Deficient of Megalin in Stable Renal Transplanted Patients with Proximal Tubular Dysfunction
Received: 06-Jun-2016 / Accepted Date: 08-Aug-2016 / Published Date: 15-Aug-2016 DOI: 10.4172/2475-7640.1000107
Abstract
Introduction: Renal-transplant patients with stable graft function and proximal tubular dysfunction (PTD) have an increased risk for IF/TA. The morphological features associated with this dysfunction are unknown. Material and methods: 54 renal transplant patients with normal and stable renal function were submitted to a biopsy and had urinary retinol binding protein (uRBP) and renal function assessment. Patients were divided according to uRBP levels: 1, these findings had no association with uRBP levels. Megalin expression was observed at BB of PTC, 13.7% of bxs presented its expression in less than 50% of tubules, 56.8% had in more than 50% of tubules but with discontinuity over the BB and in 29.5% megalin expressed in more than 50% of tubules continuouslly over the BB. Patients who presented uRBP > 0.6 mg/L had lower amount of megalin expression in their biopsies, p=0.007. Cellular RBP expression was observed diffusely over the cytoplasma of PTC, with different intensities. No correlation was found between tubular megalin expression and uRBP values with CRBP expression. Conclusions: Half of renal transplant patients with normal renal function had PTD. The deficiency of megalin expression could be the subjacent functional alteration found in patients with PTD.
Keywords: Retinol binding protein; Proximal tubular function; Kidney transplantation; Tubulointerstitial injury; Chronic allograft; Nephropathy; Allograft renal biopsy
7827Introduction
The rate of graft loss due chronic allograft tubular atrophy/ interstitial fibrosis (TA/IF) after the first year remains substantial [1-3]. This has led researchers to continuously look for early biomarkers that can actually predict graft loss and could help to optimize therapy in order to change the natural history of engrafted organs.
The functional study of proximal tubular epithelial cell (PTEC), by measuring low molecular weight proteins (LMWP), has been used in some Transplant Centres as a strategy to determine the tubular integrity in transplanted patients during follow up. LMWP are useful to identify very early acute and chronic tubule-interstitial injuries, which occur in renal transplantation. Therefore, PTD seems to be an early marker for calcineurin inhibitors nephrotoxicity, interstitial fibrosis/tubular athrophy (IF/TA) and even graft loss [4-7].
Indeed, previous reports showed that heart transplanted patients with elevated levels of urinary retinol binding protein (uRBP) had a very high risk of developing progressive renal failure probably due to cyclosporine nephrotoxicity [4,8]. In renal transplanted patients with stable renal function, PTD was found in a variable degree, ranging from 25% to 52% and it was characterized by high levels LMWP in urine [5,6,9]. The implications of these findings are still not completely understood.
LMWP are freely filtered by glomerulus and almost completely reabsorbed by PTEC at brush border (BB). Their excretion level into urine is increased in the case of reduced reabsorption by these cells, which is due to an increased reabsortive load, or to functional/ structural damage. The absorption process involves the presence of a very sophisticated calcium-dependent endocytic apparatus, and it requires high-energy expenditure. These proteins are absorbed when they reach the luminal portion of the tubular cell via endocytic receptors, called megalin and cubilin. Subsequently they are dissociated from their receptors by endosomal acidification and transported to the lysosomes, where they are degraded. The resulting aminoacids cross the contraluminal membrane and return to circulation and megalin and cubilin return to the apical membrane [10-12].
Changes in any of the steps involving the absorption of these proteins might induce the loss of these proteins in the urine. Our group has also studied the histological aspects of PTEC and of the interstitium in stable renal transplanted patients, which could be associated with uRBP elevation. No morphological variables evaluated (IF/AT, interstitial inflammation, acute tubular necrosis and tubulitis) were found in patients with high uRBP levels [13]. This finding suggests that stable renal transplanted patients who present PTD might have a functional alteration. It is not known if the endocytic apparatus in renal transplanted patients with PDT have any alteration.
In this work two hypothesis were addressed here: 1) Whether PTD in stable renal transplanted patients is associated with changes in the uRBP endocytic apparatus that are related to the expression of the megalin receptor; 2) Whether PTD correlates with histological or clinical graft dysfunction.
Material and Methods
Subjects
This was a transversal study, in which 54 renal transplant patients with stable renal function (mean serum creatinine, sCr, of 1.37 ± 0.31 mg/dL) with a mean post-transplant time of 12.48 ± 6.42 months (6- 43 months) were submitted to a protocol renal biopsy. Patients with stable renal function were those who presented sCr levels stable in 3 consecutive measurements (sCr lower than 2 mg/dL and a variability of sCr of less than 10%) before the biopsy procedure and complete absence of any clinical dysfunction such as drug toxicity, acute rejection, or infection. Kidney biopsies were performed simultaneously with uRBP measurements and renal function assessments.
Data were collected between December 2003 and November 2006. All 54 participants were followed at the Hospital and signed the informed consent, which was approved by Board Review of the Ethics Committee for Research of Hospital Israelita Albert Einstein (Identifier: 03-54).
