu, G., Fu, X., Jiang, L., Ma, X., & Yang, J.nSerum resistin concentration and insulin resistance in obese children.nChin J Pediatr., 2006;4: 114-117.
- Lu, H. L., Wang, H. W., Wen, Y., Zhang, M. X., & Lin, H. H.nRoles of adipocyte derived hormone adiponectin and resistin in insulinnresistance of type 2 diabetes.nWorld J Gastroenterol., 2006;12(11): 1747-1751.
- Al-Harithy, R. N., & Al-Ghemdi, S.nSerum resistin, adiposity and insulin resistance in Saudi.nAnn Saudi Med., 2005;25: 283-287.
- Piestrzeniewicz, K., Åuczak, K., Komorowski, J., Maciejewski, M., Wika, J. J.,n& Goch, J. H.nResistin increases with obesity and atherosclerotic risk factors in patients withnmyocardial infarction.nMetabolism., 2008;57(4): 488-493.
- Hoofnagle, A. N., Wu, M., Gosmanova, A. K., Becker, J. O., Wijsman, E. M.,nBrunzell, J. D., et al.nLow clusterin levels in high-density lipoprotein associate with insulinnresistance, obesity, and dyslipoproteinemia.nArterioscler Thromb Vasc Biol., 2010;30(12): 2528-2534.
- He, J., Dijkstra, K. L., Bakker, K., Bus, P., Bruijn, J. A., Scharpfenecker, M., etnal.nGlomerular clusterin expression is increased in diabetic nephropathy andnprotects against oxidative stress-induced apoptosis in podocytes.nSci Rep., 2020;10(1): 14888.
- MartÃnez, J. L., Bordel, S., Hong, K. K., & Nielsen, J.nGcn4p and the Crabtree effect of yeast: drawing the causal model of thenCrabtree effect in Saccharomyces cerevisiae and explaining evolutionaryntrade-offs of adaptation to galactose through systems biology.nFEMS Yeast Res., 2014;14(4): 654-662.
- Wyatt, L. H., & Ferrance, R. J.nThe musculoskeletal effects of diabetes mellitus.nJ Can Chiropr Assoc., 2006;50(1): 43-50.
- Rajala, M. W., Qi, Y., Patel, H. R., Takahashi, N., Banerjee, R., Pajvani, U. B.,net al.nRegulation of Resistin Expression and Circulating Levels in Obesity, Diabetes,nand Fasting.nDiabetes., 2004;53(7): 1671-1679.
Citation: Nassar Y, El-Azi AFA, Mohamed NM (2021) Clustrin and Resistin as Biomarkers in Metabolic Syndrome and Diabetes Mellitus in Egyptian Females. Cell Mol Biol 67: 204. DOI: 10.4172/1165-158X.1000204
Copyright: © 2021 Nassar Y, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2688
- [From(publication date): 0-2021 - Nov 25, 2025]
- Breakdown by view type
- HTML page views: 1897
- PDF downloads: 791