Research Article Open Access
Continuous Monitoring of Total Organic Carbon Based on Supercritical Water Oxidation Improved by CuSO4 Catalyst
Dongdong Han1, Hui Zhang2, Lin Fang1 and Chunmian Lin1*1College of Biological and Environmental Engineering, Zhejiang University of Technology, Hangzhou, Zhejiang, China
2Zhejiang Institute of Metrology, Hangzhou, Zhejiang, China
- *Corresponding Author:
- Chunmian Lin
College of Biological and Environmental Engineering
Zhejiang University of Technology, Hangzhou, Zhejiang, China
Tel: +86-0571-88320976
E-mail: lcm@zjut.edu.cn
Received date: September 24, 2015; Accepted date: October 26, 2015; Published date: November 02, 2015
Citation: Han D, Zhang H, Fang L, Lin C (2015) Continuous Monitoring of Total Organic Carbon Based on Supercritical Water Oxidation Improved by CuSO4 Catalyst. J Anal Bioanal Tech S13:002. doi:10.4172/2155-9872.S13-002
Copyright: © 2015 Han D, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
A device that can detect the concentration of total organic carbon (TOC) continuously was designed by combining the technology of supercritical water oxidation (SCWO) and non-dispersive infrared analyzer (NDIR). CuSO4, as one of the common homogeneous catalysts in the SCWO of wastewater, was introduced into the device to lower the reaction temperature and improve the utilization efficiency of H2O2 oxidant. Catalyst use was seen to improve destruction rate significantly, when compared with the results obtained during the non-catalytic experiments. While the oxidant stoichiometric excess needed was ten times or more under non-catalytic condition, it only needed to be two for CuSO4 catalytic system. With CuSO4 solution pumped into the reaction system, lower energy consumption was required and less thermal and mechanical stresses exist within the detecting equipment. The device could detect the concentration of TOC for practical wastewater with low proportion of inorganic carbon precisely and continuously, while for those with high proportion of inorganic carbon a pre-treatment like acidification was needed.
Keywords
CuSO4; Catalyst; Supercritical water oxidation; Total organic carbon; Continuous detection
Introduction
Supercritical water oxidation (SCWO) is a new-fashioned technology of waste treatment proposed by Modell in the 1980s [1]. It can oxidize organic pollutants, difficult to be degraded, efficiently and completely [2] and has received a great deal of attention as an effective and promising treatment method of hazardous organic pollutants [3]. Water is a kind of excellent solvent when the temperature and pressure exceed its critical point (T=374.3°C, P=22.1 MPa). One of its greatest properties is that it becomes completely miscible with gas and various organic compounds [3,4]. Heterogeneous reaction between gas and liquid will become homogeneous reaction under supercritical conditions, as the reaction then is no longer limited by interphase mass transfer, the reaction can be greatly accelerated [4-6]. For last decades, SCWO has most often been used to treat dilute organic waste streams that may be otherwise difficult to process. Studies on SCWO have been mainly focused on the oxidation of various organic compounds including PCBs and dioxins [7]. But the utilization of SCWO in TOC detection is rarely reported.
Total organic carbon (TOC), as an index assessing the degree of water environment polluted by organic matter, is widely used in water quality control, especially in many developed countries like USA and Germany. The commercially available analyzers for TOC measurement differ in the method of oxidation or the type of detector. And the tracking of CO2, obtained in oxidative process, is the core of modern TOC analyzers [8]. NDIR is a most widely used detector that can be successfully applied for the continuous monitoring of CO2 generated in chemical processes.
The traditional TOC detection not only needs to be carried out at a high temperature, but also needs to be added to the expensive solid phase catalyst. The catalyst generally has a certain service life and needs to be replaced regularly. SCWO, because of its characteristics of fast reaction speed and complete oxidation, can be used as the oxidation method in the TOC detection process and it is easy to implement continuous testing. It can not only react at a lower temperature, but also avoid the replacement of the catalyst.
In the earlier research, a new device, by combining SCWO with the technology of NDIR, was developed to monitor the TOC in water continuously [9,10]. Relative tests indicate that it’s feasible in practice. However, the equipment now still needs to run at a temperature as high as 460°C and pressure 24.0 to 26.0 MPa. Moreover, the amount of H2O2 oxidant must be 10.0 times the theoretic dosage or more [9]. Such harsh operation conditions will put forward higher requirements on the pressure-bearing components and result in the waste of resources. It will bring the drawbacks like high cost of pressure-bearing equipments and safety risks to the device inevitably.
