Cytopathological Effects of Almix Herbicide on Gill, Liver and Kidney of Oreochromis niloticus under Field and Laboratory Conditions
Received: 28-Apr-2016 / Accepted Date: 12-May-2016 / Published Date: 14-May-2016 DOI: 10.4172/2476-2067.1000112
Abstract
Objective: Present study was aimed to study the cytopathological effects of almix herbicide in Indian freshwater teleost, Oreochromis niloticus (Linn.) both under field and laboratory exposure.
Methods: O. niloticus (Linn.) was exposed to almix herbicide at sublethal concentrations of 8 g/acre and 66.67 mg/L under field and laboratory conditions respectively for 30 days. Field experiment was performed in cage, submerged in the field pond. Cytopathological study both through light and electron microscopic (scanning electron microscopy and transmission electron microscopy) observations were based on gill, liver and kidney.
Results: Histopathological observations revealed hypertrophy and proliferation in gill epithelium, curling and fusion of secondary gill lamellae (SGL), distortion in chloride and pillar cells under laboratory experiment. Scanning electron microscopy displayed severe loss of normal array of concentric microridges, swelling of microridges, damage in epithelial cells and appearance of vacuoles on the stratified epithelium, while loss of regular pattern of microridges were observed under field condition. Transmission electron microscopic study of gill showed severe damages which included degenerative changes in mitochondria, cellular vacuolation, damage in tubule vascular system, presence of lipid droplets, elongated nucleus, but in case of field experiment dilated mitochondria and cytoplasmic vacuolation were more prominent. Degenerative hepatopancreas, necrosis in hepatocytes, severe cytoplasmic vacuolation and disarrangement of hepatic cords in liver of O. niloticus were prominent seen under light microscopy in the laboratory condition, but in field condition elongated hepatocytes with increased nuclei and vacuolation in cytoplasm of hepatocytes were prominent, while TEM study showed degeneration in mitochondria, dilation in rough endoplasmic reticulum, damage in nucleus and appearance of cytoplasmic vacuolation in hepatocytes under laboratory condition. In kidney, degenerative changes in PCT and DCT, shrinkage of glomerulus, vacuolation in the haematopoietic tissues and excess fat deposition were notable changes. In TEM study necrosis in nucleus, severe vacuolation, appearance of endoplasmic reticulum as whorl pattern, degeneration in mitochondria in kidney were serious after almix exposure.
Conclusion: Present study claimed the more profound responses under laboratory condition compared to field and different significant marked changes in these two conditions were prominent. Therefore, these responses in gill, liver and kidney of O. niloticus could be considered as potential of exposure of agrochemicals.
Keywords: Almix; Cytopathology; Chronic exposure; Gill; Liver; Kidney; O. niloticus
42608Introduction
Integrated paddy-cum-fish-culture system has gained considerable attention in recent years in many developing countries of Asia, Africa and America. Paddy-cum fish-culture system is beneficial in increasing land productivity as well as eliminating weeds, molluscs and pests and subsequently can improve the economic condition of the rural people [1]. But in India such integrated paddy-cum-fish-culture system is almost non-existent because of increasing use of inorganic fertilizers and pesticides in rice fields which have deleterious effects on fish [2].
Indian economy is based on agricultural productivity and rice production is its main allied sector [3]. Weeds are the prime reason for lowering the production of rice and reducing the yield of approximately 60-70% [1]. Therefore, for increasing the productivity herbicide alone or in combination with other biological means like use of fish play an important role by controlling the weeds and a successful herbicide should not only control the weeds effectively but it also be safer to the soil flora and fauna as well as aquatic organisms. Almix is one of them and is used extensively for controlling the broad leaved weeds and sedges both in the rice fields and aquatic ecosystem. Almix® 20 WP is a selective, contact as well as systematic and both preemergent and post-emergent modern fourth generation herbicide of sulfonylurea group. It is a combination of 10.1% metsulfuron methyl, 10.1% chlorimuron ethyl and 79.80% adjuvants [4].
Fish are most sensitive to the aquatic pollutants during their early life stages [5]. Therefore, any type of aquatic pollution either by agricultural runoff or by discharge of untreated effluents etc., from manufacturing industries into natural waterways might endanger the fish population and other forms of aquatic life, and may contribute long term effects in the environment by changing the water quality. Tilapia, Oreochromis niloticus is freshwater surface feeding omnivore fish belong to the family Cichlidae. They grow fast, mature quickly, and breed easily without inducement [6]. A number of studies on toxic effect of different other agrotoxicants on different fish species including tilapia were reported by various authors [5,7-11] under laboratory condition, but limited information on almix toxicity is available on histopathological and ultrastructural responses in this fish species. Therefore, the present study is concerned with histopathological and ultrastructural examination of toxic effects of almix herbicide to Oreochromis niloticus in particular sensitive organs like gill, liver and kidney and compared the lesions as markers under laboratory and field condition to prepare baseline information for monitoring the application of this agrochemical in the aquatic environment.
Materials and Methods
Chemicals
Almix herbicide was purchased from local market (DuPont India Pvt. Ltd., Gurgaon, Haryana, India). All other reagents of analytical grade were purchased from Merck Specialities Private Limited. Osmium tetraoxide was procured from Spectrochem Pvt. Ltd., Mumbai, India.
Fish
Indian freshwater teleost, Oreochromis niloticus (Linnaeus) was procured from local fish farm and were acclimatized for 15 days under laboratory condition. The average weight and total length of concerned fish were 38.57 ± 2.47 g and 13.59 ± 0.496 cm respectively. Fish were reared at aerated water and with natural photoperiod set at 12-h light/12-h dark. Average value of water parameters during the acclimatization period, were as follows: temperature 26.49 ± 0.127°C, pH 7.94 ± 0.040, electrical conductivity 392.22 ± 0.62 μS/cm, total dissolved solids 279.33 ± 0.69 mg/L, dissolved oxygen 6.44 ± 0.05 mg/L, total alkalinity 204.00 ± 7.30 mg/L as CaCO3, total hardness 180.44 ± 3.74 mg/L as CaCO3, sodium 24.45 ± 0.56 mg/L, potassium 5.33 ± 1.02 mg/L, orthophosphate 0.03 ± 0.001 mg/L, ammoniacalnitrogen 1.66 ± 0.21 mg/L, nitrate-nitrogen 0.21 ± 0.030 mg/L. After acclimatization, fish were divided into two groups: one group was transferred to field ponds situated at University Crop Research Seed Multiplication Farm (CRSMF) premises and other group was transferred to laboratory aquarium. Fish were fed once a day with Tokyu fish pellets (32% crude protein) during acclimatization and exposure.
