Concurrent Diagnosis of a Gastric Neuroendocrine Tumor and an Adjacent Adenoma: An Approach to Diagnosis and Management
Received: 25-Oct-2018 / Accepted Date: 08-Nov-2018 / Published Date: 18-Nov-2018 DOI: 10.4172/2161-069X.1000580
Abstract
The incidence of gastric Neuroendocrine Tumors (NETs) has been rising with the increasing use of diagnostic gastroscopy. Correctly differentiating a NET from a gastric adenoma is essential prior to embarking on further endoscopic management. Both of these lesions have distinct characteristics that can be identified endoscopically. We report a case of a patient who presented with a concurrent diagnosis of a gastric NET and adenoma and contrast their respective appearances endoscopically and the experience with their subsequent endoscopic resections.
Keywords: Neuroendocrine tumor; Adenoma; Endoscopic mucosal resection; Endoscopic submucosal dissection
Introduction
Gastric Neuroendocrine Tumors (NETs) have historically been classified as rare tumors of the foregut [1]. However, with the increased use of diagnostic endoscopy and improved awareness of these tumors, the incidence of these tumors has been increasing [2,3]. Gastric NETs now account for 0.6%-2% of all gastric polyps [4].
Type 1 carcinoids represent 70%-80% of gastric NETs and are associated with chronic atrophic gastritis. Endoscopically, they are usually polypoid, small in size, multicentric and can be mistaken for erosions or adenomas [5]. Narrow-band Imaging (NBI) with magnifying endoscopy has been used to describe a disappearance of normal pit structure centrally with a yellowish hue underneath the epithelium [6].
Gastric adenomas are dysplastic, neoplastic precursors to gastric cancer that account for 6%-10% of all gastric polyps in Western populations and up to 25% in Asian populations [7,8]. Endoscopically, they appear lobulated, flat or sessile and can range from a few millimeters to centimeters in size [9].
Under magnifying chromoendoscopy, gastric adenomas were classified as having a long tubular pit pattern in the absence of dysplasia [10] and absent mucosal patterns with irregular vascular patterns with dysplasia [11]. They are most commonly located in the antrum and are solitary.
Whereas non-malignant adenomas originate and are generally confined to the mucosa, gastric NETs invade the sub mucosa [12]. Awareness of this distinction is especially important prior to embarking on attempted endoscopic resections of these lesions as traditional resection techniques can result in inadequate margins or perforation [13].
With the rising incidence of gastric NETs, the diagnosis and management of these tumors become increasingly relevant for all endoscopists. Thus, we describe a case of a patient presenting with a concurrent diagnosis of gastric NET adjacent to a gastric adenoma. The endoscopic descriptions of each and experience with their subsequent resections is contrasted and discussed.
Case Description
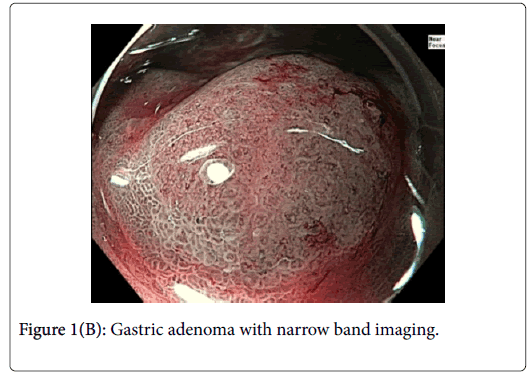
A 76-year-old gentleman was referred for further management of gastric neuroendocrine tumors discovered as part of his anemia workup. Two lesions 4 cm apart were identified at the time of followup endoscopy. Lesion 1 (Figure 1) was approximately 8 mm in diameter and had an area of architectural loss of mucosal pattern and irregular vascular pattern on NBI consistent with a dysplastic gastric adenoma.
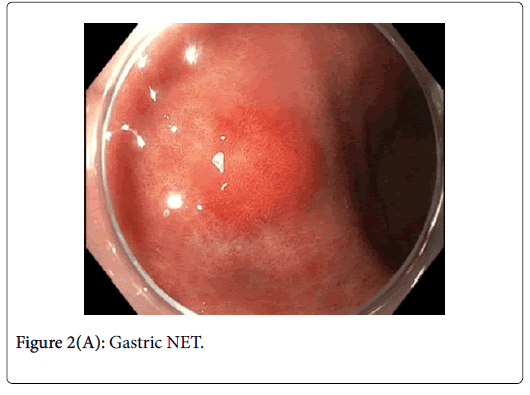
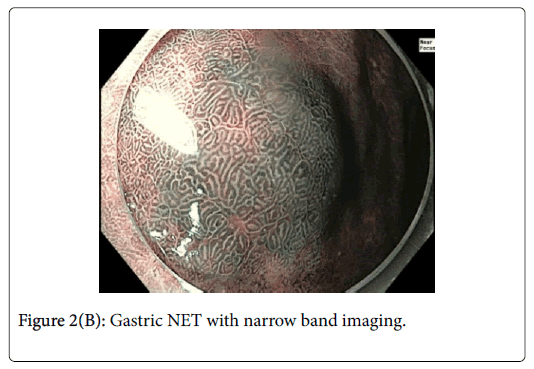
This was resected via Endoscopic Mucosal Resection (EMR) without complication. Lesion 2 (Figures 2A and B) was approximately 12 mm in diameter and had normal overlying pit pattern similar to the surrounding gastric mucosa and displayed an underlying yellowish hue consistent with a NET.