Initial immunosuppression included a calcineurin inhibitor associated with steroids and an anti-proliferative agent (mycophenolate sodium or mycophenolate mofetil). Rapamycin was used in few cases as an alternative to calcineurin inhibitor or anti-proliferative agent. As a routine in our center, all receptors from deceased donors received induction in their initial immunosuppresive schema. In this sense, 27 (50%) patients used thymoglobulin and 8 (14%) used daclizumab for induction, 21 (38%) patients used tacrolimus, 29 (53%) cyclosporine and 4 (7%) utilized rapamycin in their immunosuppressive schema at the time of biopsy. Treatment of acute rejection was based on the Banff 1997 Classification [14] and clinical presentation. Adjustment of cyclosporine and tacrolimus doses was described previously [13].
Delayed graft function (DGF) was defined as the need for dialysis during the first week after transplant, in the absence of rejection and/ or technical problems. Acute rejection (AR) was defined according to Banff 97 criteria and clinical presentation. Proximal tubular dysfunction was defined as uRBP levels > 0.6 mg/L. Cytomegalovirus (CMV) infection was defined as a positive antigenemia assay and the concomitant administration of antiviral treatment. Calcineurininhibitor nephrotoxicity (CIN) was defined by striped cortical fibrosis or new-onset arteriolar hyalinosis (not from renal ischemia or preexisting hyalinosis in the allograft) supported by tubular microcalcification.
Tubular dysfunction analysis
The uRBP was determined by immunonephelometry assay and the upper limit of normal was set at 0.6 mg/L. Urine samples were collected and frozen until RBP determination. No conservative or special precautions were considered to be necessary since RBP is very stable in urine [15]. The uRBP value considered for each patient was averaged from the last 3 measurements before biopsy. In our study, patients were divided into two groups according to uRBP values. Normal levels where those lower than 0.6 mg/L and high levels were 0.6 mg/L or higher. As showed previously, urinary concentration has no influence on RBPu, therefore the ratio RBPu/Creatinine urinary is not necessary [16].
Renal function
Renal function was measured by sCr and creatinine clearance was estimated by the simplified MDRD formula (186 × sCr-1.154 × age-0.203 × 1.210 if black × 0.742 if female) [14]. Microalbuminuria was analyzed in all patients in an isolated sample of urine by nephelometry. Urinary microalbuminuria/creatinine (uM/Cr) was determined and the normal range was set at below 0.03.
Histological studies
Renal tissue specimens from biopsies were subjected to routine staining (hematoxylin-eosin, Jone’s Silver Methanamine, periodic acid Schiff-PAS and Masson’s Trichome), Sirius red staining (for collagen I and III determination), which was assessed under polarized light. All biopsies were blindly analyzed by a renal pathologist and Banff´s 1997 Classification was used [17]. We considered adequate biopsy specimens those with at least seven glomeruli and one artery. We decided to analyze more closely the tubule-interstitial morphology, then the sum of tubular (ci) and interstitial alterations of each biopsy were calculated (ci + ct), and since our abnormalities were very subtle, biopsies were classified in 2 groups according to ci + ct > 1 or < 1.
Sirius red staining
The methodology for Sirius red staining was described previously [18]. Paraffin blocks were prepared into 3 μm thick histological sections and deparaffinized with xylol and subsequently hydrated with absolute alcohol and running water. Subsequently, the sections were immersed in saturated picric acid solution and then in Sirius red. Contra-staining was done with Harris hematoxilin.
Immunohistochemistry
For immunohistochemical analysis, the Envision Doublestain System (Dako, Carpinteria, CA) was applied. The following primary antibodies were used: Anti-vimentin (V9 clone, diluted 1:200, Dako, Carpinteria, CA), anti-collagen IV (CIV22 clone, diluted 1:80, Dako, Carpinteria, CA), anti-megalin-LRP-2 (Policlonal, diluted 1:50, Abgent San Diego, CA), anti-cellular retinol binding protein-CRBP-1 (G4E4 clone, diluted 1:10, Santa Cruz, Santa Cruz, CA), and antialpha smooth muscle actin (1A4 clone, diluted 1:40, Zymed, San Francisco, CA). In summary, for the double staining method, 3 μm sections from the paraffin blocks were obtained and pretreated with heat induced epitope retrieval. Endogenous peroxidase activity was blocked with 3% hydrogen peroxide. The slides were then incubated for 8 h with the first primary antibodies. Following incubation, the cuts were rinsed with washing buffer and incubated with the horseradish peroxidase conjugated polimer. Peroxidase activity was detected with the enzyme substrate chromogen 3,3′-diaminobenzidine tetrachloride (DAB). Afterwards, the slides were incubated with the second primary antibodies and then the alkaline phosphatase conjugated polimer. Fast red solution was used as a second substrate chromogen for visualisation of the bound antibody. Sections were counterstained with haematoxylin. For controls, negative-control reagents supplied with the double stain kit were replaced for either the first primary antibody or the second primary antibody in the dual immunolabeling scheme.
Morphometric analysis
Treatment and analysis of the images were done using the public software Image Processing and Analysis in Java, Image J provided by the National Institutes of Health (NIH). A standard point counting method was used to quantify the collagen density (sections stained with Sirius Red) and immunohistochemical markers (CRBP, megalin, vimentin, α-SMA and type IV collagen). Under high magnification (X400) consecutive non-overlapping fields were photographed from each section of the renal cortex. At least 10 fields of each section were analyzed. A grid containing 117 (13 × 9) sampling points was superimposed on each digitized image. Whenever possible, structures such as glomeruli, subcapsular cortex, large vessels, and medulla were excluded from the analysis.