Field experimental design
Fish, after acclimatization in field pond for two weeks were allocated in two groups as follows: control groups, 10 fish species distributed in three separate cages, and exposure group with 10 fish species in separate cages (triplicate) for 30 days. Recommended dose (8 g/acre) for rice cultivation was dissolved in water and was sprayed on the first day on the surface of almix-treated plots [12-15]. Special type of cage was prepared for field experiments and was installed separately at pond of university CRSMF based on Chattopadhyay et al. [16] with some modifications. Cages were rectangular in shape (2.5 m × 1.22 m × 1.83 m) and the submerged height in water was 0.83 m. Cages were prepared by using strong bamboo. Cage was fabricated with nylon net and was embraced by two types of PVC nets: the inner one (mesh sizes of 1.0 × 1.0 mm2) and outer one with mesh sizes of 3.0 ×x 3.0 mm2. Average limnological values of pond water during the exposure period were as follows: temperature 24.23 ± 0.213°C, pH 7.16 ± 0.187, electrical conductivity 342.00 ± 3.13 μS/cm, total dissolved solids 245.67 ± 2.25 mg/L, dissolved oxygen 7.00 ± 0.157 mg/L, total alkalinity 221.33 ± 3.53 mg/L as CaCO3, total hardness 140.00 ± 2.31 mg/L as CaCO3, sodium 63.40 ± 2.67 mg/L, potassium 15.96 ± 2.10 mg/L, orthophosphate 0.24 ± 0.026 mg/L, ammoniacal-nitrogen 0.74 ± 0.111 mg/L, nitrate-nitrogen 1.66 ± 0.035 mg/L.
Laboratory experimental design
After acclimatization, fish were divided into two groups (control and almix-treated) and maintained in aquaria, ten fish in each aquarium: three aquaria for control and another three for treatment (40 L capacity). Sublethal dose of 66.67 mg/L prepared and applied to the aquarium for a period of 30 days [17-19]. On every alternate day water was replaced and dose was applied. During experimentation almix-treated and control were subjected to same environmental conditions. During experimentation period, the average water parameters were as follows: temperature 26.63 ± 0.120°C, pH 7.93 ± 0.075, electrical conductivity 426.00 ± 5.93 μS/cm, total dissolved solids 302.89 ± 4.69 mg/L, dissolved oxygen 5.06 ± 0.43 mg/L, total alkalinity 209.80 ± 10.50 mg/L as CaCO3, total hardness 163.11 ± 3.04 mg/L as CaCO3, sodium 37.76 ± 1.02 mg/L, potassium 7.26 ± 1.12 mg/L, orthophosphate 0.04 ± 0.002 mg/L, ammoniacal-nitrogen 7.09 ± 2.15 mg/L, nitrate-nitrogen 1.78 ± 0.263 mg/L.
Sampling
Water quality during experimentation was measured as per APHA [20]. At the end of the experiment (i.e., 30 days), fish were collected and rapidly anesthetized with tricaine methanesulphonate (MS 222) and the desired organs namely gill, liver and kidney were dissected and immediately proceeded for histological, scanning and transmission electron microscopic study.
Histological study
After dissection of fish, gill, liver and kidney were fixed in aqueous Bouin’s fluid solution for overnight. After fixation, tissues were dehydrated through a graded series of ethanol, cleared in xylene, and embedded in paraffin. Sections were cut at 3-4 μ using Leica RM2125 microtome and stained with haematoxylin-eosin (H&E). Stained sections were observed under Leica DM2000 light microscope and images were captured.
Ultrastructural study
For scanning electron microscopic (SEM) study, tissues were fixed in 2.5% glutaraldehyde prepared in phosphate buffer (0.2 M, pH 7.4) for 24 h at 4°C followed by post-fixation in 1% osmium tetraoxide for 2 h at 4°C. After fixation, tissues were then dehydrated through a graded series of acetone followed by amyl acetate and finally subjected to critical point drying (CPD) with liquid carbon dioxide for drying the tissue. Then, tissues were mounted on metal stubs and sputter-coated by gold (thickness 20 nm) and finally examined under scanning electron microscope (Hitachi S-530) at University Science Instrumentation Centre of the University of Burdwan and images were captured.
In case of transmission electron microscopic (TEM) study, tissues were first fixed in Karnovsky fixative (mixture of 2% paraformaldehyde and 2.5% glutaraldehyde prepared in 0.1 M phosphate buffer) for 12 h at 4°C followed by post-fixation in 1% osmium tetraoxidefor 2 h at 4°C. After fixation, tissues were dehydrated through a graded series of acetone followed by infiltration, and finally embedded in resin (araldite CY212). Then, ultrathin sections (0.5-1 μm) were cut using a glass knife (Ultracut E Reichart – Jung), collected on naked copper-meshed grids, and stained with uranyl acetate and lead citrate. Stained sections were then examined under TECHNAI G2 high resolution transmission electron microscope at Electron Microscope Facility, Department of Anatomy, AIIMS, New Delhi and images were captured.
Ethical statement
Fish care, handling, and the experiment was performed following the guideline of the Institutional Animal Care and Use Committee of the University of Burdwan and approved by the ethical committee of this University.
