Correlation of Alzheimer’s Dementia Markers
Received: 15-Mar-2018 / Accepted Date: 22-Mar-2018 / Published Date: 29-Mar-2018 DOI: 10.4172/2161-0460.1000432
Abstract
Introduction: Alzheimer’s disease remains a major cause of morbidity, mortality and dependency in older patients with dementia. With increasing age in older population and dementia in western hemisphere, an inexpensive and unified modality for early diagnosis of AD is of utmost importance.
Methods: We sought to investigate single photon emission computed tomography (SPECT) imaging of the brain as a less expensive modality in a prospective single blinded study in a cohort with diagnosis of probable Alzheimer’s dementia. Patients’ demographics, family history, Mini Mental Status Exam, brain imaging, biomarkers such as Tau, Amyloid beta protein, and Apo E genotype were obtained and analyzed. We tested different possible correlations models for association of current diagnosis of Alzheimer’s disease with SPECT and biomarkers using Chi-square test.
Results: Biochemical markers (Amyloid beta 42 and tau protein) have higher sensitivity in identifying patients with AD. APOE genotype is less sensitive as a diagnostic test. SPECT did not correlate with biomarkers in early AD, but showed higher correlation in moderate and severe dementia. Further investigation is warranted to identify a more sensitive and specific yet inexpensive testing for early diagnosis of AD.
Discussion: Biochemical and genetic markers have a closer association with each other and with Alzheimer’s compared to their association with brain imaging of patients with Alzheimer’s disease.
Keywords: Alzheimer’s disease; SPECT scan; Tau protein; Aß42 protein; Apo E genotype; Mini mental status evaluation; Family history
Introduction
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder resulting in memory loss, personality and language abnormalities, global cognitive and functional impairments. In the late stages of AD, patients have disorganized thought process and become dependent on all activity of daily living (ADL). It has been reported that the cost of Alzheimer’s and other dementias total about $56,800 per year and caregivers usually pay sixty percent ($34,500 per year) of the total cost. Alzheimer’s Association projected the cost of Alzheimer’s and other dementias will total around $236 billion [1].
During the last five years of life, a patient with dementia spends in excess of $287000.00 and when is compared with heart diseases and cancer, the cost total around $175000.00 and $173000.00, respectively.
Detecting dementia is a problem in routine medical practice. The diagnosis is missed in 21 percent of demented or delirious patients on a general medical ward, while 20 percent of non-demented patients may be judged to be demented [2]. Earlier diagnosis would allow timely preparation for the disease by patient and family as well as quicker start of medication although the true effectiveness of these meds is in doubt.
History of cognitive dysfunction and memory loss, family history of AD and mini mental status exam (MMSE) are the clinical means of suspecting the disease but confirmation needs more definitive tools. Physical exam occasionally shows hyperactive tendon reflexes, myoclonic jerks and primitive sucking reflex but these are infrequent and non-specific findings.
The brains of individuals with AD are characterized by extracellular deposition of amyloid beta (Aß) protein, intracellular neurofibrillary tangles and loss of neurons. These are findings that can only be detected at autopsy. Neuritic plaques contain Aß42 as do the arterial walls in the central nervous system that in effect represents a form of amyloid angiopathy. There are also decreased nicotinic cholinergic receptors present.
Many biochemical markers have been investigated in AD. Aß protein, tau protein, phosphorylated-tau protein, melanotransferrin are amongst them. Decreased cerebrospinal fluid (CSF) Aß42 secondary to deposition of it in brain tissue and increased tau protein are traditionally considered markers of AD [3,4] (Table 1).
Genetic markers like presenilin gene mutation, AAPP gene mutation and Apo lipoprotein epsilon polymorphisms (Apo E) have aided in increasing the objectivity of diagnosis. The first genetic mutation linked to AD was found on AAPP gene on chromosome 21. Mutations on the two homologous presenilin (PS) genes, PS1 on chromosome 14 and PS2 on chromosome 1, are responsible for over half of the known familial AD cases [5]. These genes affect amyloid precursor protein (APP) and lead to an increase in its proteolytic activity, thus producing more harmful Aß peptides. Apo E allele 4/4 or 3/4 combination is thought to be a risk factor for developing AD [6].