Collagen density (type I and III) was determined by the number of points overlying the cortical interstitial space in sections processed with Sirius red staining. Using a cross polarizing filter, collagens appeared as green (mainly type III collagen) and yellow-red structures (mainly type I collagen) [18]. Type IV collagen visualization was measured by the number of points overlying the cortical interstitial space and tubular basal membrane. α-SMA myofibroblast infiltration in renal cortical interstitium was determined by the number of points overlying the cortical interstitial space. Megalin expression was determined by the number of points overlying the BB in proximal tubules. Tubular vimentin expression was determined by the number of points overlying in proximal tubules.
To quantify the CRBP that was distributed in the cytoplasm and which showed a color that could be clearly distinguished from the other findings in the field, the other colors were subtracted leaving only the color corresponding to CRBP, allowing its quantification. CRBP was also classified according to its intensity over the cytoplasm (mild, moderate, and strong) and to the percentage of cortex tubule stained with CRBP and analyzed semi-quantitatively from 0 to 100%.
Double immunostaining sections with megalin and vimentin were used to identify the EMT phenomenon. Cortical proximal tubular megalin and vimentin expressions were classified from 0 to 100%. We classified EMT biopsies as those presenting concomitant proximal tubular expression of vimentin > 20% and proximal tubular megalin expression < 70% (these values were based on their median tubular expressions).
Statistical analysis
Values are presented as mean and standard deviation (SD) and when appropriate, as median and ranges throughout the manuscript. Means of normally distributed data were compared by Student’s t-test, and by analysis of variance (ANOVA) for more than two groups. Data that were not normally distributed were compared by non-parametric tests. Pearson and Spearman correlations were used for quantitative variables according to their distribution. Chi-square was used to compare proportions. The significance level was 0.05 and the statistical software used was SPSS version 15.0.
Results
Demographic data
Among the 54 patients, 31 (57.4%) were male with mean age at enrollment 38.24 ± 13.37 years. Except for one patient who was submitted to a preemptive transplant, all patients underwent dialysis therapy prior to transplantation, with median time of 22 months (4- 96 months). Thirty-one (57.4%) patients received organ from living donors and 23 (42.6%) from deceased ones. Mean cold ischemia time was 18.80 ± 7.70 h in the transplants done from deceased donors. Mean age of donors was 35.80 ± 11.60 years. Regarding HLA compatibility (A, B, and DR), 34 (70.8%) recipients had > 3 HLA match compatibilities and 14 (29.2%) had <3 HLA compatibilities. Mean cyclosporine and tacrolimus trough levels in blood at the time of biopsy were 155.36 ± 47.08 and 8.59 ± 2.22 ng/mL, respectively. Seventeen (31.5%) patients had delayed graft function (DGF), 6 (11%) patients had acute rejection (AR), and 22 (40.7%) had CMV infection (Table 1).
| Variables | All patients (N=54) | uRBP < 0.6 mg/L (N=27) | uRBP ≥ 0.6 mg/L (N=27) | p value* |
|---|---|---|---|---|
| Receptor age (years) | 38.3±13.4 years | 42.11±14.06 | 34.48±11.76 | 0.035 |
| Donor age (years) | 35.9±11.6 years | 33.67±10.19 | 38.07±12.75 | 0.167 |
| Dialysis time (mo) | 22 (0-96) | 29.62±25.13 | 30.74±28.19 | 0.986 |
| Type of donor (deceased/living) | 23/31 | 12/15 | 11/16 | 0.783 |
| Mean cold ischemia time (hours) | 18.8±7.7 | 21.27±4.78 | 16.69±9.13 | 0.149 |
| Number of patients with > 3 HLA compatibilities | 34 (70.8%) | 14 (51.8%) | 20 (74%) | 0.145 |
| Acute rejection | 6 (11.1%) | 2 (7.4%) | 4 (14.8%) | 0.334 |
| Delayed graft function | 17 (31.5%) | 8 (29.6%) | 9 (33.3%) | 0.770 |
| Cytomegalovirus infection | 22 (40.7%) | 11 (40.7%) | 11(40.7%) | 1.000 |
| Thymoglobuline | 27 (50%) | 14 (51.8%) | 13 (48.1%) | 0.678 |
| Daclizumab | 22 (40%) | 3 (11.1%) | 4 (14.8%) | 0.725 |
p value*: High vs. normal uRBP values. Values were expressed as mean and standard deviation
Table 1: Demographic data distribution according to uRBP levels (univariate analysis).
