Communication Ability and Swallowing Function of Two Patients with Foix Chavany Marie Syndrome
Received: 31-Jul-2018 / Accepted Date: 03-Aug-2018 / Published Date: 11-Aug-2018 DOI: 10.4172/2472-5005.1000136
Keywords: Foix chavany marie syndrome; Anterior operculum syndrome; Dysarthria; Dysphagia; Rehabilitation
Introduction
Foix Chavany Marie Syndrome (FCMS) was first reported by Foix [1] and others, derived from lesions, also called anterior operculum syndrome. According to Foix et al., in the case of facial muscles leading to facial muscles, speech utterance organs, and mastication muscles, cases were limited by severe voluntary movement due to pseudobulbar paralysis, but automatic movements such as laughter and crying. It is said that the movement by automatic movement and reflection was relatively kept. In Weller’s review [2] article, 62 cases allowed the differentiation of five clinical types: (A) the classical and most common form associated with cerebro-vascular disease; (B) a subacute form caused by central nervous system infections; (C) a developmental form probably most of ten related to neuronal migration disorders; (D) a reversible form in children with epilepsy; and (E) a rare type as associated with neuro degenerative disorders. Symptoms of FCMS are articulation disorder, masticatory muscle paralysis, dysphagia, bilateral lower face paralysis. Although intentional exercise is severely impaired, the part where unintended exercise such as laughter, crying is relatively kept is different from pseudosphere paralysis [3]. A particular functional deficit to be addressed in rehabilitation includes impairments to communication secondary to dysarthria and dysphagia, both of which are obstacles to activities of daily living (ADL). There have been reported rehabilitative interventions focused on impairments of vocalization in previous studies from overseas [2], whereas in recent years in Japan there have been reports of rehabilitative measures for dysphagia [4]. Although it is a relatively rare syndrome, there are differences in progress, pathology and prognosis, but in the case we experienced this time, improvement in swallowing function was observed by intervention of rehabilitation, so we report it compared with previous study.
Case Studies
This study was approved by the ethics committee of Nittazuka Medical Welfare Center (approval no. 29-113 for new projects). Also, at the time of this study, patient consent was obtained.
Case 1
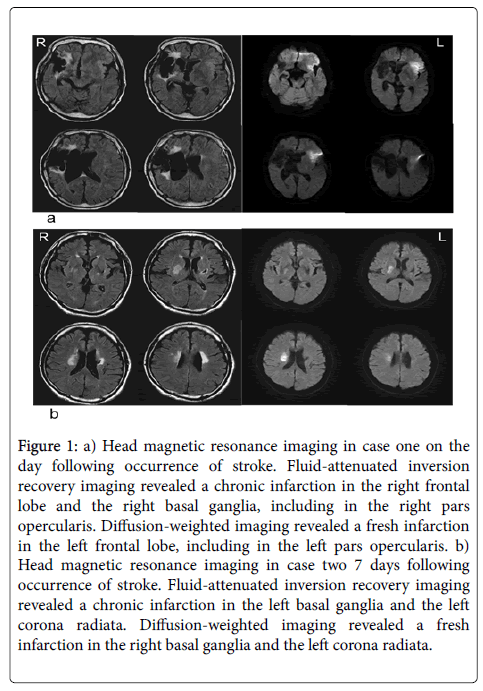
The patient was a 73-year-old man with a medical history of cerebral infarction 11 years ago, which resulted in paralysis of the entire left side of the body, but walking was possible using a cane, independence in ADL. In mid-April 2017, the patient was transported by ambulance and hospitalized following an incident of loss of consciousness. Cranial magnetic resonance imaging (MRI), right frontal cortex/frontal lobe, including the right pars opercularis, indicated a recent cerebral infarction of the left frontal lobe. A previous cerebral infarction of the basal ganglia and the left pars opercularis was further identified (Figure 1a).
Figure 1: a) Head magnetic resonance imaging in case one on the day following occurrence of stroke. Fluid-attenuated inversion recovery imaging revealed a chronic infarction in the right frontal lobe and the right basal ganglia, including in the right pars opercularis. Diffusion-weighted imaging revealed a fresh infarction in the left frontal lobe, including in the left pars opercularis. b) Head magnetic resonance imaging in case two 7 days following occurrence of stroke. Fluid-attenuated inversion recovery imaging revealed a chronic infarction in the left basal ganglia and the left corona radiata. Diffusion-weighted imaging revealed a fresh infarction in the right basal ganglia and the left corona radiata.