The role for neuroimaging in the evaluation of patients suspected to have a dementing disorder has yet to be solidified. Although (99m) Tc-HM-PAO Single Photon Emission Computed Tomography (SPECT) has long been studied, newer research is always looking for a better alternative. Regular computed tomography might show cortical atrophy, ventricular enlargement and atrophy of the hippocampus, especially in more advanced cases. Positron emission tomography (PET) will show decreased function in temporal cortex and hippocampus area as well as plaques counting, however this modality is not widely available and expensive [7].
Methods
In a group of patients with cognitive decline, we investigated the correlation amongst SPECT, spinal fluid biochemical markers (Aß42 and tau protein), Apo E allele, family history and MMSE. Our objective was to further define the role of these modalities in the early diagnosis of AD. Although many of these modalities have been studied separately, comprehensive correlation studies are rare. We hoped to evaluate different correlations and find out if there is a combination of tests that can determine diagnosis of AD with significant sensitivity and specificity. The protocol was approved by IRB.
In a prospective blinded single center study, 108 patients with cognitive dysfunction were evaluated with SPECT. Forty-four (44) of them also consented (or the consent was given by caregivers/power of attorney) to undergo spinal tap and CSF analysis for Aß42 and tau protein. Apo E allele was also measured for the latter group. Each of these individuals was questioned about family history of AD, then a single physician performed MMSE for all willing/capable participants. Clinically, they were evaluated using the National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA) as well as Diagnostic and Statistical Manual of Mental Disorders (DSM V). Many of the patients had other imaging studies including computed tomography (CT) and magnetic resonance imaging (MRI) of the head. Those results were noted for ruling out vascular dementia and other causes of cognitive dysfunction. AD patient population data were assessed and multiple possible correlations between these parameters (or different combinations of them) were hypothesized and evaluated with Chi-square test. Statistical significance of different possible correlations was then determined and relevant factors were measured.
SPECT images
All SPECTs were done in our institution with one machine under similar conditions. They were HMPAO-TC99 (Ceretec) 120-degree acquisition orbit studies using 3° steps for 30 s per step on a 128 × 128 matrix. Brain images were acquired on a multi head Siemens scanner with fan beam collimators at a photo peak of 140 KeV and 15% symmetric window. Image processing protocol incorporated an order 7 low pass Butterworth filter with a cutoff frequency of 0.6. Images were reviewed in multi view with a display zoom of 136%.
Image interpretation
Each image was reviewed twice. Multiple readers did the initial reading and the medical records were available to them if they opted to use them. For the second blind reading a single expert neuroradiologist reviewed the SPECT images. He had the most experience with SPECTS in the department. Each image was noted to be either consistent or inconsistent with AD. Any one of the following findings was considered consistent with the diagnosis: Symmetric bilateral posterior temporal and parietal perfusion defects (posterior association cortex), unilateral temporal parietal hypo perfusion, or frontal hypo perfusion. If there was disparity between the two readings the result was considered equivocal and non-diagnostic.
CSF analysis
CSF samples were tested in a single tertiary diagnostic laboratory (Athena diagnostic, Worcester, MA). Determination of the Tau and Aß42 concentrations in CSF were performed by enzyme linked immunosorbent assay (ELISA) methodology using highly specific antibodies. Concentrations of antibodies are determined from standard curves using human brain-derived tau and synthetic Aß42. Cut offs were developed using Aß42 and tau together utilizing classification tree statistics in S-plus software. Table 1 was used for interpretation.
| Aß42 (PG/ML) | Not AD | Non-diagnostic |
|---|---|---|
| 1125 | Non-diagnostic | Alzheimer’s Disease |
| 761 | 503 | 558 |
Table 1: Interpretation of Tau and Aß42 (Athena diagnostic, Worcester, MA).
Apo E genotyping
Performed by restriction endonuclease digestion of polymerase chain reaction (PCR) amplified genomic DNA in a single tertiary diagnostic laboratory (Athena diagnostic, Worcester, MA). Allele 1/ allele 2 combination of 4/4 or 3/4 were correlated to AD but not the other variations (3/3, 2/3, 2/4, 2/2).
Results
Of 108 cases with cognitive deficit and a SPECT scan, 97 were confirmed to have AD based on clinical, CSF biomarkers and imaging studies. The rest had other diseases such as vascular dementia.