Proximal tubular dysfunction analysis-urinary RBP levels
The median value of uRBP was 0.59 (range 0.55-10.80). Among the 54 patients, 27 (50%) had normal uRBP levels (0.55, range 0.55-0.58) and 27 (50%) had high levels of uRBP (0.87, range 0.60-10.80). The only demographic characteristic associated with higher uRBP levels was the recipient age (34.48 ± 11.76 vs. 42.11 ± 14.06; p=0.035). Except for that, patients with high and normal levels of uRBP had similar demographics, laboratory values (including microalbuminuria), and were under similar imunossupressor regime (Table 1). Both groups were very similar in terms of renal function, calcineurin inhibitors levels, AR, DGF, and occurrence of CMV infection (Table 2).
| Variables | All patients (N=54) | uRBP < 0.6 mg/L (N=27) | uRBP ≥ 0.6 mg/L (N=27) | p value* |
|---|---|---|---|---|
| 1/serum creatinine at biopsy, mg/dL | 0,77±0.19 | 0.81±0.15 | 0.73±0.22 | 0.148 |
| Cr Cl at biopsy, ml/min | 60.92±18.02 | 60.81±12.36 | 61.00±22.41 | 0.968 |
| Cyclosporine level at biopsy time, mg/L (n=29) | 155.36±47.08 | 159.45±42.89 | 148.69±54.78 | 0.560 |
| Tacrolimus level at biopsy time, mg/L (n=21) | 8.59±2.22 | 7.69±2.89 | 14.18±19.02 | 0.067 |
| Microalbuminuria ≥0.03 | 12 cases (22.2%) | 6 cases (22.2%) | 6 cases (22.2%) | 0.845 |
p value*: High vs. normal uRBP values. Values were expressed as mean and standard deviation
Table 2: Laboratory data according uRBP levels (univariate analysis).
Association of histological findings according to Banff 97 with uRBP levels
Among the 54 patients, 23 (43%) biopsies presented CAN according Banff 97 score and this finding had no association with uRBP levels (Table 3). Ci + Ct > 1 was found in 76% of our sample and in 58% of them the uRBP levels were higher than 0.6 mg/L (p=0.026) (Table 3). Six (11%) biopsies presented CIN and all patients had high levels of uRBP at the time of biopsy (> 0.6 mg/L). Among these 6 cases, 2 of them had high levels of calcineurin inhibitor. One patient presented borderline changes and another patient had Banff type 1A acute rejection (Banff 1997). Values for uRBP were 2.06 and 0.55 mg/L, respectively. For the remaining biopsies, 17 were normal, 6 (35.3%) of them had high levels of uRBP and 6 had non-acute rejection diagnosis according to Banff Score (non-specific changes, pre-existing disease, and de novo glomerulonephritis), and 4 (66.6%) had uRBP > 0.6 mg/L.
| Variables | All patients (N=54) | RBPu < 0.6 mg/L (N=27) | RBPu > 0.6 mg/L (N=27) | p value* |
|---|---|---|---|---|
| CAN according to Banff 97 Classification | 23 (43%) | 10 | 13 | 0.409 |
| Ci+Ct > 1 | 41 (76%) | 17 (41.5%) | 24 (58.3%) | 0.026 |
| Megalin expression (morphometric analysis) | 7.42±3.17 | 8.53±2.92 | 6.22±3.03 | 0.007 |
| Sirius red staining quantification | 3.20±2.30 | 3.07±2.50 | 3.35±2.09 | 0.707 |
| a-SMA Myofibroblast expression quantification | 6.24±5.82 | 5.98±6.69 | 6.54±4.77 | 0.757 |
| Vimentin expression quantification | 5.49±4.89 | 5.08±4.74 | 5.96±5.12 | 0.527 |
p value*: High vs. normal uRBP values. Values were expressed as mean and standard deviation
Table 3: Association between histological findings and uRBP levels.
Immunohistochemistry
Collagen deposition, interstitial α-SMA myofibroblast expression, tubular vimentin expression, and EMT phenomenon according to uRBP levels: To further investigate the degree of tubule-interstitial compartment injury associated with uRBP levels, collagen deposition, α-SMA myofibroblast infiltration, vimentin expression, and EMT phenomenon were studied. In normal kidney, type IV collagen is seen as a linear pattern along the tubular and glomerular basement membranes, glomerular mesangium, and renal arteriolar walls. In our study, we found presence of type IV collagen in all the usual sites but also occasionally in the interstitium. The α-SMA myofibroblast is normally found only in smooth muscle cells of renal arteries and arterioles in the kidney. In our study, α-SMA myofibroblast was not found in PTEC but essentially in the interstitium, particularly in areas with tubule-interstitial injury and adjacent to areas with PTEC expressing vimentin. Usually, vimentin is present in glomeruli, arterioles, and interstitial fibroblasts but is not found in the PTEC. In our study, however, vimentin was also found in PTEC and in interstitium where its expression was greater in areas with chronic tubule-interstitial injury. It was also found in places where collagen deposition was detected by Sirius red (r=0.469; p=0.003) and where interstitial α-SMA myofibroblasts expression was observed (r=0.477; p=0.001) (Table 3).
The EMT phenomenon was also analyzed here, as it is part of the fibrogenic mechanism. Eighteen (35%) biopsies presented EMT (characterized by presence of both tubular vimentin and megalin expression).
Levels of uRBP had no association with collagen deposition, α-SMA myofibroblast infiltration, tubular vimentin expression, and EMT phenomenon (Table 3).