The activation energy in chemical reaction can be lowered or the path of relative chemical reaction can be changed by using catalyst [11], the process to achieve complete oxidation of organic components can be also greatly accelerated. The catalytic SCWO process is expected to be much more feasible for practical application in the aspects of device safety, durability and economy [12]. So catalyst was adopted aiming to lower the operating temperature, the energy consumption of the device, as well as to improve the formation efficiency of CO2. The possibility to increase the utilization efficiency of oxidants was also tested herein. As earlier studies have shown that the influence of pressure on the destruction rate was relatively not obvious, and the optimized pressure was 24.0 to 26.0 MPa [9], no experiments was specially designed to explore the influence of pressure on TOC destruction rate.
The catalysts used in SCWO can be mainly divided into noble metals, metal oxides and transition metal salts. Besides, H4Si12O40 [11] and activated carbon [13] were also investigated as catalysts in the SCWO of organic components. Maintaining catalyst activity is even more critical in supercritical water, because interactions between the catalyst and water may be extensive and irreversible. Heterolytic reactions can be strengthened in the presence of an ionic environment, i.e., the addition of acids, alkali, salts or catalysts and the formation of dimerization products can be minimized in this way [11]. So it seems much more suitable to adopt some kind of homogeneous catalyst. As Cu2+, frequently-used in homogeneous catalytic SCWO, has been proven to be an efficient catalyst [14,15], CuSO4 was introduced to provide the Cu2+ needed.
The aim of this study is to investigate the catalytic effect of Cu2+ in the oxidation of organic chemicals in supercritical water and the feasibility of the experimental device in the continuous detecting of TOC.
Materials and Methods
Chemicals
Potassium biphthalate (AR) was used in the experiment as a model pollutant, the standard substance used in the correction of TOC analyzer. Hydrogen peroxide (H2O2, 30% (w/w), AR) was chosen as oxidant without any further treatment, which can decompose easily and turn into oxygen and water when heated. CuSO4∙5H2O (AR) was chosen to provide Cu2+ needed in the preparation of homogeneous catalyst.
Experimental device and procedure
A schematic diagram of the device used in the experiment is shown in Figure 1. The reactor used in the experiment was consist of an electric stove and a spiral tubular reactor made of stainless steel (SS) tube with 0.125’’ o.d. and 0.060’’ i.d. The temperature of the stove can be precisely regulated by a PID controller. A back pressure valve was adopted in the system to control the pressure. Organic and CuSO4 solution with a certain concentration, super pure water and oxidizer were mixed proportionally by using the low pressure gradient mixer, and then pumped into the reaction tube by high-pressure metering pump. Redox reaction occurred in the mixed liquid in the reactor under given temperature and pressure. The liquid after reaction is cooled by a water bath cooling device and then released to normal pressure by the back pressure valve. The effluent then flowed into the gas-liquid separation device, in which CO2 generated and dissolved was blown-off by high purity N2 (99.999% (V/V)). The mixed gas of N2 and CO2 flowed through an electronic dehumidifier to remove residual water. At last the gas mixture flowed into the NDIR detector to get the amount of CO2 generated. As the signal intensity of NDIR reflects the concentration of CO2, and there is a linear relation between TOC and CO2 generated, there is also a linear relation between TOC and NDIR signal. Then the amount of TOC in water can be obtained by such a relation.
Figure 1: Schematic diagram of device used in the experiment. 1. Low pressure gradient mixer; 2. High-pressure metering pump; 3. Electric stove; 4. Spiral tubular reactor; 5. PID controller; 6. Cooling device; 7. Back pressure valve; 8. Pressure gage; 9. Gas-liquid separator; 10. Mass flowmeter; 11. Nitrogen cylinder; 12. Electronic dehumidifier; 13. NDIR detector; 14. Computer.
Experiments were also conducted under optimized conditions to assess the performance of the device. Practical samples of wastewater used in the experiments were got from several different industries. Another TOC analyzer used to offer the comparative data was made by Shimadzu with a type of TOC-VCPH/CPN.