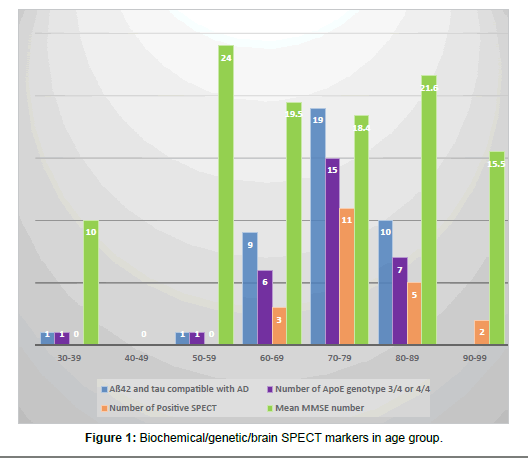
Of the 97 patients with AD, 18 (18.5%) were male and 79 (81%) female. Age ranged from 39 to 94 years old with 34 patients (35%) in both 70-79 and 80-89 years old groups (Table 2). Eighty-five (85) patients had MMSE results. Their mean MMSE was 19.3 (Table 2 and Figures 1 and 2).
| Age group | Number of patients | Percent of patients | Family history of AD in 1 immediate member | Family history of AD in >1 immediate member | Number of patients with MMSE | Mean MMSE number |
|---|---|---|---|---|---|---|
| 30-39 | 1 | 1% | 1 (100%) | 0 (0%) | 1 (100%) | 10 |
| 40-49 | 0 | 0% | ||||
| 50-59 | 2 | 2% | 1 (50%) | 0 (0%) | 1 (50%) | 24 |
| 60-69 | 17 | 17.5% | 3 (17%) | 2 (11%) | 12 (70%) | 19.5 |
| 70-79 | 34 | 35% | 6 (17%) | 5 (14%) | 32 (94%) | 18.4 |
| 80-89 | 34 | 35% | 6 (17%) | 1 (3%) | 30 (88%) | 21.6 |
| 90-99 | 9 | 9.5% | 1 (11%) | 1 (11%) | 9 (100%) | 15.5 |
| Total | 97 | 100% | 18 (18%) | 9 (9%) | 85 (87%) | 19.3 |
Table 2: Demographics of the cases.
Family history was positive in 27 patients (28%) with several people having more than one case of AD in immediate family members. Nine had mothers with AD, 5 had sisters, 4 had multiple family members, 2 had fathers, 2 had uncles (one with 4 of uncles), 1 had an aunt, 1 had a grandfather, and 1 had a brother. Two reported family history but were not sure of the exact relationship. Four of the cases who did not have AD also had positive family history (36%). Positive family history was not correlated to positive chemical or genetic biomarkers, nor was it related or proportional to positive SPECT results.
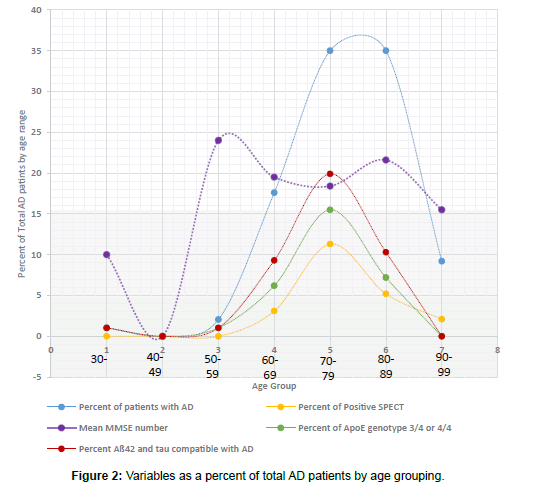
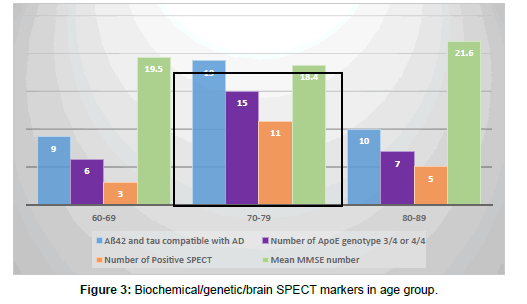
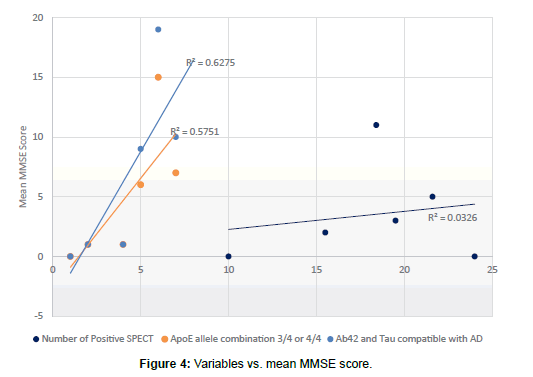
Forty-two patients with AD (43%) had CSF studied for Tau and Aß42 testing. Only 3 of these patients had results that were negative for AD while all others tested positive. Of those patients found to have a diagnosis other than AD, 2 had the test: one positive and one negative. Different statistical values were obtained as shown in Table 3 and Figures 3-5. Mean MMSE for patients with positive Aß42-tau were 19.4 (compared to overall MMSE of 19.3).