Initial SLP assessment
Initial assessment of the patient found that his eyes could not be kept open independently, indicating the II-10 level of consciousness per the Japan coma scale [5]. The patient responded to questions with the statement ‘yes’; however, it was difficult for him to speak meaningful words and he expressed no comprehension of word meaning. The patient further exhibited difficulty performing voluntary movements including opening and closing his lips. A significant increase in muscle tone was noted upon palpation of the face. Bilateral paralysis of the upper and lower limbs and face was observed. The patient maintained a relative sense of decorum and there was no decline in language comprehension. The patient answered questions primarily with the use of ‘yes’ or ‘no’ responses.
When spontaneous conversation and repetition were suggested, the patient became nervous and articulation became impossible. The patient experienced difficulty in conditions that urged vocalization in unison with the therapist, such as the expression of greetings or idiomatic expressions. Expression of vowel sounds was barely possible, and a remarkable restriction of voluntary movement of the oral structures, such as the opening and closing of the lips and movement of the tongue, was also observed. When told to ‘Please open your mouth’, increased muscle tension was only observed in the lower part of the face, and the patient experienced difficulty in opening the mouth. However, the patient’s response to other movement commands was more favourable. For example, when told to ‘Please lick your upper teeth with your tongue’, the patient was able to open his mouth. Additionally, when the patient was asked to hold a cylinder or rodshaped oral hygiene item in the right hand and independently bring it to the mouth, he could perform a general cleaning of the oral cavity. A further absence of muscle tension was noted when yawning or laughing, though a sufficient range of motion was observed in the lower jaw.
With regards to swallowing function, the patient was able to swallow both air and saliva after receiving oral care. A swallowing reflex reaction was obtained in the food test, and there was a further absence of choking, changes in breathing, and a small accumulation of food was observed in the mouth. As a result of poor movement of the tongue and lips, imp airment of food transfer was also observed. Despite this deficit, a swallowing response was observed upon food reaching the pharynx. The severity of this patient’s condition was determined to be a grade 3 on the Fujishima oral intake scale [6], and swallowing function training, including direct training, were initiated. With the International Dysphagia Diet Standardization Initiative [7], the foods level 4 and drinks level 4.
Intervention and course of rehabilitation
Training in speech and swallowing function was conducted by a speech-language-hearing therapist for 60 min a day, 7 days a week. Exercise training of the tongue, lip, and lower jaw, and breathing/vocal training and direct swallowing were carried out. Two months after the start of rehabilitation, the patient was able to ingest not just ‘swallowing jelly’, but also 30% of a paste meal. Although there was a lack of vocalization in certain cases, the patient could express simple idioms and greetings with relative ease. In situations where conscious focus on vocalization was required, the patient became nervous and had difficulty vocalizing. Although non-verbal, everyday conversation was possible through conversation partner observation of mouth movement. Approximately 4 months after the start of rehabilitation, almost an entire paste meal could be ingested with assistance that brings the spoon to the mouth, and a portion could be ingested independently. Articulation ability assessments identified an improvement in clearly understood vocalization and increased volume of the voice.
Case 2
The second patient discussed here is a 64-year-old man. The patient had experienced a cerebral infarction 6 years ago, after which he experienced mild motor aphasia and partial paralysis on the right side. As a result of paralysis on the left side of his body in early December 2016, the patient was transferred to the hospital by ambulance and hospitalized with a diagnosis of cerebral infarction. Cranial MRI revealed a previous cerebral infarction in left basal ganglia, which radiated in a crown shape. A further, new cerebral infarction was also identified in the right basal ganglia, which also radiated in a crown shape (Figure 1b).
Initial SLP assessment
The patient was clearly conscious at the time of initial intervention. Patient was non-verbal but able to respond to questions appropriately by nodding the head to indicate 'yes' and shaking to indicate 'no'. Writing was possible but there was a lack of expression of meaningful words. There was difficulty in the intentional opening and closing of the mouth and there was a remarkable increase in muscle tone of the face when touched. Brunnstrom stage [8] characterization of body function, the upper limb, fingers and lower limb of the left side were indicated at stage V. The upper limb, fingers and lower limb of the right side were indicated at stage VI of the Brunnstrom stage. Patients sometimes needed light assistance to be able to support the body by their carers, with the exception of consuming meals, required either minimal assistance or were self-sustained.