Results
Gill
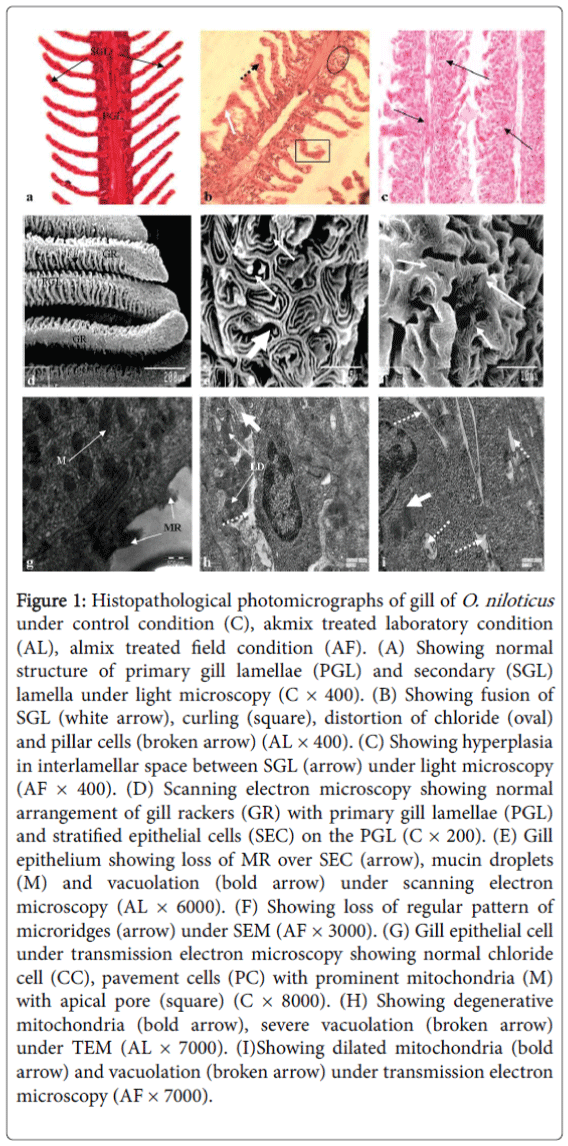
Histologically, gill of control fish is composed of primary and secondary gill lamellae. The free edges of the lamellae are extremely thin, covered with stratified epithelium and contain a vast network of capillaries supported by pilaster cells. The primary lamellar epithelium is provided with many chloride cells at the base of the secondary gill lamella and secondary lamellae are present on gill filaments (Figure 1a).
Figure 1: Histopathological photomicrographs of gill of O. niloticus under control condition (C), akmix treated laboratory condition (AL), almix treated field condition (AF). (A) Showing normal structure of primary gill lamellae (PGL) and secondary (SGL) lamella under light microscopy (C × 400). (B) Showing fusion of SGL (white arrow), curling (square), distortion of chloride (oval) and pillar cells (broken arrow) (AL × 400). (C) Showing hyperplasia in interlamellar space between SGL (arrow) under light microscopy (AF × 400). (D) Scanning electron microscopy showing normal arrangement of gill rackers (GR) with primary gill lamellae (PGL) and stratified epithelial cells (SEC) on the PGL (C × 200). (E) Gill epithelium showing loss of MR over SEC (arrow), mucin droplets (M) and vacuolation (bold arrow) under scanning electron microscopy (AL × 6000). (F) Showing loss of regular pattern of microridges (arrow) under SEM (AF × 3000). (G) Gill epithelial cell under transmission electron microscopy showing normal chloride cell (CC), pavement cells (PC) with prominent mitochondria (M) with apical pore (square) (C × 8000). (H) Showing degenerative mitochondria (bold arrow), severe vacuolation (broken arrow) under TEM (AL × 7000). (I)Showing dilated mitochondria (bold arrow) and vacuolation (broken arrow) under transmission electron microscopy (AF × 7000).
The evident of alterations as observed under light microscopy after almix intoxication under laboratory condition were hypertrophy and proliferation in gill epithelium and secondary lamellae, curling and fusion of secondary lamellae, distortion in chloride and pillar cells in O. niloticus (Figure 1b), while under field condition gill epithelium showed almost normal appearance without any marked alterations (Figure 1c).
Scanning electron microscopic study also confirmed the damages as seen under light microscopy such as severe loss of normal array of stratified epithelial cells with concentric micro ridges, (Figure 1d) swelling of micro ridges, damage in epithelial cells and appearance of vacuoles in between the stratified epithelial sheet in gill of O. niloticus (Figure 1e); there were also loss of regular pattern of microridges under field condition (Figure 1f).
Transmission electron microscopic observation showed severe damages in gill like degenerative changes in mitochondria, severe vacuolation, damage in tubule vascular system, presence of lipid droplets and elongated nucleus in laboratory condition (Figure 1h). But in case of field experiment there was less damage such as dilation in mitochondria and vacuolation after almix exposition in field condition (Figure 1i).
Liver
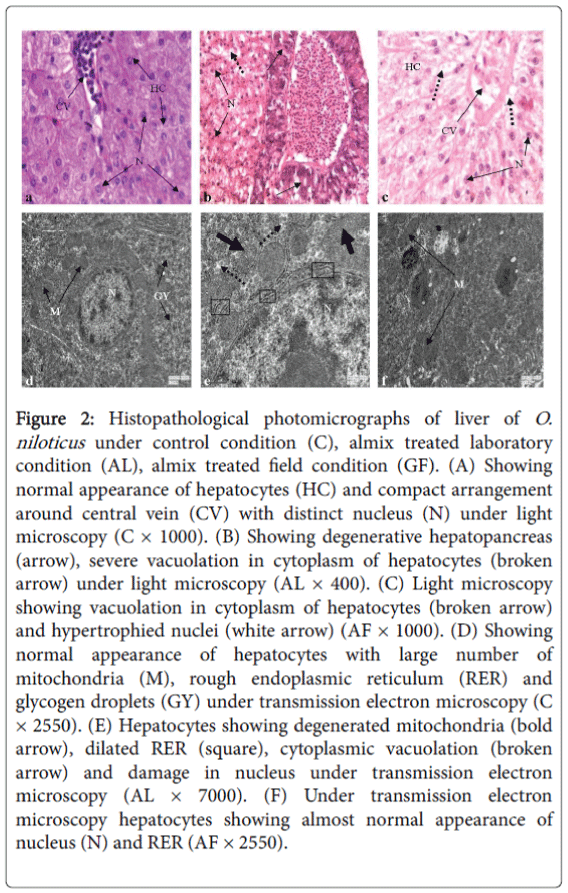
Histologically, the hepatic cells are arranged in cords surrounding a central vein. Each hepatic cell provided with deeply stained centrally placed nucleus and granular cytoplasm. Connective tissue matrix occurs in between the space of the hepatic cords and normal arrangement of blood cells in the blood vessel (Figure 2a).