Apo E allele determination showed 32 patients with allele1/allele2 combination of either 3/4 or 4/4 (which are associated with increased risk of AD). Ten remaining patients showed 3/3, 2/3, 2/4, 2/2 variances. Two people with diagnosis other than AD had Apo analysis and one was positive. Based on these results, the data on tables were tabulated (Table 3 and Figures 3 and 4). Mean MMSE for people who had positive Apo E pattern for AD was 19.5 (Overall MM SE of 19.3).
|
|
Sensitivity % |
Specificity % |
Positive predictive value % |
Negative predictive value % |
Positive likelihood |
Negative likelihood |
|---|---|---|---|---|---|---|
| Aß42-Tau | 87 | 50 | 95 | 25 | 1.71 | 0.26 |
| Apo E | 76 | 50 | 97 | 9 | 1.52 | 0.48 |
| SPECT | 21 | 90 | 95 | 11 | 2.1 | 0.87 |
Table 3: Statistical values of different tests for purpose of determining presence of AD.
Of 97 patients with AD, 21 of them (22%) had positive SPECT scan for AD. There were 5 equivocal results (5%) and 71 patients (73%) were negative for AD patterns. Of the 11 patients with diagnoses other than AD, one had a positive reading and 10 were negative. Statistical values were tabulated and are shown (Figure 5). Patients with positive SPECT had a mean MMSE of 17.1 (compared to overall MMSE of 19.3). SPECT was positive between 60-69 years in 3 cases, 70-79 years in 11 cases, 80-89 in 5 cases and 90-99 in 2 cases. No one younger than 64 had positive SPECT results although some of the same younger group had very positive biochemical/genetic marker profile (Table 4 and Figure 5).
| Age group | Number of CSF analysis | Tau protein mean (PG/ML) | Aß42 mean (PG/ML) | Aß42 and tau compatible with AD | Number of Apo E genotype 3/4 | Number of Apo E genotype 4/4 | Number of Positive SPECT |
|---|---|---|---|---|---|---|---|
| 30-39 | 1 | 454 | 347 | 1 (100%) | 1 (100%) | 0 (0%) | 0 |
| 40-49 | 0 | 0 | |||||
| 50-59 | 1 | 732 | 529 | 1 (100%) | 1 (100%) | 0 (0%) | 0 |
| 60-69 | 10 | 537 | 557 | 9 (90%) | 3 (30%) | 3 (30%) | 3 |
| 70-79 | 19 | 605 | 498 | 19 (100%) | 13 (68%) | 2 (10%) | 11 |
| 80-89 | 12 | 567 | 564 | 10 (83%) | 6 (50%) | 1 (8%) | 5 |
| 90-99 | 0 | 2 | |||||
| Total | 43 | 578 | 527 | 40 (93%) | 24 (55%) | 6 (14%) | 21 |
Table 4: Biochemical/genetic/SPECT marker in age group data.
Four of the 21 patients with positive SPECT were male (19%) which is compatible with the overall ratio of males in this study (18.5%). Ten of the 39 AD patients with positive Aß42-tau were male (25%). There was no statistically significant correlation between age and race in patients who had positive tests and they followed the overall pattern of age and race in this study.