The destruction rate X, as an assessing index used in the experiment, was used in later discussion of the result, which was defined as equation (1), of which, TOCm (mg L-1) was the value of TOC detected by the device and TOCt (mg L-1) was the theoretical value transformed from the concentration of potassium biphthalate in the solution.
X=TOCm/TOCt × 100% (1)
The index of oxidant stoichiometric excess was used to measure the amount of H2O2 oxidant used in the experiment. It represents the ratio of practical and theoretical oxidant dosage.
Results and Discussion
Feasibility of detecting the TOC in water based on SCWO
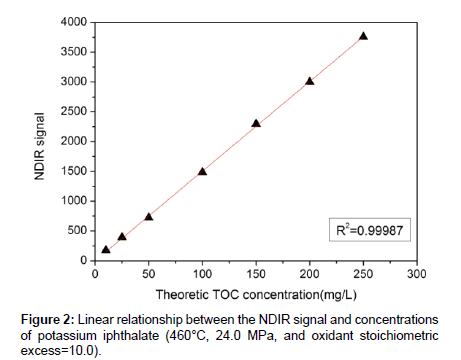
Experiments testing the feasibility of the method in detecting of TOC were conducted with temperature of 460°C, pressure of 24.0 MPa and hydrogen peroxide oxidants 10.0 times the amount calculated by the chemical reaction equation. It can be firmly considered that under such conditions organic substance have been thoroughly oxidized. The relationship between NDIR signal value and potassium biphthalate concentration is shown in Figure 2. It is obvious that the NDIR signal value present a nice linearity with the concentration of potassium biphthalate pumped into the device. Actually, the value of R square in the linear fitting of the two variables reaches 0.9996. So it is greatly believed the feasibility in theory and practice to adopt this device to detect the amount of TOC.
| Number | Wastewater | pH | TOC-LCPH/CPN | Experimental device | ||
|---|---|---|---|---|---|---|
| TC (mg/L) | IC (mg/L) | TOC (mg/L) | TOC (mg/L) | |||
| 1 | Pharmaceuticala | 6 | 81.1 | 54.1 | 27.0 | 82.4 |
| 2 | Chemicala | 7 | 284.9 | 115.5 | 169.4 | 279.7 |
| 3 | Chemicalb | 8 | 11914.8 | 260.8 | 11654.0 | 10578.8 |
| 4 | Pharmaceuticalb | 2 | 321.8 | 0.7 | 321.1 | 319.6 |
| 5 | Sewage outfall | 7 | 56.4 | 3.0 | 53.4 | 56.9 |
| 6 | Dyeing | 8 | 874.2 | 0.1 | 874.1 | 889.4 |
aTOC detected by experiment device were tested at T=420°C, P=23.2 Mpa, [Cu2+]=1.0 mg/L and residence time=217.3 s.
Table 1: TOC concentration of practical wastewater detected by Shimadzu TOC analyzer and experimental devicea.
Effect of temperature on the destruction rate
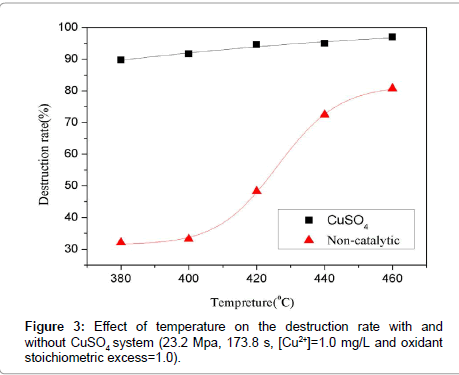
Temperature has been proven to be the main controlling variable of the reaction; essentially the higher the temperature, the more effective the oxidation reaction is in terms of the removal of the organic compound and the production of ultimate product. However, the harsh conditions of the reaction can accelerate the corrosion of the reactor. Therefore, the inclusion of a catalyst was envisaged as a mean to diminish the thermal stress as well. A series of experiments were performed at 23.2 MPa to explore the effect of CuSO4 system in improving the destruction rate, with an initial TOC concentration of 180 mg L-1 and oxidant stoichiometric ratio of 1.0. The experimental temperature varied from 380 to 460°C. In order to elucidate the efficiency of the catalytic reaction more intuitively, contrast tests without catalyst and with other conditions the same were also conducted. Figure 3 shows the trend between the temperature and destruction rate with and without CuSO4 in the reaction system. The temperature did not show evident effect on the destruction rate of TOC within CuSO4 catalytic system. The destruction rate reached a value close to 90.00% at 380°C, the lowest temperature studied in the experiment. In contrast, under noncatalytic condition the destruction rate was only 32.12%. Without catalyst the destruction rate overall was low and had no evident rise when the temperature increased from 380 to 400°C. Then it increased rapidly when the temperature increased from 400°C to 420°C and drew near to the value of catalytic reaction at 460°C. Taken together, it is viable to obtain an ideal destruction rate at lower temperatures with CuSO4 as a catalyst.