Figure 2: Histopathological photomicrographs of liver of O. niloticus under control condition (C), almix treated laboratory condition (AL), almix treated field condition (GF). (A) Showing normal appearance of hepatocytes (HC) and compact arrangement around central vein (CV) with distinct nucleus (N) under light microscopy (C × 1000). (B) Showing degenerative hepatopancreas (arrow), severe vacuolation in cytoplasm of hepatocytes (broken arrow) under light microscopy (AL × 400). (C) Light microscopy showing vacuolation in cytoplasm of hepatocytes (broken arrow) and hypertrophied nuclei (white arrow) (AF × 1000). (D) Showing normal appearance of hepatocytes with large number of mitochondria (M), rough endoplasmic reticulum (RER) and glycogen droplets (GY) under transmission electron microscopy (C × 2550). (E) Hepatocytes showing degenerated mitochondria (bold arrow), dilated RER (square), cytoplasmic vacuolation (broken arrow) and damage in nucleus under transmission electron microscopy (AL × 7000). (F) Under transmission electron microscopy hepatocytes showing almost normal appearance of nucleus (N) and RER (AF × 2550).
The notable changes under laboratory condition were severe degeneration in hepatopancreas, necrosis in hepatocytes, severe cytoplasmic vacuolation and disarrangement of hepatic cord (Figure 2b), but under field condition elongated hepatocytes with increased nuclear volume and vacuolation in cytoplasm of hepatocytes were the notable changes (Figure 2c).
Ultrastructural alterations showed degenerated mitochondria, dilation in rough endoplasmic reticulum, damage in nucleus and appearance of cytoplasmic vacuolation in hepatocytes of O. niloticus after almix exposure in laboratory condition as seen under TEM observation (Figure 2e) as compared to control (Figure 2d). On the other hand, hepatocytes of O. niloticus showed almost normal appearance under field condition after exposure to almix (Figure 2f).
Kidney
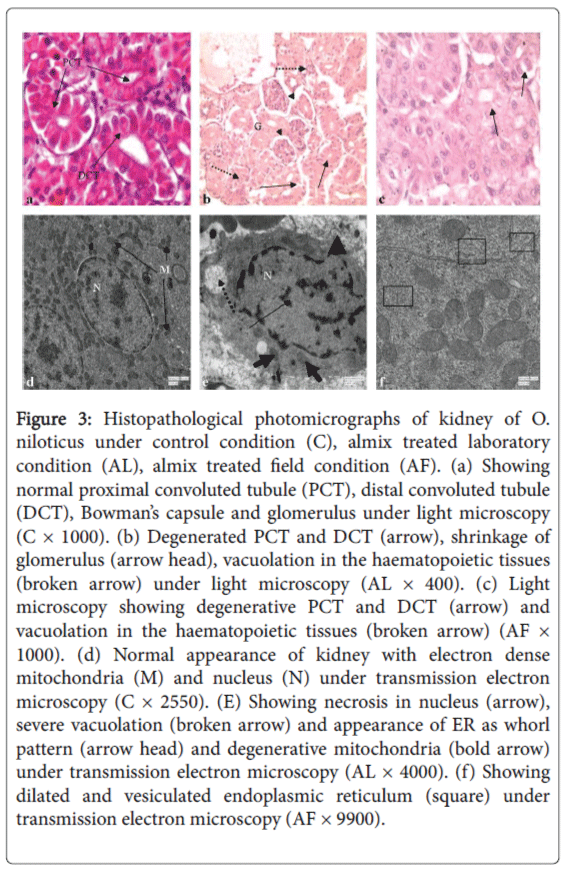
Histologically, kidney is made up of a large number of nephrons, each consisting of a renal corpuscle or the Malpighian body and the renal tubules. Renal capsules are numerous in number, spherical or oval in shape and contain vascularised glomerulus. Renal tubules are consisted of columnar epithelial cells and are differentiated into proximal convoluted tubule (PCT), distal convoluted tubule (DCT) and collecting ducts (Figure 3a). The nephropathic effects due to almix toxicosis under the laboratory condition included severe degenerative changes in PCT and DCT, severe shrinkage of glomerulus, vacuolation in haematopoietic tissues and excess fat deposition (Figure 3b), while in field condition, lesions in PCT and DCT, and vacuolation in haematopoietic tissues were noticed (Figure 3c).
Figure 3: Histopathological photomicrographs of kidney of O. niloticus under control condition (C), almix treated laboratory condition (AL), almix treated field condition (AF). (a) Showing normal proximal convoluted tubule (PCT), distal convoluted tubule (DCT), Bowman’s capsule and glomerulus under light microscopy (C × 1000). (b) Degenerated PCT and DCT (arrow), shrinkage of glomerulus (arrow head), vacuolation in the haematopoietic tissues (broken arrow) under light microscopy (AL × 400). (c) Light microscopy showing degenerative PCT and DCT (arrow) and vacuolation in the haematopoietic tissues (broken arrow) (AF × 1000). (d) Normal appearance of kidney with electron dense mitochondria (M) and nucleus (N) under transmission electron microscopy (C × 2550). (E) Showing necrosis in nucleus (arrow), severe vacuolation (broken arrow) and appearance of ER as whorl pattern (arrow head) and degenerative mitochondria (bold arrow) under transmission electron microscopy (AL × 4000). (f) Showing dilated and vesiculated endoplasmic reticulum (square) under transmission electron microscopy (AF × 9900).