Manipulation of items with the left hand resulted in increased tension in the fingers until it became noticeable and separation of the fingers became difficult. This characteristic was not observed in the right hand, with which writing movements were conducted smoothly. Decorum was maintained and the patient was able to clearly express themselves in writing, writing, for example, ‘I want to be able to talk’ and ‘I want to be able to eat’. The ability to articulate in the context of intentional speech was lacking. Voluntary movement of the tongue, lips and organs of vocalization was also poor. The lower jaw could only be opened to 1 cm; however, during a yawn 5 cm could be achieved. The use of vocal sounds was confirmed during laughter and while the body was moving. Upon introspection, the patient related this phenomenon in writing with the following statement: ‘No sound will come out if I think about speaking’.
The patient was able to manipulate a paste meal independently with the use of a spoon in the right hand. During eating, muscle tension in the face would increase and the patient was able to direct the spoon into his slightly agape mouth. Transfer activity was further observed when food was placed on the tongue. Swallowing was performed with an absence of observable chewing movement. Videofluoroscopic examination of swallowing findings it was difficult to form a bolus and transport the food to the pharynx, so it was in a state of swallowing food in its original shape. Swallowing reflex is delayed for a while, but the ascending range of the larynx is normal and no aspiration occurs. A small amount of food residue was found around the pear-shaped recess and it was difficult to spit out own. Meal intake was approximately one-half of a serving. With the International Dysphagia Diet Standardization Initiative, the foods level 4 and drinks level 4.
Intervention and course of rehabilitation
Training on speech function and swallowing function was conducted by a speech-language-hearing therapist for 60 min a day, 7 days a week. Two months after the start of rehabilitation, there was a reduction in facial muscle tone and the opening range of the lower jaw was found to be 2 cm. Vocalization of oral speech became possible by the appropriation of humming sounds while shifting weight during walking. Despite the fact that the patient was able to bite through gum consistency items during training, paste meals were continued. The volume ingested was 80% of the full meal.
Discussion
In this study, we present two cases of FCMS, in which oral intake became possible consequent to the introduction of rehabilitative measures and temporal changes in the pathophysiology of the syndrome. Based on the 1926 report by Foix et al., FCMS was recognized as resulting from bilateral lesions of the central aspect of the lower anterior region of the operculum of the frontal cortex. In agreement with this, bilateral anterior opercular lesions were also observed in the two cases presented here. As a result of these bilateral lesions, paralysis occurs in various muscle groups, including vocalization organs, although symptoms of pseudobulbar paralysis are also present. The autonomic involuntary movements characteristic of cortical lesions are also reported in cases of FCMS [3,9].
In the cases presented here, there was a significant restriction of voluntary movements of the organs involved in vocalization, as well as exacerbated facial muscle tone. Interestingly, however, no abnormalities were found with yawning or laughter, in both cases a normal range of motion is achieved, which is a pathological condition distinguished from pseudobulbar paralysis. This particular dissociation of symptoms is explained by Alajouanine and the Baillarger-Jackson principle [10]. Aphasia and apraxia patients who experience difficulties in verbal and movement expression in a training room are able to freely express themselves when relaxing in their own rooms, a phenomenon commonly experienced in the clinical sphere [11]. In other words, while the voluntary movement behaviours characterized by Baillarger [12] are lost, some involuntary movements are preserved. Jackson [13] outlined a hierarchical conceptualization of the nervous system where the higher-order nervous system is unstable and purposeful while the lower-order nervous system is more stable and autonomic/automatic. In applying this principle here, a consequence of cortical damage could be loss of voluntary movement of the organs involved in speech/vocalization while those involuntary movements that mediate ‘lower’ emotional responses, such as laughing or yawning, are preserved. Difficulty in intentional, purposeful movement is usually mild in cases of unilateral damage. This is because the region of the lower half of the face, including the vocal organs, receives bilateral inputs. If the injury extends bilaterally, however, pseudobulbar paralysis and severe dysarthria and dysphagia will be observed [14]. FCMS is said to be a subtype of pseudobulbar paralysis, though individuals with FCMS exhibit characteristically unintentional automatic movements and, depending on the particular lesions present, may also experience apraxia of speech and buccofacial apraxia. While it is known that these symptoms are caused by an injury to the left central anterior region of the brain, the cases outlined in the current report demonstrate a remarkable impairment in the voluntary movement of the vocalization organs after bilateral injury at multiple time points. Particularly in the second case, which featured previous damage to the left hemisphere after which noticeable symptoms of verbal apraxia were not observed, only after an injury to the contralateral frontal lobe was increased muscle tone and impairments in voluntary movement was observed. Using functional MRI and a task in which words are repeatedly vocalized at a constant pace, Riecker et al. [15] reported increased blood flow to the left central anterior region only during vocalization of multi-syllable words with no meaning. A similar level of activation was observed with repetition of monosyllables and bilateral left and right movements of the tongue. During repetition of words, left dominant bilateral activation was observed around the midline.