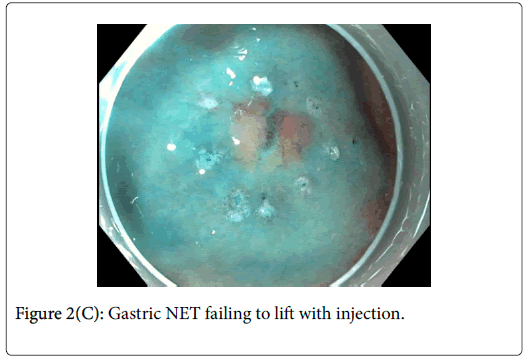
As can be seen in Figure 2C, it was difficult to achieve an adequate lift of the NET after multiple injections. In light of this, Endoscopic Submucosal Dissection (ESD) was performed.
Final histology revealed lesion 1 to be a gastric tubular adenoma with low and high grade dysplasia. Lesion 2 was found to be a grade 1 NET with no dysplasia, no lymph vascular emboli and Ki67 of 7%. There was no invasion of the NET into the overlying gastric epithelium.
Discussion
Gastric NETs are rare foregut neoplasms. The concurrent presentation of a gastric NET with a gastric adenoma in the same patient is even more uncommon, providing a unique opportunity to contrast their respective endoscopic appearances and subsequent resections. In this case, the gastric adenoma had an area of architectural loss of mucosal pattern and irregular vascular pattern with magnified NBI. In contrast, the NET had a normal overlying pit pattern consistent with the surrounding unaffected gastric mucosa but exemplified an underlying yellowish hue. The appearance of both of these lesions is consistent with previous descriptions [6,10,11].
Gastric adenomas are typically confined to the mucosa making subsequent resections relatively straightforward. In this case, the adenoma lifted well off of the muscle and was resected without issue. Gastric NETs differ from this in that they have a tendency to involve the submucosal layer. Figure 2C exemplifies this issue clearly and ultimately, ESD was required for its resection. Through this case, the importance of distinguishing between gastric NETs and adenomas prior to embarking on endoscopic resection is shown. In the event of a NET diagnosis, the requirement of a more complex resection with increased risk needs to be considered by the endoscopist and relayed to the patient.
References
- Modlin IM, Kidd M, Latich I, Zikusoka MN, Shapiro MD (2005) Current status of gastrointestinal carcinoids. Gastroenterol 128 :1717-51.
- Modlin IM, Oberg K, Chung DC, Jensen RT, de Herder WW, et al. (2008) Gastroenteropancreatic neuroendocrine tumors. The Lancet Oncology 9: 61-72.
- Hassan M, Phan A, Li D, Dagohoy CG, Leary C, et al. ( 2008) Risk factors associated with neuroendocrine tumors: A U.S.-based case-control study. Int J Cancer 123: 867-873.
- Lawrence B, Gustafsson BI, Chan A, Svejda B, Kidd M, et al. (2011) The epidemiology of gastroenteropancreatic neuroendocrine tumors. Endocrinol Metab Clin North Am 40: 1-18.
- Basuroy R, Srirajaskanthan R, Prachalias A, Quaglia A, Ramage JK (2014) Review article: the investigation and management of gastric neuroendocrine tumours. Aliment Pharmacol Ther 39: 1071-84.
- Singh R, Yao K, Anagnostopoulos G, Kaye P, Ragunath K (2008) Microcarcinoid tumor diagnosed with high-resolution magnification endoscopy and narrow band imaging. Endoscopy 40: E12.
- Chandrasekhara V, Ginsberg GG (2011) Endoscopic management of gastrointestinal stromal tumors. Curr Gastroenterol Rep 13: 532–539.
- Stolte M, Sticht T, Eidt S, Ebert D, Finkenzeller G (1994) Frequency, location, and age and sex distribution of various types of gastric polyp. Endoscopy 26: 659-665.
- Islam RS, Patel NC, Lam-Himlin D, Nguyen CC (2013) Gastric Polyps: A review of clinical, endoscopic, and histopathologic features and management decisions. Gastroenterol Hepatol 9: 640-51.
- Ohnita K, Isomoto H, Shikuwa S, Yamaguchi N, Nakayama T, et al. (2011) Magnifying chromoendoscopic findings of early gastric cancer and gastric adenoma. Dig Dis Sci. 56: 2715-22.
- Boeriu A, Boeriu C, Drasovean S, Pascarenco O, Mocan S, et al. (2015) Narrow-band imaging with magnifying endoscopy for the evaluation of gastrointestinal lesions. World J Gastrointest Endosc 7: 110-20.
- Yoshikane H, Tsukamoto Y, Niwa Y, Hase S, Mizutani K, et al. (1993) Carcinoid tumors of the gastrointestinal tract: evaluation with endoscopic ultrasonography. Gastrointest Endosc 39: 375-83.
- Sato Y, Hashimoto S, Mizuno K, Takeuchi M, Terai S (2016) Management of gastric and duodenal neuroendocrine tumors. World J Gastroenterol 22: 6817-28.
Citation: Kwan M, Hoong CK, Singh R (2018) Concurrent Diagnosis of a Gastric Neuroendocrine Tumor and an Adjacent Adenoma: An Approach to Diagnosis and Management . J Gastrointest Dig Syst 8:580. DOI: 10.4172/2161-069X.1000580
Copyright: © 2018 Kwan M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 4285
- [From(publication date): 0-2018 - Jun 28, 2025]
- Breakdown by view type
- HTML page views: 3475
- PDF downloads: 810