Biopsies with higher tubule-interstitial lesion (ci + ct ≥ 1)had higher amount of collagen deposition, higher expression of vimentin, of α-SMA myofibroblast expressions and more EMT phenomenon, indentified as vimentin expression and α-SMA myofibroblasts expressions (Table 4). The mean of Sirius red staining was 3.54 ± 2.44 in biopsies with ci + ct > 1 vs. 2.02 ± 1.19 in biopsies with ci + ct < 1 (p=0.016). The mean of myofibroblast expression was 7.50 ± 6.12 in biopsies with ci + ct > 1 vs. 2.59 ± 2.52 in biopsies with ci + ct < 1 (p=0.001). The mean of vimentin-positive tubules was 6.34 ± 5.10 in biopsies with ci + ct > 1 vs. 3.02 ± 3.34 in biopsies with ci + ct < 1 (p=0.033). Twenty one biopsies with EMT phenomenon had ci+ct > 1 while only 2 biopsies with EMT phenomenon had ci + ct < 1 (p=0.022) (Table 4).
| ci+ct < 1 (N=13) | ci+ct ≥ 1 (N=41) | p value* | |
|---|---|---|---|
| EMT phenomenon | 2 biopsies (15.4%) | 21 biopsies (51.2%) | 0.022 |
| Sirius red staining | 2.02±1.19 | 3.54±2.44 | 0.016 |
| Vimentin expression | 3.02±3.25 | 6.34±5.10 | 0.033 |
| a-SMA myofibroblast expression | 2.59±2.52 | 7.50±6.12 | 0.001 |
| Type IV collagen deposition | 13.95±4.67 | 15.24±7.54 | 0.595 |
| Megalin expression | 8.37±3.13 | 7.10±3.15 | 0.212 |
| CrCL (mL/min) at biopsy time | 63.74±12.51 | 60.00±19.52 | 0.521 |
p value*: High vs. normal uRBP values. Values were expressed as mean and standard deviation
Table 4: Association between tubulointerstitial injury (ci+ct ≥ 1) and others morphological findings and renal function at biopsy time.
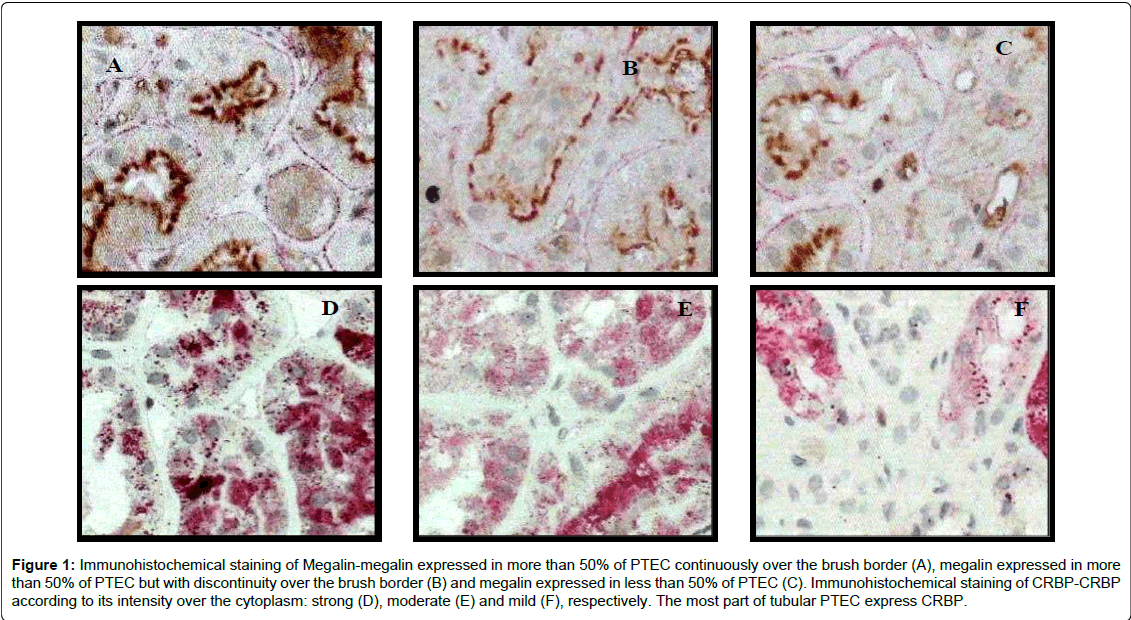
Megalin and CRBP expressions according to uRBP levels: To investigate functional impairment of proximal tubules, immunostaining of megalin, involved in the RBP receptor-mediated endocytocis process, was performed. Megalin expression was observed at the BB of PTEC. One biopsy showed no expression of megalin. In this case, the RBPu level was 1.02 mg/L. The remaining biopsies presented the following distribution of megalin expression in proximal tubules: 56.8% had discontinuous expression of megalin in more than 50% of the tubules, 29.5% had the megalin continuously expressed over the BB in more than 50% of the tubules, and 11.4% had megalin in less than 50% of the tubules (Figure 1: Panels A-C). Patients who presented higher levels of uRBP had lower megalin expression (according to morphometric quantification) in their biopsies with significant difference between groups according to uRBP (6.22 ± 3.03 vs. 8.53 ± 2.92, p=0.007) (Table 3). Megalin and CRBP expressions did not present any correlation with other morphological markers here analysed (EMT phenomenon, Sirius red staining, vimentin expression, α-SMA myofibroblast expression and type IV collagen deposition) (Table 5).