Suzuki et al. [16] reported that in seven brain tumour cases, after performing intraoperative functional mapping by cortical electrical stimulation, when the lower gyrus precentralis was stimulated, speech stoppage, speech delay, and other related symptoms were seen. In most of these cases, impaired mouth and tongue movements were also observed. This has seldom been reported by prior research from overseas, however. In one report of a unilateral injury to the pars opercularis, FCMS, left-sided unilateral injury [17,18], and right-sided unilateral injury were reported. Based on this report, areas surrounding the pars opercularis, including central anterior regions, have been tied to functions related to higher-order movements such as executing situationally appropriate movements and intentional speech. Ogawa et al. [4] reported on the feeding and swallowing rehabilitation progress across four cases of difficult palpable or incompetent severe pseudobulbar paralysis and reported that only one patient was able to maintain oral intake. In this report, all four cases experienced bilateral facial paralysis, disordered lingual movement, and severe vocal disturbance, while their pharyngeal and oral function was maintained. Furthermore, in the three cases where oral intake became possible, these individuals experienced slightly better function in mouth opening.
While significant restrictions to oral function were observed in both cases reported here, swallowing was possible of a food bolus was transferred to the pharynx using a force, such as gravity. Table 1 compares the two cases described here with those introduced by Ogawa et al. [4]. With the exception of maintenance of the pharyngeal stage of swallowing, both cases are similar to those introduced by Ogawa et al., with severe impairments and restriction of purposeful movement of the organs of vocalization and dysphonia until the time of discharge. While there is a history of differing scientific opinions regarding the concept of swallowing apraxia, complications resulting from apraxia of the oral region has been identified. There have further been case reports of maintenance of the pharyngeal stage [19]. In the current cases too, dysphagia is thought to arise from impairments to voluntary movement of the organs of vocalization, and only if food is transferred to the pharyngeal region can swallowing be achieved through automatic reflexive movement. Further examination of compensatory movements in food transferal actions and the format of meals may be an important factor in the improvement of rehabilitative measures moving forward.
| Previous study (Ogawa, 2016) | This research | |||||
|---|---|---|---|---|---|---|
| Case 1 | Case 2 | Case 3 | Case 4 | Case 1 | Case 2 | |
| Age (y) | 65 | 70 | 63 | 83 | 74 | 64 |
| Gender | male | female | male | male | male | male |
| Disease | cardiogenic embolism | lacunar stroke | intracerebral haemorrhage | cardiogenic embolism | cerebral infarction | cerebral infarction |
| Site of lesion (new) | pars opercularis, the left frontal lobe | right putamen–corona radiata | right putamen | deep white matter of the left frontal lobe | right and left frontal lobes including the left pars opercularis | radiation from the right basal ganglia |
| Site lesion (old) | right frontal and temporal lobes | left radial coronary lacunar infarction | right putaminal haemorrhage | right frontal lobe ~ insular cortex, right occipital lobe | from the right frontal lobes to the basal ganglia and including the right pars opercularis | from the left basal ganglia to the corona radiate |
| Body function (at the start of rehabilitative intervention) | Brunnstrom stage right upper limb V, finger V, lower limb-VI left upper limb I, finger-I, lower limb I | light paralysis on both sides of the body | Brunnstrom stage right upper limb VI, finger V, lower limb VI, left upper limb I, finger I, lower limb I | no paralysis of any of the limbs | paralysis on both sides of the body | Brunnstrom stage right upper limb stage VI, finger VI, lower limb VI left upper limb V, finger V, lower limb V |
| ADL at the time of discharge from the hospital | completely assisted | completely assisted, supervised walking at short distances | completely assisted | supervised walking possible but primarily at short distances, completely assisted due to brain dysfunction | supervised walking at short distances, completely assisted | independence in walking, squatting position possible, independence |
| FIM at the time of Discharge from the hospital | FIM total: 46 FIM motor: 21 FIM cognitive: 25 | FIM total: 50 FIM motor: 33 FIM cognitive: 17 | FIM total: 36 FIM motor: 13 FIM cognitive: 23 | FIM total: 29 FIM motor: 22 FIM cognitive: 7 | FIM total: 27 FIM motor: 12 FIM cognitive: 15 | FIM total: 116 FIM motor: 20 FIM cognitive: 96 |