Effect of oxidant stoichiometric excess on the destruction rate
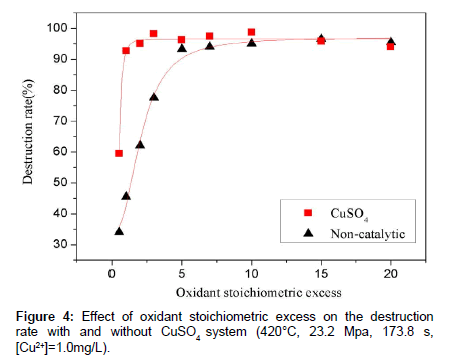
Oxidant stoichiometric excess has been proven to be another main controlling variable of the reaction [11]. Actually the more oxidant is used the more effective the oxidation reaction is within limits. Earlier study has demonstrated that the dosage of oxidant must be ten times the amount of 1.0 stoichiometric ratio to obtain an ideal TOC destruction rate. So it is clear that the utilization efficiency of oxidant is low. So the possibility of decreasing the oxidant stoichiometric excess was also studied. A series of experiments were performed at 420°C and 23.2 MPa with the flow rate of 2.5 mL min-1, and Cu2+ catalyst concentration of 1.0 mg L-1. The results were depicted in Figure 4. It was obviously that by utilizing CuSO4 as catalyst, the oxidant stoichiometric excess needed to ensure the maximum TOC destruction rate decreased greatly than that without catalyst. The biggest difference happened when the oxidant stoichiometric excess is 1.0, where the value of destruction rate is 45.45% without catalyst and 92.76% with CuSO4 respectively. When oxidant stoichiometric excess is 10.0 times or more, the destruction rate with and without CuSO4 showed no obvious difference and lower than the value which means the organic compound was completely oxidized.
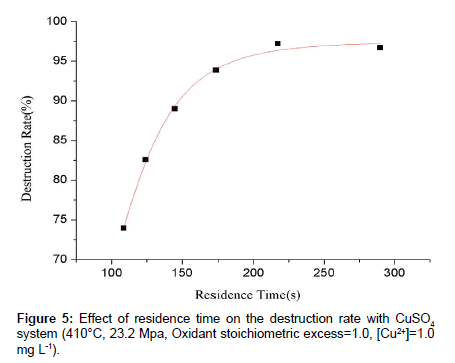
Effect of residence time on the destruction rate
Residence time can affect the oxidation efficiency of redox reactions obviously, especially when the operation condition is not very harsh. Experiments aiming to reveal the law between residence time and destruction rate were performed as well. The residence time was controlled by changing the flow rate of high-pressure metering pump and increased with the decrease of flow rate. It can be seen from Figure 5 that the destruction rate increased apparently with the increase of residence time, though the increase speed of destruction rate become slower. The destruction rate reached a value as high as 97.21% when the residence time is 217.3 s. With other conditions unchanged, a higher destruction rate can be obtained by extending the residence time.
Performance in TOC detecting of practical wastewater
Practical wastewater used in the experiments was obtained from several pharmaceutical, chemical industries and a sewage outfall. Table 1 shows the TC, IC and TOC concentration detected by Shimadzu TOC analyzer and TOC value detected by the experimental device.
It can be seen from Table 1 that when there was little inorganic carbon in the water, TOC concentration detected by the experimental device was extremely close to that by Shimadzu TOC analyzer (pharmaceutical b and dyeing wastewater). When the proportion of inorganic carbon in total carbon was pronouncedly big, a great discrepancy can be seen between the two values detected by experimental device and Shimadzu TOC analyzer. To be more precisely, under such conditions, the detection values by the experimental device should be considered to be the total organic carbon as there was no acidizing stage to remove the inorganic carbon. The test results of wastewater from pharmaceutical industry and Chemical industry just well verified the speculation.