Transmission electron micrograph of normal kidney showed electron dense mitochondria and nucleus and abundant vesicular structures in the capillary epithelial cell cytoplasm. Mitochondria were surrounded by few interdigitations of plasma membrane in some places and nucleus was next to a few interdigitations. Myelin-like structures was prominent in the capillary endothelial cell cytoplasm (Figure 3d). After almix intoxication under the laboratory condition, TEM study confirmed the pathological lesions such as necrosis in nucleus, severe vacuolation, appearance of endoplasmic reticulum as whorl pattern, degeneration in mitochondria in kidney of O. niloticus (Figure 3e), while only dilated and vesiculated endoplasmic reticulum were noticed after almix exposure in field condition (Figure 3f).
Discussion
In the present study, histopathological and ultrastructural effects in gill, liver and kidney of freshwater omnivorous fish, O. niloticus were investigated and compared between natural and laboratory conditions after almix intoxication. The study aimed at assessing the suitability of histological and ultrastructural responses as biomarkers of environmental contamination in aquatic ecosystem. Cellular biomarkers including histological and ultrastructural alterations represent an intermediate level of biological organization between lower-level biochemical effects and higher-level population effects [21,22], which mainly occur earlier than reproductive changes and are more sensitive for evaluation of organism health than a single biochemical response [23,24].
In this context, histopathological study through light microscopy is a rapid investigation method to detect the toxic effects of different xenobiotics, especially chronic ones, in various tissues and organs. On the other hand, ultramorphological analysis depicts topological characterization of cell surface under scanning electron microscope (SEM), while transmission electron microscope (TEM) allows the observation of alterations in the cellular and subcellular levels. Three organs namely gills, liver and kidney investigated under present study are major sites of respiration, accumulation and biotransformation, and excretion of xenobiotic substances in fish. All these three organs showed significant differences in the cytopathological response to the almix exposure under different conditions. Generally, the responses were more pronounced in the laboratory condition than field condition and this may be due to prevalence of natural condition under field treatment.
Present results exhibited severe histopathological lesions in gill of O. niloticus including hypertrophy and proliferation in gill epithelium and secondary lamellae, curling and fusion of secondary lamellae along with distortion in chloride and pillar cells. Similar results were also reported by Hued et al. [25] as lifting of secondary lamellar epithelium, oedema formation, hypertrophy of epithelial cells, severe lamellar aneurysm and lamellar fusion in gill of Jenynsia multidentata after Roundup exposure. Jiraungkoorskul et al. [26] have shown fusion of secondary lamellae, hyperplasia and oedema. Hypertrophy as seen in this study represents adaptation by the organism to protect underlying tissues from any toxicant [27]. Epithelial lifting is another most important pathological sign also observed by Cardoso et al. [28] in Pacama, Lophiosilurus alexandri. Damage in chloride and pillar cells can result in increased blood flow inside the lamellae which ultimately causing dilation of the marginal channel, blood congestion or even an aneurysm [29,30]. Damage in pillar cells indirectly indicates development of aneurysm [31,32] due to direct effects of contaminants on these cells. Scanning electron microscopic study showed loss of normal array of concentric microridges, swelling of microridges, damage in epithelial cells and appearance of vacuoles on the stratified epithelium. Similar results of loss of microridge and swelling of microridges were also reported by Johal et al. [33] in gill of Cyprinus carpio communis (Linn.) exposed to monocrotophos. Loss of microridges of the gills of test fish species was also observed by Wong and Wong [34], Mazon et al. [35] and Biagini et al. [36]. Mallatt [37] in their study reported that microridges are related with the retention of mucous on the gill epithelium as a way to protect them against environmental contaminants. Transmission electron micrograph showed degenerative changes in mitochondria, severe vacuolation, damage in tubular vascular system, presence of lipid droplets and nuclear deformity after almix exposure under both conditions but lesions were less in case of field experiment. Vacuolation as observed under present study impede gas exchange capacity as well as indicate swelling of mitochondria and rough endoplasmic reticulum was also reported by Ultsch et al. [38] and Pawert et al. [39]. Mitochondrial damage is responsible for impairment of ionic transport was also described by Perry and Laurent [40] and Goss et al. [41] in gill of fish species after intoxication of toxicant. Damage in tubular vascular network is another notable change after almix exposition under laboratory condition. Therefore, these changes in gill may interfere with the fundamental process such as maintenance of osmoregulation and antioxidant defence of gills as well as be considered as fast and most valid method to mark the damages caused by pollutants in aquatic ecosystem.
Liver showed diversity of pathological lesions including severe degenerative changes in zymogen granules of acinar cells of hepatopancreas, necrosis in hepatocytes, and severe vacuolation in cytoplasm along with disarrangement of hepatic cord under both conditions. Necrosis represented the most evident hepatic lesion found in this study and have been considered as common lesion. Presence of this lesion indicates that effect of almix herbicide is very impactful; therefore caused functional and structural impairments [42]. Rahman et al. [43] also observed severe necrosis, appearance of large number of vacuoles in cytoplasm and pyknotic nuclei in liver of C. punctatus and A. testudineus after intoxication of Diazinon 60 EC. Vacuolation, infiltration of leukocytes and pyknotic nuclei were also reported by Jiraungkoorskul et al. [5] in liver of Oreochromis niloticus after Roundup exposure. Vacuolization as observed in hepatocytes of test fish species indicate imbalance between the rate of synthesis of substances in the parenchymal cells and the rate of their release into the systemic circulation within the body [44]. Nuclear hypertrophy, cellular atrophy, irregular contour of cells and nucleus, cytoplasmic vacuolation, cytoplasmic and nuclear degeneration, cellular rupture, pyknotic nucleus, necrosis and melanomacrophages aggregations in the liver of Cyprinus carpio were also reported after chlorpyrifos exposure by Pal et al. [45]. In our study, transmission electron micrograph of liver showed degenerative changes in mitochondria, dilation in rough endoplasmic reticulum, damage in nucleus and appearance of cytoplasmic vacuolation under laboratory condition. These alterations in hepatocytes indicate development of toxic stress condition. The mitochondrial degeneration seen under present study may account for the impaired oxidative capability of hepatocytes by inhibiting normal function of respiratory chain of enzymes which oxidises the formation of ATP molecule during phospholipid metabolism and fatty acid synthesis. Marked ultrastructural changes including the presence of swollen mitochondria with loss of functional cristae have already been reported in the liver tissue of catfish exposed to methyl parathion by Tripathi and Shukla [46]. Alterations of the rough ER, including dilation and vesiculation, are the common response to the herbicide exposure. Braunbeck and Völkl [47] and Au et al. [48] correlated these alterations of the rough ER with a higher biotransformation capacity of hepatocytes, and Ghadially [49] interpreted the dilation of ER cisternae as enhanced storage of proteins due to a reduced secretory activity. Along with this, altered RER indicate induction of mixed-function oxidases (MFO) which can also well be interpreted as the morphological counterpart of ethoxycoumarin-O-deethylase (ECOD) and ethoxyresorufin-Odeethylase (EROD) induction [50]. Similar findings were reported in rainbow trout after exposure to endosulfan and disulfoton [51], and in the demersal fish following intraperitoneal injection of benzo(a) pyrene [48]. Cytoplasmic vacuolation in hepatocytes of test fish species was also reported by Li et al. [52] indicating imbalance in the synthesis of substances in parenchymal cells. In general, the cytopathological changes were more pronounced in fish exposed under laboratory condition as compared to field and this may be assumed that in case of field experiments existence of fish in natural environment along with dilution effects of herbicidal action may induct general metabolic adaptation under this natural environment.