| Vocalization | nervousness, vocalization possible only with effort, absence of lisp | hoarse voice as a result of nervousness, hoarse wet voice, absence of lisp | inefficient | sometimes there is vocalization | nervousness, nervous voice as a result of effort, normal voice when laughing or in the event of a yawn | nervousness, nervous voice as a result of effort |
| Paralysis of the face | severe in both sides of the lower region | severe in both sides of the lower region | severe in both sides of the lower region | paralysis of both sides of the upper lip on dominant right | severe in both side of the lower lip | paralysis of the upper lip |
| Movement of the tongue | almost impossible | only slight movement within the oral cavity | only slight movement within the oral cavity | possible movement of the tongue to the lips | only slight movement within the oral cavity | only slight movement within the oral cavity |
| Opening | 1 finger | 1 finger | 1 finger | 1 finger | 1 finger | 1 finger |
| Pharyngeal Reflex | eliminated | eliminated | eliminated | eliminated | reduced | slightly reduced |
| Mandibular (lower jaw) reflex | negative (absent) | positive (present) | hyperactive | positive (present) | not described | not described |
| RSST | not possible | not possible | once | not possible | once | once |
| WST | swallowing present, choking present | swallowing present, choking present | swallowing present, choking present | swallowing present, absence of choking, food remnants within the oral cavity | swallowing present, choking present | swallowing present, choking absent, food remnants within the oral cavity |
| Profile | 3b | 3b | 3b | 5 | 3b | 5 |
| Findings of swallowing angiography | severe impairment in the oral phase, moderate impairment observed in the pharyngeal stage, small amount of aspiration, reflex choking was absent, no effect on posture, no effect of thickener | severe impairment in the oral phase, moderate impairment in the pharyngeal phase, error in swallowing of jelly when reclined at 30°-45°, increase in pharyngeal remains of food, choking condition with increased food remains | poor closing of the lips, poor delivery of food bolus to the oral cavity, in a reclining position at 45° pharyngeal influx with swallowing reflex, presence of choking with swallowing reflex | high level impairment was moderate, pharyngeal function was maintained | pharyngeal function was maintained, transfer of bolus formation not required | bolus formation, poor bolus transfer, slight delay in swallowing reflex with pear shaped bolus, small amounts of food residue, difficulty with self-expectorant function |
| GR | 2 | 3 | 2 | 5 | 3 | 4 |
| DSS | 2 | 2 | 2 | 5 | 3 | 4 |
| Consequence of swallowing function | gastrostomy due to dysfunction in oral intake | gastrostomy due to dysfunction in oral intake | gastrostomy due to dysfunction in oral intake | gastrostomy due to dysfunction in oral intake | one meal independent as a result of adjustment of swallowing | one meal independent as a result of adjustment of swallowing |
| Pneumonia | absent | absent | absent | absent after rehabilitation for swallowing | absent | absent |
Table 1: Comparison of case studies across reports. The data in the cases presented here was compared with the data reported in a previous study (Ogawa, 2016). RSST: Repetitive saliva swallowing test, WST: Water swallowing test, GR: Fujishima swallowing grade (Ichiro, 2012), DSS: Dysphagia severity scale (Nishiyama, 2015), ADL: Activities of daily living, FIM: Functional Independence Measure.
While nearly a century has passed since the first reported case of FCMS, the existent number of reported cases remains low. Although this disease was originally thought to exhibit dyskinetic disorder of persistent speech organs due to poor prognosis, in recent years, cases where diet oral intake became possible little by little, improvement in dysarthria was seen and have been reported [20]. Two cases reported here had also allowed meals to be self-reliant through intervention of rehabilitation. Pseudobulbar paralysis is not a rare disorder, but it seems that some patients who have problems with higher-order exercise treatment that will dissociate into intentionality and automation as in this case are also included. By appropriate intervention of intensive evaluation and intensive rehabilitation intervention, we should pick up cases like this case and consider expansion of ADL and improvement of quality of life. We hope that this report will improve diagnostic accuracy in the future and lead to rehabilitation intervention based on appropriate prognostic prediction.