Figure 1: Immunohistochemical staining of Megalin-megalin expressed in more than 50% of PTEC continuously over the brush border (A), megalin expressed in more than 50% of PTEC but with discontinuity over the brush border (B) and megalin expressed in less than 50% of PTEC (C). Immunohistochemical staining of CRBP-CRBP according to its intensity over the cytoplasm: strong (D), moderate (E) and mild (F), respectively. The most part of tubular PTEC express CRBP.
| Sirius red staining quantification | CRBP expression quantification | Megalin expression quantification | Interstitial a-SMA myofibroblast quantification | Tubular Vimentin expression quantification | Type IV collagen expression quantification | Creatinine Clearance at bx time | |
|---|---|---|---|---|---|---|---|
| Sirius red staining quantification | 1 | r=-0.104 p=0.557 | r=-0.077 p=0.646 | r=0.319 p=0.085 | r=0.469 p=0.003 | r=-0.145 p=0.412 | r=-0.168 p=0.307 |
| CRBP expression quantification | r=-0.104 p=0.557 | 1 | r=0.199 p=0.190 | r=-0.043 p=0.797 | r=-0.180 p=0.244 | r=-0.280 p=0.080 | r=-0.096 p=0.534 |
| Megalin expression quantification | r=-0.077 p=0.646 | r=0.199 p=0.190 | 1 | r=-0.168 p=0.282 | r=-0.135 p=0.344 | r=0.025 p=0.872 | r=0.088 p=0.538 |
| Interstitial a-SMA myofibroblast quantification | r=0.319 p=0.085 | r=-0.043 p=0.797 | r=-0.168 p=0.282 | 1 | r=0.477 p=0.001 | r=0.020 p=0.904 | r=-0.220 p=0.147 |
| Tubular Vimentin expression quantification | r=0.469 p=0.003 | r=-0.180 p=0.244 | r=-0.135 p=0.344 | r=0.477 p=0.001 | 1 | r=-0.127 p=0.413 | r=-0.282 p=0.047 |
| Type IV collagen expression quantification | r=-0.145 p=0.412 | r=-0.280 p=0.080 | r=0.025 p=0.872 | r=0.020 p=0.904 | r=-0.127 p=0.413 | 1 | r=-0.098 p=0.529 |
| Creatinine Clearance at biopsy time | r=-0.168 p=0.307 | r=-0.096 p=0.534 | r=0.088 p=0.538 | r=-0.220 p=0.147 | r=-0.282 p=0.047 | r=-0.098 p=0.529 | 1 |
Table 5: Correlations between morphological findings and renal function.
To investigate absorption by PTEC, we looked for the presence of RBP inside of the tubular epithelial cytoplasm. In normal circumstances, RBP inside the tubules is dissociated from megalin and redistributed to the rest of the body. Here, the cellular retinol binding protein (CRBP) expression was observed diffusely over the cytoplasma of PTECs in granules, with different degrees of intensity (mild, moderate, and strong), and in some cases with strong RBP stainning of the subapical membrane. Considering staining intensity, 4.4% showed mild expression, 62.2% moderate and 33.3% strong. Most biopsies (84%) presented CRBP expression in more than 70% of the PTEC (Figure 1: Panels: D-F). None of the characteristics of the CRBP here analyzed, such as staining intensity, percentage of CRBP stained tubules, and morphometric analysis showed any existing association among uRBP values and megalin expression (Table 5). No expression of CRBP was found in the glomeruli and interstitium, as showed by others [19].
Graft function and microalbuminuria at time of biopsy according to histological findings: Patients with higher tubular vimentin expression had a lower CrCl at the time of biopsy (r=-0.282; p=0.047) (Table 5). The presence of EMT in biopsies was associated with lower CrCl levels at the time of biopsy (52.03 ± 8.77 vs. 60.73 ± 20.61 mL/min; p=0.0004). Patients with ci + ct > 1 had a lower Cr Cl at the time of biopsy The presence of microalbuminuria did not correlate with histological findings (EMT phenomenon, Sirius red staining, vimentin expression, α-SMA myofibroblast expression and type IV collagen deposition, megalin expression and CRBP expression).
Discussion
In this study we showed that in stable renal transplanted patients with PTD (those with higher levels of uRBP) megalin expression is lower at brush border. Likewise, in megalin-deficient knockout mice there is a tubular reabsorption deficiency and a high excretion of LMWP in the urine [12,20]. These suggest the direct role of megalin receptor on LMWP binding and on their cellular uptake [12]. In accordance, recently Storm el al., described the clinical and morphological presentation of a very rare syndrome in a family, named Donnai-Barrow/Facio-Oculo- Acustico-Renal, caused by a pathological mutation in the megalinencoding gene (LRP2), leading to a dysfunctional megalin deficiency. Clinically, patients had hearing impairment, high-grade myopia and low-molecular weight proteinuria. Renal consequences of megalin deficiency were characterized by an increased urinary excretion of megalin-ligands, as vitamin D-binding protein, retinol binding protein and albumin. Their biopsies showed in light microscopy no major morphological abnormalities initially, however focal glomerulosclerosis has developed lately, in association with progressive renal dysfunction. In immunohistochemistry analysis, there was absence or reduced expression of megalin in proximal tubules and in the urine [21]. This is a clinical evidence of the relevant role of megalin in receptor-mediated endocytosis and the consequences of its deficiency.