In kidney, almix intoxication caused severe pathological alterations both under laboratory and field condition, although the pathological responses were more severe under laboratory condition. Degenerative changes in PCT and DCT, shrinkage of glomerulus, severe vacuolation in the haematopoietic tissues and excess fat deposition were evident. Similar results were also reported by Oulmi et al. [53] who showed small cytoplasmic vacuoles, nuclear deformation in the epithelium of the first and second segments of the proximal tubule. Vacuolization of tubular epithelium, enlargement of nuclei and degeneration of the kidney as observed in the present study also reported by several authors after exposure of different contaminants [54–56]. Butchiram et al. [57] in their study also noticed degenerative changes in haemopoietic tissue which include severe necrosis, cloudy swelling in renal tubules, cellular hypertrophy and granular cytoplasm in kidney of Channa punctatus after alachlor exposure. Cytopathological alterations such as necrosis in nucleus, severe vacuolation, appearance of endoplasmic reticulum as whorl pattern and degeneration in mitochondria were prominent under transmission electron microscopy in laboratory condition. Cytoplasmic vacuolation is the most prominent alterations seen under present study after herbicide exposure have also been reported in kidney of gold fish exposed to hexachlorobutadiene by Reimschüssel et al. [58] and by Segnini de Bravo et al. [59] in two Venezuelan cultured fish, Caquetaia kraussii and Colossoma macropomum after triazine exposure. Fischer-Scherl et al. [60] in their study also reported degeneration and vacuolation in epithelial cells, and fragmentation in RER in kidney of rainbow trout exposed to atrazine. These lesions also resembled with the symptoms of the present study in the laboratory condition in concerned test fish. Chaudhuri et al. [61] in their study reported presence of hyaline droplets in renal tubules. In the field condition dilation of endoplasmic reticulum in some places was prominent and this different response in two conditions may be attributed to different feeding habits and the habitat.
In conclusion, the present study revealed that almix herbicide is toxic to fish and causes histopathological and ultrastructural changes in gill, liver and kidney under laboratory condition. The results presented herein demonstrated that almix at rice-paddy field concentrations caused lesions in the respective fish organs were comparatively less than laboratory condition. Therefore, these responses could be considered as potential biomarkers for risk assessment of these drain-off agrochemicals in aquatic ecosystem.
Acknowledgements
The authors like to thank the INSPIRE Program Division, Department of Science & Technology, Govt. of India (DST/INSPIRE Fellowship/2011/164, Dt. 29.09.2011) for the financial assistance. We also like to thank the Head, Department of Environmental Science, the University of Burdwan, Burdwan, and West Bengal, India for providing the laboratory facilities and library facilities during the course of research.
References
- Rekha GB, Ghosh C, Mitra A, Jana MK, Mitra BN (1994) Techniques of rice-cum-fish culture for increasing productivity in lowlands. Indian Fmg 44: 23-26
- Ramah K (2011) Histopathological study on the effect of rice herbicides on grass carp (Ctenopharyngodanidella). Afr J Biotechnol 10: 1112-1116
- Sachdeva S (2007) Pesticides and their socio-economic impact on agriculture. Southern Economist 41: 42-53.
- DuPont Safety Data Sheet (2012) DuPont™ Almix® 20 WP. Version 2.1, Revision Date 27.07.2012 (Ref. 130000029001).
- Jiraungkoorksul W, Upatham ES, Kruatrachue M, Sahaphong S, Vichasri-Grams S, et al. (2002) Histopathological effects of Roundup, a glyphosate herbicide, on Nile tilapia (Oreochromisniloticus). Sci Asia 28: 121-127
- Fagbenro OA, Adedire CO, Owoseni EA, Ayotunde EO (1993) Studies on the biology and aquacultural potential of feral catfish, Heterobranchusbidorsalis (Geoffroy Saint Hilaire 1809) (Clariidae). Trop Zool 6: 67-79
- Oloruntuyi OO, Mulero O, Odukale B (1992) The effects of two pesticides on Clariasgiriepinus. Proceeding of the 10th Annual Conference of the Fisheries Society of Nigeria. 173-177
- Koviznych JA, Urbancikova M (1998) Acute toxicity of Acetachlor pollution for zebra fish (Daniorerio) and Guppy (Paccilia reticulate). Aquacult Environ Qual 17: 449-456
- Abd El-Gawad AM (1999) Histopathological studies on the liver and gills of Tilapia nilotica (Oreochromisnilticus) exposed to different concentrations of lead acetate and zinc sulphate. J Egypt German SocZool 30: 3-22.
- Visoottiviseth P, Thamamaruitkum T, Sahaphong S, Reingroipitak S, Kruatrachua M (1999) Histological Effects of Triphemyltin hydroxide on liver, kidney and gill of Nile tilapia (Oreochrominniloticus). Appl Organometallic Chem 13: 749-763
- Babatunde MM, Oludimiji AA, Balogun JK (2001) Acute toxicity of Gamaxone to Oreochromisniloticus (Treweva) in Nigeria. Water Air Soil Pollut 13: 1-10.