When SPECT scan and Aß42-tau combination were both compatible with AD diagnosis (7 cases) all the patients were found to have AD. But that was a very low percentage of the patients (7.2%). Of these, 4 had compatible Apo E genotyping and 3 had Apo E that were not considered to be associated with AD (3/3, 3/3, 4/2). Therefore, no real correlation with Apo E existed.
There was no statistically meaningful correlation between patients with positive SPECT and those with positive Aß42-tau. Positivity of SPECT also did not correlate with Apo E positivity.
Of the 39 persons with AD and positive Aß42-tau, 27 had positive Apo E pattern for AD (69.2%) but only 7 had positive SPECT (17.9%). There were 14 people (14.4%) with AD and positive SPECT that were negative for biochemical markers and genetic markers. 31 patients (31.9%) had AD but no family history, biochemical/genetic marker or positive imaging (Figure 5).
Discussion
To our knowledge this is one of few studies that evaluate the correlation among SPECT, Aß42-Tau, Apo E, MMSE, and family history in AD. Several other studies have evaluated some of these parameters but rarely all of them in conjunction.
We found high sensitivity of Aß42-tau protein for diagnosis of AD (92%) although the specificity was not as great (50%). These numbers have been suggested in past years as well [4-8]. All the data suggests that although invasive, CSF biomarkers can be a reliable screening test for AD especially when early clinical picture is puzzling. High accuracy of 90% and PPV of 97% also supports CSF data values.
SPECT on the other hand was specific (90%) with 95% PPV but not at all sensitive (21%) for AD diagnosis. Although the lack of sensitivity might be related to the fact that the disease was in early stages and not fully diagnosed in these patients, the fact remains that SPECT is aimed at being an early diagnostic tool. There is not much value to it when the clinical picture is completely advanced and clear. SPECT positive patients did have lower MMSE compared to Aß42-tau matched patients showing that it is positive when the disease is further advanced [9]. Also SPECT was never positive before 64 years of age, although several patients in those same groups had very positive biochemical/genetic markers. This might indicate that no SPECT should be performed for patients under 64 years of age, perhaps because the structural changes that SPECT detects need time to settle in.
Overall it seems that CSF changes can be detected faster and in the earlier stages compared to SPECT changes that need time and progression of the disease. A study by Tsolaki et al. [10] suggested that there is a statistical correlation between degree of hypo perfusion and deteriorating neuropsychological tests. Shih et al. [11] used brain SPECT surface three-dimensional display and showed more significant dementia and lower MMSE with more advance brain SPECT score. Overall conclusion was that SPECT images can measure loss of brain function over time and could be useful for assessing the efficacy of therapeutic interventions for AD patients.
Apo E allele was found to be a dismal parameter in our study. Different allelic combinations were present and no statistically significant correlation was found with either presence of AD, MMSE or SPECT results. Other researchers have noted this as well. Engelborghs [12] reported that Apo lipoprotein epsilon 4 alleles is a risk factor for AD but not a diagnostic marker, as many individuals who inherit epsilon 4 do not develop the disease.
Of great interest to us was the finding that significant family history of dementia exists in patients who have AD (28%). Several patients had more than one immediate family member with AD. This deserves more in detail studies especially with recent emphasis on value of family history and current knowledge about genetic markers of AD.
Aß42-tau marker is much more aligned with Apo E than with brain imaging. It seems fair to postulate that genetic markers are probably affecting biochemical markers and that is why there is a stronger correlation between them. On the other hand, SPECT and other imaging are indicative of a different process and show structural changes that are not necessarily originating from the same roots and therefore have a very weak association with biochemical and genetic factors.
To identify all cases of AD, it would make sense to add SPECT to biochemical/genetic markers. Then we have a combination that is greatly enhanced in sensitivity with great specificity. They will pick up different processes and are therefore complementing each other. In fact, 100% of patients who have had positive results for both were found to have AD in our study. Of course, the subset with no markers that has AD (31.9%) will always exist but the combined format will decrease the rate of non-diagnosis.