Important substracts were identified as megalin-ligands including insulin, albumin, vitamin protein, RBP, B2-microglobulin and furthermore some toxic substances interact with megalin and undergo endocytosis, as glycated proteins, myeloma light chain, amino glycosides, leading to proximal tubule epithelial cells (PTECs) injury [12]. In a rat model of aristolochic acid nephropathy, tubular proteinuria and early reduction of megalin expression were also seen, indicating to be a process very sensitive to toxic insult and probably the involvement of megalin as a ligand of toxic substances [22].
In our study, we found no association between chronic histological findings and megalin expression, which suggests that even those cells with chronic morphological alterations might continue expressing the megalin receptor. However, in our study the level of chronicity of tubule-interstitial compartment is low. It is possible that megalin expression in transplanted patients with moderate to severe IF/TA shows an association with tubule-interstitial damage that are more chronically affected. However, Vinge et al., in a canine model of Alport Syndrome, characterized by a progressive glomerular disease, showed an impairment of megalin mediated endocytosis caused by protein overload, which lead to proximal dysfunction. The loss of LMW is due to the large amount of proteins in urine exceeding the reabsorption capacity of megalin [23].
In contrast with our initial hypothesis, CRBP was not affected by megalin expression. Besides being found in the apical granules, homogeneous staining for CRBP over the cytoplasm was also found. In general, CRBP was found in most PTECs in 80% of our biopsies. The presence of CRBP may reflect the plasma RBP that has been filtered and reabsorbed or it may also be a mechanism to conserve some of the retinol that is filtered and reabsorbed, as the retinol-RBP complex [19,24].
In our transplanted patients presenting stable renal function there are multiple morphological evidences of active fibrogenic processes occurring, with some of them being related with renal function, such as EMT presence and tubular vimentin expression. However, chronic histologic findings had no association with uRBP levels, confirming our previous findings, suggesting a functional substract for the PTD in stable renal transplant patients [13].
Considering the stability of our patients, the reason that led 50% of our population to have PTD is still unknown, however it could reflect the consistently lack of monitoring of PTD or the insults do exist but they are not clinically relevant and therefore are not identified. Previous study showed that the presence of biomarkers for tubular injury after renal transplantation was higher in patients with clinical presentation of tubular damage (tubulitis Ia/Ib, poliomavirus nephropathy, moderate to severe IF/TA) than in patients with no tubular pathologies or in patients with subclinical tubulitis [9].
In summary, chronic histologic findings can be seen early after transplantation in stable renal transplant patients, however they had no association with PTD in this sample of patients. The incidence of IF/TA rapidly progresses after renal transplantation, following an exponential curve. Its presence is associated with a decreased graft survival and its predictive value on outcome is independent from other predictors of survival, such as sCr or acute rejection. In clinical practice, the usual indicator for chronic histological findings is the progressive elevation of sCr, however this alteration occurs lately, when the damage of the tubule-interstitial compartment is already significant. Early detection of PTD may offer a more sensitive alternative to predict chronic tubule-interstitial allograft abnormalities before any other clinical and laboratorial sign can be observed.
In a separate analysis, including 50 renal transplant patients who used calcineurin inhibitors in their imunossupressor schema, and considering uRBP levels as >1 ml/L or <1 mg/L, we found 12 (24%) patients with RBP > 1 mg/L. Among 21 patients who used FK, 8 had uRBP > 1 mg/L, (p=0.047 RR: 3.9), while only 4 of 29 patients who used Csa had uRBP > 1 mg/L. According to Banff 97 Classification, 42% bxs had CAN, 74% had ci + ct > 1 and 12% of biopsies presented calcineurin inhibitor nephrotoxicity (CINT). These features did not show association with the type of imunossupressor used (FK or Csa) and with CIN levels. Fibrosis measured by Sirius red staining was similar in both groups. Among immunohistochemical markers studied, patients who used FK had a lower amount of megalin in their biopsies (5.8 ± 3.4 vs. 8.3 ± 2.6 p=0.007).
Therefore, patients who used FK was associated with a worse proximal tubular function and independently of its level, it was also associated with a reduced megalin expression in brush border of PTC.
The process of uRBP absorption by PTEC, megalin-mediated, was also analyzed in our study, with reduction of the megalin receptor representing the subjacent functional alteration found in patients with high uRBP levels. The absorption of uRBP by PETC requires a multistep high energy consuming process. Therefore, any interference in this process caused by known insults, such as the renal transplantation (ischemic, immunological, infectious or CIN), could interfere with megalin expression and consequently with uRBP absorption.
Experimental models of CIN, rejection or ischemia-reperfusion could be done to evaluate their effect, alone or together, on the expression of megalin. It would be interesting to investigate whether loss of megalin expression is irreversible and what its role is in chronic damage. A better understanding of this process may open new avenues to alternative treatments to counteract the progression of renal disease.
References
- Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, et al.(1999) Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med 341: 1725-1730.
- Campistol JM, Boletis IN, Dantal J, de Fijter JW, Hertig A, et al.(2009) Chronic allograft nephropathy--a clinical syndrome: early detection and the potential role of proliferation signal inhibitors. Clin Transplant 23: 769-777.
- Nankivell BJ, Borrows RJ, Fung CL, O'Connell PJ, Allen RD, et al. (2003) The natural history of chronic allograft nephropathy. N Engl J Med349: 2326-2333.