Acknowledgment
I would like to express my sincere gratitude to the members of the Fukui General Hospital for their cooperation.
References
- Foix C, Chavany JA, Marie J (1926) Diplégic facio-linguo-masticatrice d’origine cortico sous-corticale sans paralysis des. Review Neurology 33: 214-219.
- Weller M (1993) Anterior opercular cortex lesions cause dissociated lower cranial nerve palsies and anarthria but no aphasia: Foix-Chavany-Marie syndrome and “automatic voluntary dissociation†revisited. J Neurol 240:
- Bakar M, Kirshner HS, Niaz F (1998) The opercular-subopercular syndrome: Four cases with review of the literature. Behav Neurol 11: 97-103.
- Ogawa T (2016) Functional outcome of swallowing rehabilitation in 4 dysphagic patients with stroke presenting with dysphonia, severe pseudobulbar palsy, and trismus. Nosotchu 38: 319-325.
- Ohta T (1975) New grading of level of consciousness in acute stage. Nosochuno gekakenkyukai koenshu 3: 61-68.
- Ichiro F (2012) Dysphagia and Disease: An Encyclopedia. Ishiyaku Publishers, INC, Tokyo.
- Cichero JA, Lam P, Steele CM, Hanson B, Chen J, et al. (2017) Development of International Terminology and Definitions for Texture-Modified Foods and Thickened Fluids Used in Dysphagia Management: The IDDSI Framework. Dysphagia 32: 293-314.
- Brunnstrom S (1966) Moter testing procedures in hemiplegia: based on sequential recovery stages. Phys Ther 46: 357-375.
- Lang C, Reichwein J, Iro H, Treig T (1989) Foix-Chavany-Marie-syndrome--neurological, neuropsychological, CT, MRI, and SPECT findings in a case progressive for more than 10 years. Eur Arch Psychiatry Neurol Sci 239: 188-193.
- Alajouanine T (1960) Baillarger and Jackson: the principle of Baillarger-Jackson in aphasia. J Neurol Neurosurg Psychiatry 23: 191-193.
- Yamadori A (1985) Introduction to Neuropsychology. IGAKU-SHOIN, Tokyo. Pp: 4-6
- Baillarger JGF (1865) De l’aphasie au point de vie psychologique. Paris Masson.
- Jackson H (1884) Evolution and Dissolution of the Nervous System. Br Med J Lang C Pp: 660-663.
- Duffy JR (2005) Motor Speech Disorders: Substrates, Differential Diagnosis, and Management. Elsevier Mosby. Pp: 143-144
- Riecker A, Ackermann H, Wildgruber D, Meyer J, Dogil G, et al. (2000) Articulatory/phonetic sequencing at the level of the anterior perisylvian cortex: A functional magnetic resonance imaging (fMRI) study. Brain Lang 75: 259-276.
- Suzuki K (2004) Neuronal Bases of “Apraxia of Speechâ€. The Japan Journal of Logopedics and Phoniatrics 45: 300-304.
- Brandão E, Ferreria A, Loureiro LJ (2013) Anterior biopercular syndrome caused by unilateral infarction. Acta Med Port 26: 177-179.
- Moragas GM, Cardona PP, MartÃnez-Yélamos S, Rubio BF (2007) Heterogeneous topography of Foix-Chavany-Marie syndrome. NeurologÃa 22: 333-336.
- Meadows JC (1973) Dysphasia in unilateral cerebral lesions. J Neurol Neurosurg Psychiatry 36: 853-860.
- Fukuoka T (2010) A Case of Foix-Chavany-Marie Syndrome with Dysphagia That Automatic Masticatory Movement Was Preserved. Japanese Society of Dysphagia Rehabilitation 14: 155-161.
Citation: Shintani J, Yamakawa M, Sato M, Kobayashi Y (2018) Communication Ability and Swallowing Function of Two Patients with Foix Chavany Marie Syndrome. J Speech Pathol Ther 3: 136. DOI: 10.4172/2472-5005.1000136
Copyright: © 2018 Shintani J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 6429
- [From(publication date): 0-2018 - May 01, 2025]
- Breakdown by view type
- HTML page views: 5566
- PDF downloads: 863