- Samanta P, Pal S, Mukherjee AK, Senapati T, Kole D, et al. (2014) Effects of Almix herbicide on alanine aminotransferase (ALT), aspartate aminotransferase (AST) and alkaline phosphatase (ALP) of three teleostean fishes in rice field condition. Global J Environ Sci Res 1: 1-9
- Samanta P, Pal S, Mukherjee AK, Senapati T, Kole D, et al. (2014) Effects of Almix herbicide on profile of digestive enzymes of three freshwater teleostean fishes in rice field condition. Toxicol Rep 1: 379-384.
- Samanta P, Pal S, Mukherjee AK, Ghosh AR (2014) Biochemical effects of almix herbicide in three freshwater teleostean fishes. In HydroMedit - 2014, 1st International Congress of Applied Ichthyology & Aquatic Environment
- Samanta P, Bandyopadhyaya N, Pal S, Mukherjee AK, Ghosh AR (2015) Histopathological and ultramicroscopical changes in gill, liver and kidney of Anabas testudineus (Bloch) after chronic intoxication of almix (metsulfuron methyl 10.1%+chlorimuron ethyl 10.1%) herbicide. Ecotoxicol Environ Saf 122: 360-367
- Chattopadhyay DN, Mohapatra BC, Adhikari S, Pani PC, Jena JK, et al. (2013) Effects of stocking density of Labeorohita on survival, growth and production in cages. AquacultInt 21: 19-29
- Samanta P, Pal S, Mukherjee AK, Senapati T, Ghosh AR (2014a) Effects of Almix herbicide on metabolic enzymes in different tissues of three teleostean fishes Anabas testudineus, Heteropneustesfossilis and Oreochromisniloticus. Int J Sci Res Environ Sci 2: 156-163
- Samanta P, Pal S, Mukherjee AK, Senapati T, Ghosh AR (2014g) Alterations in digestive enzymes of three freshwater teleostean fishes by Almix herbicide: A comparative study. ProcZoolSoc doi:10.1007/s12595-014-0122-7
- Samanta P, Pal S, Mukherjee AK, Senapati T, Ghosh AR (2015a) Evaluation of enzymatic activities in liver of three teleostean fishes exposed to commercial herbicide, Almix 20 WP. ProcZoolSoc 68: 9-13
- Young JC, Clesceri LS, Kamhawy SM (2005) Changes in the biochemical oxygen demand procedure in the 21st edition of Standard Methods for the Examination of Water and Wastewater. Water Environ Res 77: 404-410.
- Adams SM, Shepard KL, Greeley MS, Jimenez BD, Ryon MG, et al. (1989) The use of bioindicators for assessing the effects of pollutant stress on fish. Mar Environ Res 28: 459-464
- Adams SM, Giesy JP, Tremblay LA, Eason CT (2001) The use of biomarkers in ecological risk assessment: recommendations from the Christchurch conference on Biomarkers in Ecotoxicology. Biomarkers 6: 1-6.
- Segner H, Braunbeck T (1988) Hepatocellular adaptation to extreme nutritional conditions in ide,Leuciscusidusmelanotus L. (Cyprinidae). A morphofunctional analysis. Fish PhysiolBiochem 5: 79-97.
- Triebskorn R, Kahler HR, Honnen W, Schramm M, Adams SM, et al. (1997) Induction of heat shock proteins, changes in liver ultrastructure, and alterations of fish behaviour: Are these biomarkers related and are they useful to reflect the state of pollution in the field?. J AquatEcosyst Stress Recovery 6: 57-73
- Hued AC, Oberhofer S, de los Angeles Bistoni M (2012) Exposure to a commercial glyphosate formulation (Roundup®) alters normal gill and liver histology and affects male sexual activity of Jenynsiamultidentata (Anablepidae, Cyprinodontiformes). Arch Environ ContamToxicol 62: 107-117
- Jiraungkoorskul W, Upatham ES, Kruatrachue M, Sahaphong S, Vichasri-Grams S, et al. (2003) Biochemical and histopathological effects of glyphosate herbicide on Nile tilapia (Oreochromisniloticus). Environ Toxicol 18: 260-267
- Meissner MA, Diamandopoulos GTH (1977) Neoplasia. In: Anderson WAD, Kissane JM (ed.) Pathology. p. 640-691.
- Cardoso EL, Chiarini-Gracia H, Ferreira RMA, Poli CR (1996) Morphological changes in the gills of Lophiosilurusalexandri exposed to unionized ammonia. J Fish Biol 49: 778-787
- Takashima F, Hibya T (1995) An atlas of fish histology: normal and pathological features. Kodansha, Tokyo.
- Rosety-RodrÃguez M, Ordoñez FJ, Rosety M, Rosety JM, Rosety I, et al. (2002) Morpho-histochemical changes in the gills of turbot, Scophthalmusmaximus L., induced by sodium dodecyl sulfate. Ecotoxicol Environ Saf 51: 223-228.
- Heath AG (1987) Water pollution and fish physiology. CRC Press Inc., Florida.
- Martinez CB, Nagae MY, Zaia CT, Zaia DA (2004) Acute morphological and physiological effects of lead in the neotropical fish Prochiloduslineatus. Braz J Biol 64: 797-807.
- Johal MS, Sharma ML, Ravneet (2007) Impact of low dose of organophosphate, monocrotophos on the epithelial cells of gills of Cyprinuscarpiocommunis Linn.--SEM study. J Environ Biol 28: 663-667.
- Wong CK, Wong MH (2000) Morphological and biochemical changes in the gills of Tilapia (Oreochromismossambicus) to ambient cadmium exposure. AquatToxicol 48: 517-527.
- Mazon AF, Cerqueira CC, Fernandes MN (2002) Gill cellular changes induced by copper exposure in the South American tropical freshwater fish Prochilodusscrofa. Environ Res 88: 52-63.
- Biagini FR, de Oliveira David JA, Fontanetti CS (2009) The use of histological, histochemical and ultramorphological techniques to detect gill alterations in Oreochromisniloticus reared in treated polluted waters. Micron 40: 839-844.