- Câmara NO, Matos AC, Rodrigues DA, Pereira AB, Pacheco-Silva A(2001) Early detection of heart transplant patients with increased risk of ciclosporin nephrotoxicity. Lancet 357: 856-857.
- Câmara NO, Silva MS, Nishida S, Pereira AB, Pacheco-Silva A(2004) Proximal tubular dysfunction is associated with chronic allograft nephropathy and decreased long-term renal-graft survival. Transplantation 78: 269-275.
- Teppo AM, Honkanen E, Finne P, Törnroth T, Grönhagen-Riska C (2004) Increased urinary excretion of alpha1-microglobulin at 6 months after transplantation is associated with urinary excretion of transforming growth factor-beta1 and indicates poor long-term renal outcome. Transplantation 78: 719-724.
- Camara NO, Williams WW, Pacheco-Silva A (2009) Proximal tubular dysfunction as an indicator of chronic graft dysfunction. Braz J Med Biol Res 42: 229-236.
- Chinen R, Câmara NO, Nishida S, Silva MS, Rodrigues DA, et al. (2006) Determination of renal function in long-term heart transplant patients by measurement of urinary retinol-binding protein levels. Braz J Med Biol Res 39: 1305-1313.
- Schaub S, Mayr M, Hönger G, Bestland J, Steiger J, et al.(2007) Detection of subclinical tubular injury after renal transplantation: comparison of urine protein analysis with allograft histopathology. Transplantation 84: 104-112.
- Christensen EI,Gburek J (2004) Protein reabsorption in renal proximal tubule-function and dysfunction in kidney pathophysiology. PediatrNephrol 19: 714-721.
- Christensen EI1, Moskaug JO, Vorum H, Jacobsen C, Gundersen TE, et al. (1999) Evidence for an essential role of megalin in transepithelial transport of retinol. J Am SocNephrol 10: 685-695.
- De S, Kuwahara S, Saito A (2014) Theendocytic receptor megalin and its associated proteins in proximal tubule epithelial cells. Membranes (Basel) 4:333-355.
- de Matos AC, Câmara NO, de Oliveira AF, Franco MF, Moura LA, et al. (2010) Functional and morphologic evaluation of kidney proximal tubuli and correlation with renal allograft prognosis. TransplInt 23: 493-499.
- Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, et al. (1999) A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med 130: 461-470.
- Pereira AB, Nishida SK, Vieira JG, Lombardi MT, Silva MS, et al. (1993) Monoclonal antibody-based immunoenzymometric assays of retinol-binding protein. ClinChem 39: 472-476
- MastroianniKirsztajn G, Nishida SK, Silva MS, Ajzen H, Pereira AB (2000) Urinary retinol-binding protein as a prognostic marker in the treatment of nephrotic syndrome. Nephron 86: 109-114.
- Racusen LC, Solez K, Colvin RB, Bonsib SM, Castro MC, et al. (1999) The Banff 97 working classification of renal allograft pathology. Kidney Int 55: 713-723.
- Lavaud S, Poirier B, Mandet C, Bélair MF, Irinopoulou T, et al. (2001) Inflammation is probably not a prerequisite for renal interstitial fibrosis in normoglycemic obese rats. Am J Physiol Renal Physiol 280: 683-694.
- Beneden KKV, Grunsven LALV, Geers CC, Pauwels MM, Desmoulière AA, et al.(2008) CRBP-I in the renal tubulointerstitial compartment of healthy rats and rats with renal fibrosis. Nephrol Dial Transplant 23: 3464-3471.
- Leheste JR, Rolinski B, Vorum H, Hilpert J, Nykjaer A, et al. (1999) Megalin knockout mice as an animal model of low molecular weight proteinuria. Am J Pathol 155: 1361-1370.
- Storm T, Tranebjærg L, Frykholm C, Birn H, Verroust PJ, et al. (2013)Renal phenotypic investigations of megalin-deficient patients: novel insights into tubular proteinuria and albumin filtration. Nephrol Dial Transplant 28: 585-591.
- Lebeau C, Debelle FD, Arlt VM, Pozdzik A, De Prez EG, et al. (2005) Early proximal tubule injury in experimental aristolochic acid nephropathy: functional and histological studies. Nephrol Dial Transplant 20: 2321-2332.
- Vinge L, Lees GE, Nielsen R, Kashtan CE, Bahr A, et al. (2010) The effect of progressive glomerular disease on megalin-mediated endocytosis in the kidney. Nephrol Dial Transplant 25: 2458-2467.
- Kato M, Kato K, Goodman DS (1984)Immunocytochemical studies on the localization of plasma and of cellular retinol-binding proteins and of transthyretin (prealbumin) in rat liver and kidney. J Cell Biol 98: 1696-1704.
Citation: Matos ACC, Câmara NOS, Maurano A, Durão M, Tonato EJ, et al. (2016) Deficient of Megalin in Stable Renal Transplanted Patients with Proximal Tubular Dysfunction. J Clin Exp Transplant 1: 107. DOI: 10.4172/2475-7640.1000107
Copyright: © 2016 Matos ACC, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 13317
- [From(publication date): 11-2016 - Aug 23, 2025]
- Breakdown by view type
- HTML page views: 12343
- PDF downloads: 974