Conclusion
Temperature is the main factor affecting the destruction rate. With the increase of temperature, the destruction rate is increasing. Catalyst use can obviously improve the destruction rate when compared with the results obtained during the non-catalytic experiments. Excess oxidant has a great impact on destruction rate; it was obviously that by utilizing CuSO4 catalyst, the oxidant stoichiometric excess needed to ensure the higher TOC destruction rate decreased greatly compared with the situation without catalyst. It was also seen that the destruction rate increased apparently with the increase of residence time and reached a value as high as 97.2% when the residence time was 217.3 s during the experiment. When there was no interaction from inorganic carbon (i.e., pharmaceutical b), TOC detection value by experimental device (319.6 mg L-1) was nearly the same as that detected by Shimadzu TOC analyzer (321.1 mg L-1). That is to say, organics can be considered to be completely oxidized under such a pressure as 23.2 MPa.
Acknowledgements
This work was supported by the Zhejiang Provincial Natural Science Foundation of China under Grant LY12B07009.
References
- Modell M (1982) Processing methods for the oxidation of organics in supercritical water. US 4338199 A.
- Xu D, Wang S, Huang C, Tang X, Guo Y (2014) Transpiring wall reactor in supercritical water oxidation. Engineering Research and Design 92: 2626-2639.
- Takahashi F, Sun ZR, Fukushi K, Oshima Y, Yamamoto K (2012) Catalytic oxidation of acetic acid over sodium titanate synthesized hydrothermally in supercritical water. J Supercrit Fluid 61: 126-133.
- Civan F, Özaltun DH, Kipçak E, Akgün M (2015) The treatment of landfill leachate over Ni/Al2O3 by supercritical water oxidation. J Supercrit Fluid 100: 7-14.
- Erkonak H, Sögüt OÖ, Akgün M (2008) Treatment of olive mill wastewater by supercritical water oxidation. J Supercrit Fluid 46: 142-148.
- Sögüt OÖ, Akgün M (2010) Treatment of dyehouse wastewater by supercritical water oxidation: a case study. Journal of Chemical Technology and Biotechnology 85: 640-647.
- Park TJ, Lim JS, Lee YW, Kim SH (2003) Catalytic supercritical water oxidation of wastewater from terephthalic acid manufacturing process. J Supercrit Fluid 26: 201-213.
- Visco G, Campanella L, Nobili V (2005) Organic carbons and TOC in waters: An overview of the international norm for its measurements. Microchem J 79: 185-191.
- Zhang H, Xia XQ, Qiu Y, Lin CM, Yu J (2011) Study on detecting of total organic carbon in water by supercritical water oxidation. Chemical Reaction Engineering and Technology 27: 467-471.
- Zhang H, Xia XQ, Qiu Y, Lin Z, Zhang X (2012) Application of supercritical water oxidation in detecting of total carbon organic carbon. Journal of Henan Normol Universitu (Natural Science Edition) 40: 100-102.
- Arslan-Alaton I, Ferry JL (2002) H4SiW12O40-catalyzed oxidation of nitrobenzene in supercritical water: Kinetic and mechanistic aspects. Appl Catal B-Environ 38: 283-293.
- Tomita K, Oshima Y (2004) Stability of manganese oxide in catalytic supercritical water oxidation of phenol. Ind Eng Chem Res 43: 7740-7743.
- Nunoura T, Lee G, Matsumura Y, Yamamoto K (2003) Reaction engineering model for supercritical water oxidation of phenol catalyzed by activated carbon. Ind Eng Chem Res 42: 3522-3531.
- Chen JH, Ma CY, Xi DL, Li Q (2011) Study on catalytic supercritical water oxidation process for treating the perfume waste water. Environmental Engineering 29: 36-39.
- Lin KS, Wang HP (2000) Supercritical water oxidation of 2-chlorophenol catalyzed by Cu2+ cations and copper oxide clusters. Environ Sci Technol 34: 4849-4854.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 11638
- [From(publication date):
specialissue-2015 - Aug 31, 2025] - Breakdown by view type
- HTML page views : 10637
- PDF downloads : 1001