- Mallatt J (1985) Fish gill structural changes induced by toxicants and other irritants: a statistical review. Can J Fish AquatSci 42: 630-648
- Ultsch GR, Ott ME, Heisler N (1980) Standard metabolic rate, critical oxygen tension and aerobic scope for spontaneous activity of trout (Salmogairdneri) in acidified water. Comp BiochemPhysiol 67: 329-335
- Pawert M, Müller E, Triebskorn R (1998) Ultrastructural changes in fish gills as biomarker to assess small stream pollution. Tissue Cell 30: 617-626.
- Perry SE, Laurent P (1993) Environmental effects on fish gill structure and function. In Rankin JC, Jenseu FB (ed.) Fish ecophysiology. Chapman and Hall, London, p. 231-264.
- Goss GG, Perry SF, Laurent P (1995) Ultrastructural and morphometric studies on ion and acid-base transport processes in freshwater fish. In Hoar WS, Randall DJ, Farrell AP (ed.) Fish physiology. Academic Press, New York, p. 257-284
- Stentiford GD, Longshaw M, Lyons BP, Jones G, Green M, et al. (2003) Histopathological biomarkers in estuarine fish species for the assessment of biological effects of contaminants. Mar Environ Res 55: 137-159.
- Rahman MZ, Hossain Z, Mollah MFA, Ahmed GU (2002) Effect of Diazinon 60 EC on Anabas testudineus, Channapunctatus, Barbodesgoniontus. NAGA, The ICLARM Quart 25: 8-11
- Gingerich WH (1982) Hepatic toxicology in fish. In Weber LJ (ed.) Aquatic toxicology. Raven Press, New York, p. 55-105.
- Pal S, Kokushi E, Koyama J, Uno S, Ghosh AR (2012) Histopathological alterations in gill, liver and kidney of common carp exposed to chlorpyrifos. J Environ Sci Health B 47: 180-195.
- Tripathi G, Shukla SP (1990) Enzymatic and ultrastructural studies in a freshwater catfish: impact of methyl parathion. Biomed Environ Sci 3: 166-182.
- Braunbeck T, Valkl A (1993) Toxicant-induced cytological alterations in fish liver as biomarkers of environmental pollution? A case study on hepatocellular effects of dinitro-o-cresol in golden ide (Leuciscusidusmelanotus). In Braunbeck T, Hanke W, Segner H (ed.) Fish eecotoxicology and ecophysiology. VCH Verlagsgesellschaft, Weinheim, p. 55-80
- Au DWT, Wu RSS, Zhou BS, Lam PKS (1999) Relationship between ultrastructural changes and EROD activities in liver of fish exposed to Benzo[a]pyrene. Environ Pollut 104: 235-247
- Ghadially FN (1989) Ultrastructural pathology of the cell and matrix. Butterworths 139: 91
- Hawkes JW (1980) The effects of xenobiotics on fish tissues: morphological studies. Fed Proc 39: 3230-3236.
- Arnold H, Plutab HJ, Braunbeck T (1995) Simultaneous exposure of fish to endosulfan and disulfoton in-vivo: ultrastructural, stereological and biochemical reactions in hepatocytes of male rainbow trout (Oncorhynchusmykiss). AquatToxicol 33: 17-43
- Li X, Liu Y, Song L (2001) Cytological alterations in isolated hepatocytes from common carp (Cyprinuscarpio L.) exposed to microcystin-LR. Environ Toxicol 16: 517-522.
- Oulmi Y, Negele RD, Braunbeck T (1995) Cytopathology of iver and kidney in raindow trout (Oncorhynchusmykiss) after long-term exposure to sub-lethal concentrations of linuron. Dis Aquat Org 21: 35-52
- Thurston RV, Russo RC, Luedtke RJ, Smith CE, Meyn EL, et al. (1984) Chronic toxicity of ammonia to rainbow trout. Trans Am Fish Soc 113: 56-73
- Meade JW, Herman RL (1986) Histopathological changes in cultured lake trout, Salvelinusnameycush, subjected to cumulative loading in a water reuse system. Can J Fish AquatSci 43: 228-231
- Ravindra K (2000) Chronic ammonia induced histopathological changes in Indian subtropical freshwater murrel, Channapunctatus (Bloch). Pollut Res 19: 611-613.
- Butchiram MS, Tilak KS, Raju PW (2009) Studies on histopathological changes in the gill, liver and kidney of Channapunctatus (Bloch) exposed to Alachlor. J Environ Biol 30: 303-306.
- Reimschuessel R, Bennet RO, May EB, Lipsky MM (1989) Renal histopathological changes in the goldfish (Carassiusauratus) after sublethal exposure to hexachlorobutadiene. AquatToxicol 15: 169-180
- Bravo MI, Medina J, Marcano S, Finol HJ, Boada-Sucre A (2005) Effects of herbicide on the kidneys of two Venezuelan cultured fish: Caquetaiakraussii and Colossomamacropomum (Pisces: Ciclidae and Characeae). Rev Biol Trop 53: 55-60
- Fischer-Scherl T, Veeser A, Hoffmann RW, Kühnhauser C, Negele RD, et al. (1991) Morphological effects of acute and chronic atrazine exposure in rainbow trout (Oncorhynchusmykiss). Arch Environ ContamToxicol 20: 454-461.
- Chaudhuri BN, Kleywegt GJ, Björkman J, Lehman-McKeeman LD, Oliver JD, et al. (1999) The structures of alpha 2u-globulin and its complex with a hyaline droplet inducer. Acta Crystallogr D Biol Crystallogr 55: 753-762.
Citation: Samanta P, Pal S, Mukherjee AK, Senapati T, Ghosh AR (2016) Cytopathological Effects of Almix Herbicide on Gill, Liver and Kidney of Oreochromis niloticus under Field and Laboratory Conditions. Toxicol Open Access 2: 112. DOI: 10.4172/2476-2067.1000112
Copyright: © 2016 Ghosh AR, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 14216
- [From(publication date): 5-2016 - Aug 17, 2025]
- Breakdown by view type
- HTML page views: 13129
- PDF downloads: 1087