Based on these findings, we recommend screening with CSF Aß42- tau for people with cognitive deficits. Addition of brain imaging can then be used for further evaluation if doubts exist. We also recommend large-scale prospective studies to further investigate and delineate the diagnostic dilemmas of Alzheimer’s. We are getting very close to prompt recognition of the disease. Although at some point a single blood test might be sufficient for diagnoses, currently we need markers for it and we do have very useful markers at our disposal currently.
Conclusion
CSF study for Aß42-tau protein is very sensitive with great positive predictive value for diagnosis of AD and can be used for screening. SPECT scan or Apo E genotyping is not sufficient for this purpose alone. SPECT specificity is significant and as AD gets more advanced, SPECT becomes more positive and shows more extensive evidence of disease and decline in MMSE.
Addition of SPECT scan increases Aß42-tau protein sensitivity and statistical significance. If both studies are positive in a demented patient suspected of having AD, the chances of that patient having AD are around 100%.
Biochemical markers and genetic markers of AD seem to be more in concordance with each other than with brain imaging results. It might be postulated that they highlight different processes and end-results. Therefore, combination of them will broaden the range for identifying AD.
References
- Alzheimer’s Association (2017) 2017 Alzheimer’s disease facts and figures. Alzheimers Dement 13: 325-373.
- Ritchie K, Ropacki M, Albala B, Harrison J, Kaye J, et al. (2017) Recommended cognitive outcomes in preclinical Alzheimer's disease: Consensus statement from the European Prevention of Alzheimer's Dementia project. Alzheimer’s Dement 13: 186-195.
- Scarano S, Lisi S, Ravelet C, Peyrin E, Minunni M (2016) Detecting Alzheimer's disease biomarkers: From antibodies to new bio-mimetic receptors and their application to established and emerging bioanalytical platforms - A critical review. Anal Chim Acta 940: 21-37.
- Jack CR Jr, Holtzman DM (2013) Biomarker modeling of Alzheimer's disease. Neuron 80: 1347-1358.
- Spodzieja M, Kalejta K, Kolodziejczyk AS, Maszota-Zieleniak M, Rodziewicz-Motowidlo S, et al. (2016) Characteristics of C-terminal, ß-amyloid peptide binding fragment of neuroprotective protease inhibitor, cystatin C. J Mol Recognit 30.
- Singh N, Wang AY, Sankaranarayanan P, Fletcher PT, Joshi S, et al. (2012) Genetic, structural and functional imaging biomarkers for early detection of conversion from MCI to AD. Med Image Comput Assist Interv 15: 132-140.
- Zimmer ER, Leuzy A, Benedet AL, Breitner J, Gauthier S, et al. (2014) Tracking neuroinflammation in Alzheimer’s disease: The role of positron emission tomography imaging. J Neuroinflammation 11: 120.
- Mattsson N, Insel PS, Palmqvist S, Portelius E, Zetterberg H, et al. (2016) Cerebrospinal fluid tau, neurogranin and neurofilament light in Alzheimer's disease. EMBO Mol Med 8: 1184-1196.
- Osorio RS, Pirraglia E, Gumb T, Mantua J, Ayappa I, et al. (2014) Imaging and cerebrospinal fluid biomarkers in the search for Alzheimer's disease mechanisms. Neurodegener Dis 13: 163-165.
- Tsolaki M, Sakka V, Gerasimou G, Dimacopoulos N, Chatzizisi O, et al. (2001) Correlation of rCBF SPECT, CSF tau, and cognitive function in patients with dementia of the Alzheimer’s type, other types of dementia and control subjects. AM J Alzheimer’s Dis Other Demen 16: 21-31.
- Atri A (2016) Imaging of neurodegenerative cognitive and behavioral disorders: Practical considerations for dementia clinical practice. Handb Clin Neurol 136: 971-984.
- Engelborghs S, De Deyn PP (2001) Biological and genetic markers of sporadic Alzheimer's disease. Acta Med Okayama 55: 55-63.
Citation: Akhondi H (2018) Correlation of Alzheimer’s Dementia Markers. J Alzheimers Dis Parkinsonism 8: 432. DOI: 10.4172/2161-0460.1000432
Copyright: © 2018 Akhondi H. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 5841
- [From(publication date): 0-2018 - Dec 09, 2025]
- Breakdown by view type
- HTML page views: 4869
- PDF downloads: 972